Introduction
Pulmonary fibrosis results from different types of
lung injuries and is characterized by an excessive deposition of
extracellular matrix (ECM) proteins, including collagens type I–V
in the alveolar wall. Pulmonary fibrosis is a potentially lethal
disorder with currently no effective therapies (1). Our current understanding of the
mechanisms of pulmonary fibrosis is predominantly derived from
studies of bleomycin-induced lung fibrosis in mice and rats
(2–4). The underlying molecular mechanisms of
lung fibrosis remain to be fully elucidated, however, certain
important pathological features of pulmonary fibrosis have been
identified, including increased ECM synthesis and deposition and
replacement of normal functional lung tissue with an abnormal
accumulation of fibroblasts and collagen (2). Certain profibrotic molecules,
including transforming growth factor-β1 (TGF-β1) (3), Smad3 (4), heat shock protein 47 (HSP47)
(2), α-smooth muscle actin (α-SMA)
(5,6) and collagen type I are involved in
pulmonary fibrosis initiation and progression. Although significant
progress has been made in understanding the molecular mechanisms of
the pathogenesis of pulmonary fibrosis (7), there are no effective therapies for
treating this disease and the five-year survival rate is <50%
according to clinical studies (8–10).
Thus, it is necessary to identify new drugs with an improved
efficacy and tolerability for pulmonary fibrosis.
Artesunate, which is recognized as being a vital
cornerstone in the control of malaria (11), has been used in the treatment of
severe and complicated malaria around the world (12,13).
Notably, a previous study demonstrated that artesunate conjugated
with a single-chain variable fragment from the monoclonal antibody
NP11-4 inhibited liver fibrosis induced by schistosomiasis
(14). Therefore, it was
hypothesized that artesunate may attenuate pulmonary fibrosis.
Based on these previous findings, the present study used a rat
model of pulmonary fibrosis stimulated by bleomycin instillation
and treated with artesunate, to examine whether artesunate
attenuates bleomycin-induced pulmonary fibrosis, and whether
artesunate affects the expression of profibrotic molecules,
including TGF-β1, Smad3, HSP47, α-SMA and collagen type I, which
are important in the initiation and development of pulmonary
fibrosis.
Materials and methods
Materials
Bleomycin was purchased from Zhejiang Hisun
Pharmaceutical Co., Ltd. (Taizhou, China). Artesunate was purchased
from Guilin Pharmaceutical Co., Ltd. (Guilin, China). The
Hydroxyproline assay kit (#A030-2) was obtained from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). Primers were
synthesized by Invitrogen Life Technologies (Shanghai, China). The
cDNA synthesis kit was purchased from Takara Biotechnology Co.,
Ltd. (Dalian, China). The total RNA extraction kit and 2X Taq PCR
Master mix were obtained from Tiangen Biotech Co., Ltd. (Beijing,
China). Western blot and immunoprecipitation tissue and cell lysis
solution were purchased from Beijing Biosynthesis Biotechnology
Co., Ltd. (Beijing, China). The bicinchoninic acid protein assay
kit was obtained from Beyotime Institute of Biotechnology
(Shanghai, China). The TGF-β1 antibody and horseradish
peroxidase-conjugated goat anti-rabbit/mouse secondary antibody
were purchased from Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd. (Beijing, China). Smad3 antibody was purchased from
Abzoom Biolabs, Inc. (Dallas, TX, USA). α-SMA antibody and the
Hsp47 antibody were purchased from Epitomics (Burlingame, CA,
USA).
Animals
Male Sprague Dawley (SD) rats weighing 180–250 g
(Guilin Medical University, Guilin, China) were used in all
experiments. SD rats were housed in specific-pathogen-free
conditions with free access to food and water.
Experimental protocols
All experimental protocols performed on all rats
were approved by the Guilin Medical University Animal Experiment
Ethics Committee. Animals were randomly assigned to one of the
following four groups: i) Control group (n=24), which received
intratracheal administration of 0.9% NaCl solution alone; ii)
bleomycin group (n=39), which received intratracheal administration
of bleomycin (5 mg/kg); iii) artesunate group (n=24), which
received daily intraperitoneal injections of artesunate (100 mg/kg)
and the iv) bleomycin + artesunate group (n=39), which received
intratracheal administration of bleomycin (5 mg/kg) and daily
intraperitoneal injection of artesunate (100 mg/kg). Bleomycin was
injected into the trachea exposed through a midline anterior neck
incision using a 24-gauge needle. At 7, 14, 21 or 28 days after
bleomycin treatment, rats were sacrificed by decapitation under
anesthesia by inhaling isofluorane (3.5% in oxygen; sc-363629Rx;
Santa Cruz Animal Health, Paso Robles, CA, USA), and lung tissue
was quickly removed and processed as described below.
Histopathological analysis
Left lungs obtained from rats were instilled with
10% formalin. Tissues were embedded in paraffin and then cut into 4
μm thickness sections that were used for hematoxylin and
eosin (H&E), Masson staining and immunohistochemistry analysis.
The severity of interstitial fibrosis was observed and assessed by
a blinded pathologist using the Ashcroft score (15). Masson staining was performed in the
present study to observe collagen deposition. Right lungs obtained
from rats were immediately frozen in liquid nitrogen and then
stored at −80°C in a freezer until use for immunoblot analysis,
reverse transcription-polymerase chain reaction (RT-PCR) and
hydroxyproline measurement.
Hydroxyproline measurement
The total collagen content of the right lung was
determined by hydroxyproline measurement using a hydroxyproline
determination kit (Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer’s instructions. Data are expressed as
milligrams of hydroxyproline per gram of protein in the lungs
(mg/g).
Immunoblotting
The lung tissues were homogenized in WIP tissue and
cell lysis solution with the protease inhibitor cocktail
(#04693159001; Roche Diagnostics, Basel, Switzerland) using a
tissue grinder. The concentration of protein was determined using a
protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Approximately 30 μg protein from each sample was
electrophoresed in a 10 or 12% polyacrylamide gel. Following
transferring proteins onto a polyvinylidene fluoride membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), immunoblotting was
performed using monoclonal rabbit anti-rat HSP47 (1:1,000 dilution;
#3198-1), TGF-β1 (1:1,000 dilution; #RS-0105R), α-SMA (1:500
dilution; #5264-1) and polyclonal rabbit anti-rat Smad3 (1:500
dilution; #AM4061) antibodies. The proteins were visualized by
incubating the membrane with chemiluminescence reagent (NEN Life
Science Products, Boston, MA, USA) and exposing the membrane to
X-ray films.
RT-PCR
RT-PCR was used in the present study to determine
the mRNA expression of HSP47 and collagen type I mRNA in the lung.
In brief, total RNA was isolated from the lung tissues using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). PCR was
performed using a DNA thermal cycler in a 25 μl reaction
volume, containing the cDNA template (2 μl), forward and
backward primers (1 μl each), 12.5 μl 2X Master mix
and 8.5 μl ddH2O for 30 cycles via GeneAmp PCR
system 9700 (Applied Biosystems, Foster City, CA, USA). The primer
sequences were as follows: HSP47, forward 5′-AGA ACC AAG GCA GAC
TTA TCGC-3′ and reverse 5′-CCG TAG ATG TCC TGG TCA AAG-3′; collagen
type I, forward 5′-TGC CGT GAC CTC AAG ATG TG-3′ and reverse 5′-CAC
AAG CGT GCT GTA GGT GA-3′; GAPDH, forward 5′-GTG CTG AGT ATG TCG
TGG AG-3′ and reverse 5′-ACC AGT GGA TGC AGG GAT-3′. The rat GAPDH
housekeeping gene was used as an internal control.
Immunohistochemical analysis
Slides were rinsed in 1X phosphate-buffered saline
(PBS) and then incubated in 0.3% H2O2
dissolved in methanol for 30 min in order to quench endogenous
peroxidase. Following being rinsed with 1X PBS several times,
slides were incubated with 3% normal serum in 1X PBS for 1 h to
block nonspecific binding. Slides were then incubated with HSP47
antibody (1:400) or α-SMA antibody (1:500) overnight at 4°C.
Following being rinsed with 1X PBS several times, slides were
incubated with biotinylated goat anti-rabbit polyclonal secondary
antibody (1:200; #BA1000; Vector Laboratories, Burlingame, CA, USA)
for 1 h and then avidinbiotin complex reagent (Vector Laboratories)
for 30 min. Slides were rinsed in 1X PBS and covered with
diaminobenzidine peroxidase substrate solution from the Impact DAB
kit (Vector Laboratories) for 2 min and then rinsed in water.
Counterstaining was performed with hematoxylin. Slides were then
dehydrated using increasing concentrations of ethanol and xylenes
and mounted. Finally, images of tissue sections were captured using
an Olympus BX53 digital microscope (Olympus, Tokyo, Japan).
Statistical analysis
Statistical analyses were performed with Sigmaplot
software (SPSS) version 17.0 software (IBM, Armonk, NY, USA). The
data are presented as the mean ± standard deviation. Statistical
differences were determined by two-way analysis of variance and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Artesunate reduces bleomycin-induced
mortality in rats
As shown in Table
I, ~25.64, 8.70 and 11.11% of the bleomycin group rats died
within 7, 14 and 28 days after the administration of bleomycin,
however, administration of artesunate reduced mortality to 20.51,
4.00 and 0.00% within 7, 14 and 28 days, respectively. The data
demonstrated that the mortality rate was highest during the first 7
days after the administration of bleomycin. Following that, the
mortality rate remained at ~10.00% in the bleomycin group, while
the mortality rate reduced to 4.00% at 14 days. No mortality was
observed between 14 and 28 days in the bleomycin+artesunate group.
No mortality in the saline and artesunate group was observed during
the whole experimental period. Notably, between 14 and 21 days, no
mortality was found in all groups.
 | Table IArtesunate reduces bleomycin-induced
mortality in rats (%). |
Table I
Artesunate reduces bleomycin-induced
mortality in rats (%).
| Group | Day 7 | Day 14 | Day 21 | Day 28 |
|---|
| Con | 0.00 | 0.00 | 0.00 | 0.00 |
| Art | 0.00 | 0.00 | 0.00 | 0.00 |
| Ble | 25.64a | 8.70 | 0.00 | 11.11 |
| Ble+Art | 20.51a | 4.00a | 0.00 | 0.00a |
Artesunate attenuates bleomycin-induced
pulmonary fibrosis in rats
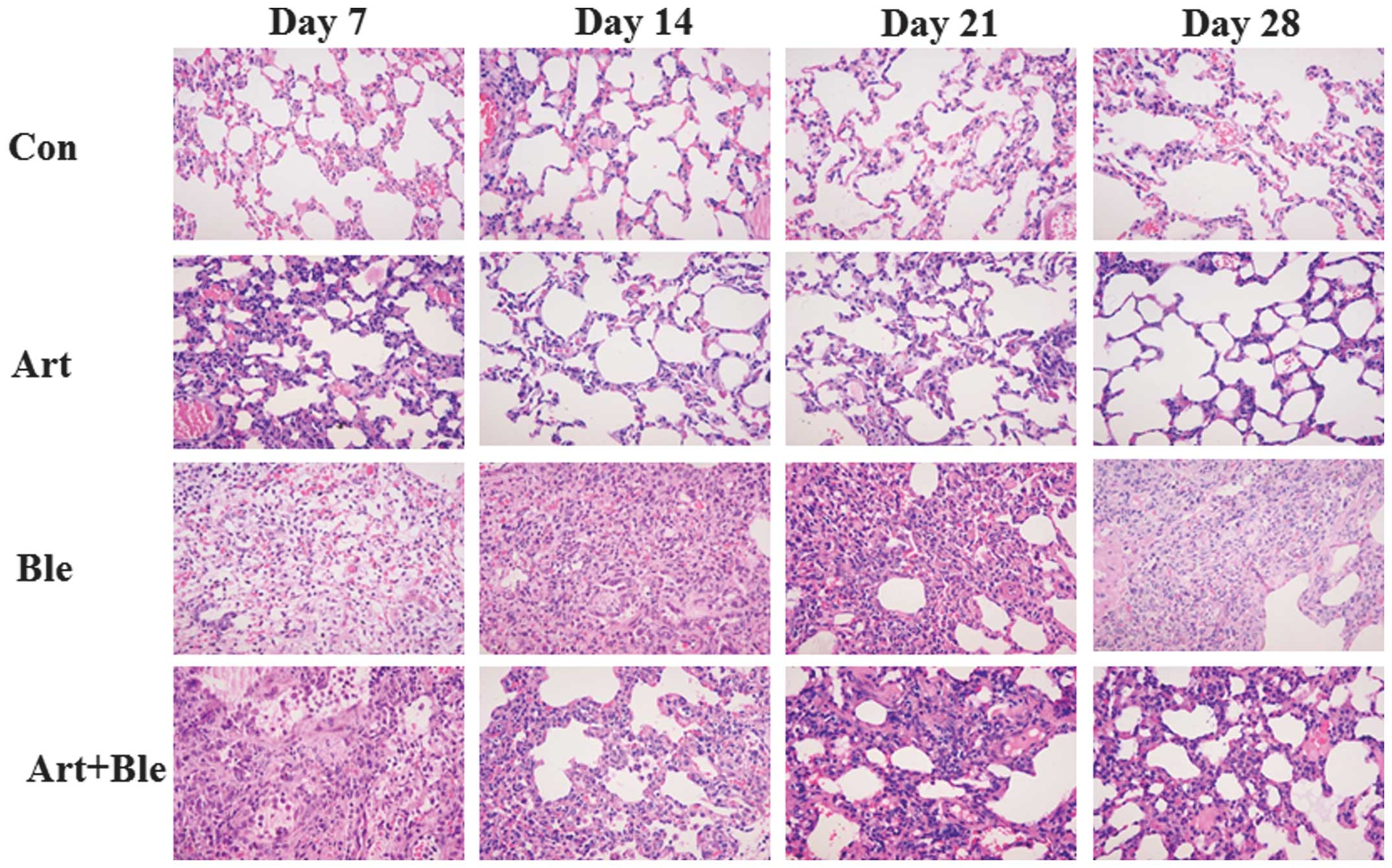
In order to determine the pathological alterations
in the lung, H&E staining was performed (Fig. 1) and Masson staining was used to
visualize the collagen fibrils (blue color) in the tissue (Fig. 2). The histological evaluation of
lung sections at 7, 14, 21 and 28 days after bleomycin treatment
revealed evidence of marked infiltration of inflammatory cells
(particularly at day 7), excessive deposition of mature collagen in
the interstitium (particularly at 14, 21 and 28 days), diffuse
consolidation of parenchyma with loss of alveolar architecture and
increased cell number, clear alveolar wall thickening and finally
lung tissue was severely damaged. However, following artesunate
treatment, the pathological alterations in the lung tissues were
attenuated. The rats in the control group and artesunate alone
group demonstrated no histological alterations. Similar to Masson
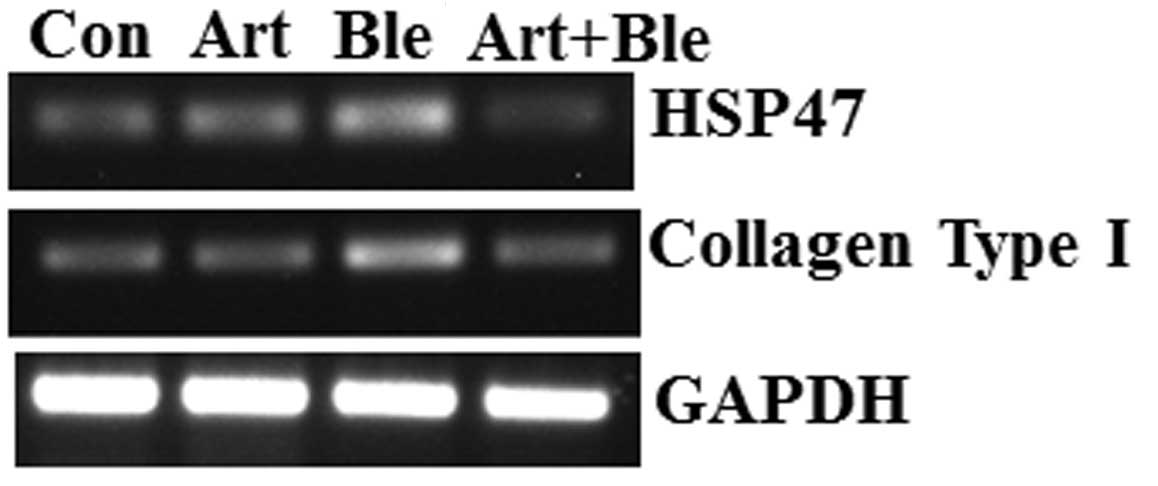
staining, the RT-PCR results demonstrated that the mRNA expression
of collagen type I (at day 28) was higher in bleomycin-treated rats
compared with that in the control rats, and that the addition of
artesunate then reduced this effect of bleomycin (Fig. 3). As for pulmonary fibrosis
evaluation, the hydroxyproline content was measured. On days 7 and
14, the hydroxyproline contents were similar between the four
groups. Out of all time points, the highest hydroxyproline contents
were observed at day 14 in all groups. On day 21, the rats
receiving bleomycin demonstrated a higher hydroxyproline content
than the control group. However, artesunate did not significantly
reduce bleomycin-induced increases in hydroxyproline content on day
21. On day 28, the bleomycin group had a higher hydroxyproline
content than the control group. However, artesunate significantly
reduced increases in bleomycin-induced hydroxyproline content
(Table II). Additionally,
artesunate alone had no effect on hydroxyproline content. The
severity of interstitial fibrosis among the four groups was
compared using the Ashcroft score. As shown in Table III, on day 14, 21 and 28, the
Ashcroft score was higher in the bleomycin group than that in the
control group. Administration of artesunate could reduce
bleomycin-induced increases in the Ashcroft score on day 21 and 28,
but not on day 14. In addition, artesunate alone had no effect on
the Ashcroft score.
 | Table IIHydroxyproline content in the four
groups at different time points (μg/g). |
Table II
Hydroxyproline content in the four
groups at different time points (μg/g).
| Group | Day 7 | Day 14 | Day 21 | Day 28 |
|---|
| Con | 388.33±60.30 | 644.00±88.60 | 355.50±47.72 | 435.00±65.41 |
| Art | 424.67±37.50 | 650.00±84.53 | 354.67±47.58 | 424.17±47.26 |
| Ble | 346.80±68.10 | 699.33±79.75 | 513.17±72.72a | 584.50±60.85a |
| Ble+Art | 335.33±56.24 | 719.33±108.84 | 489.33±58.81a | 492.17±75.54b |
 | Table IIIAshcroft score in the four groups at
different time points. |
Table III
Ashcroft score in the four groups at
different time points.
| Group | Day 14 | Day 21 | Day 28 |
|---|
| Con | 2.17±0.42 | 2.33±0.97 | 2.99±0.59 |
| Art | 2.22±0.47 | 2.37±0.64 | 3.06±0.59 |
| Ble | 6.15±0.60a | 6.25±0.71a | 5.98±0.87a |
| Ble+Art | 6.29±0.36a | 3.72±0.92b | 4.01±1.25b |
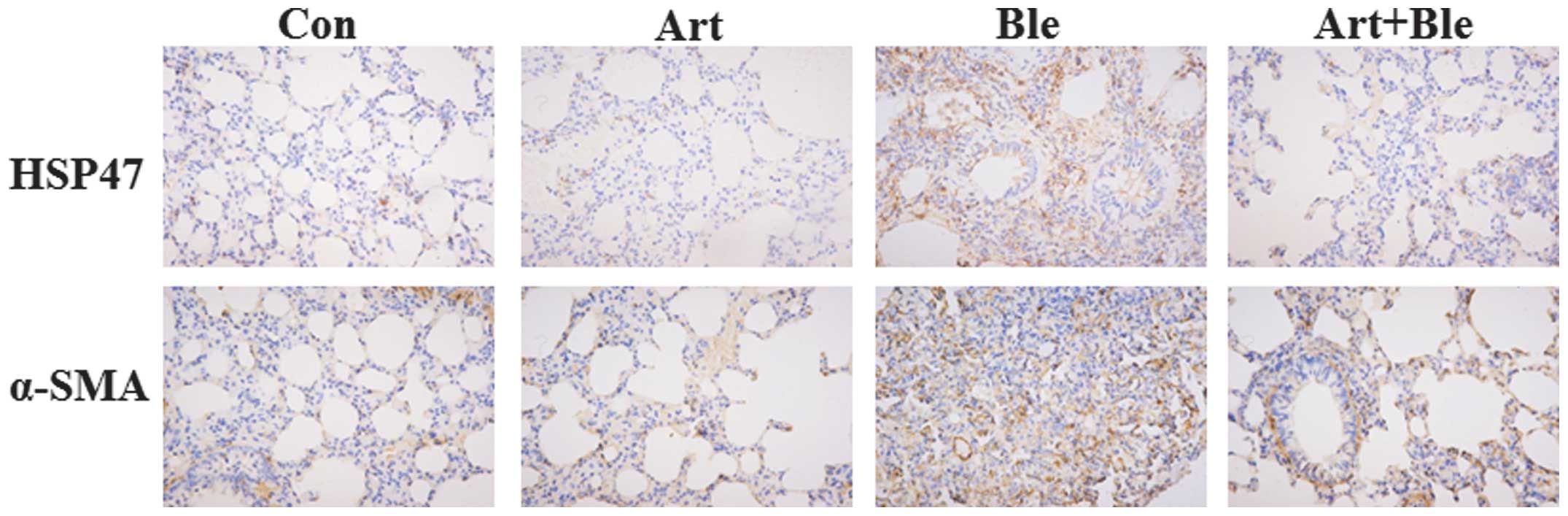
Artesunate inhibits HSP47 expression in
bleomycin-induced pulmonary fibrosis
As stated above, bleomycin induced collagen
deposition in lung tissues and artesunate inhibited this. HSP47, a
collagen-binding glycoprotein, is associated with collagen
accumulation and disease progression in an experimental pulmonary
fibrosis model (2). Therefore, in
the present study, the effect of artesunate on HSP47 was
investigated. The data demonstrated that bleomycin induced HSP47
upregulation and artesunate inhibited HSP47 upregulation, which is
evidenced by the results of RT-PCR (Fig. 3), immunohistochemistry (Fig. 4) and western blotting (Fig. 5).
 | Figure 5Western blotting to detect HSP47,
TGF-β1, α-SMA and Smad3 expression in bleomycin-treated rat lungs
with and without artesunate. At 28 days after bleomycin injection
with or without artesunate treatment, the rats were sacrificed and
their lungs were removed. Western blotting was performed to detect
HSP47, TGF-β1, α-SMA and Smad3 protein expression in the lung
tissues. Con, control group; Art, artesunate group; Ble, bleomycin
group; Both, bleomycin+artesunate group; HSP47, heat shock protein
47; α-SMA, α-smooth muscle actin; TGF-β1, transforming growth
factor-β1. |
Artesunate inhibits TGF-β1, Smad3 and
α-SMA expression in bleomycin-induced pulmonary fibrosis in
rats
TGF-β1 and its downstream molecule α-SMA are known
to be critical for pulmonary fibrosis (5,6).
Western blotting confirmed enhanced protein expression of TGF-β1 on
day 28 after bleomycin injection compared with the control group
(Fig. 5). In addition, Fig. 5 also shows that bleomycin-induced
increases in TGF-β1 were inhibited by artesunate. Western blotting
demonstrated that bleomycin treatment led to increased Smad3
expression and artesunate administration attenuated this increase
(Fig. 5). Similar to TGF-β1, α-SMA
protein was enhanced by bleomycin treatment and this enhancement
was also inhibited by artesunate administration, which was
confirmed by immunohistochemistry (Fig. 4) and western blotting (Fig. 5).
Discussion
As a progressive and largely untreatable group of
disorders, pulmonary fibrosis is caused by exposure to
radiotherapy, chemotherapeutic drugs, viral infection or other
conditions. Despite numerous drugs being identified and developed
to treat pulmonary fibrosis, the prognosis remains poor and new
drugs with improved efficacy and tolerability are required.
A previous study confirmed that collagen deposition
must be controlled in order to reverse pulmonary fibrosis and
improve the mortality rate (2). In
the present study, it was found that artesunate reduced
bleomycin-induced mortality in rats. Artesunate also attenuated
bleomycin-induced collagen deposition, which was confirmed by
H&E staining, Masson staining, hydroxyproline content
measurement, Ashcroft score evaluation and collagen type I mRNA
determination by RT-PCR. Decreases in the mRNA expression of
collagen type I in artesunate-treated bleomycin-induced pulmonary
fibrosis rats indicated that the anti-fibrotic effect of artesunate
may be mediated through direct inhibition of collagen type I
expression. However, artesunate ameliorated the increases in
TGF-β1, Smad3, HSP47 and α-SMA induced by bleomycin implying that
the anti-fibrotic effect of artesunate may also be mediated by
inhibition of these pro-fibrotic proteins with a resultant
reduction of collagen synthesis in lung fibrosis.
TGF-β1 is a profibrotic cytokine (3). The finding in the present study that
bleomycin induced increases in TGF-β1 protein expression, together
with a previous study that TGF-β1 expression in lung tissues was
markedly elevated 21 days after bleomycin treatment (7) indicated that the upregulation of
TGF-β1 is important in the pathogenesis of bleomycin-induced
pulmonary fibrosis (16,17). Generally, TGF-β1 performs its
profibrotic effects by stimulation of downstream Smad protein.
Smad3 was increased by bleomycin treatment in the current study. It
is established that Smad3, a downstream protein of TGF-β1, is
necessary for TGF-β1 signal transduction (18,19).
A previous study confirmed that TGF-β1/Smad3 affected the
expression of HSP47 in bleomycin-induced pulmonary fibrosis
(4), suggesting that a
TGF-β1/Smad3/HSP47 signaling pathway exists affecting collagen
deposition. However, a study in human lung fibroblasts revealed
that TGF-β1 induced trimer formation of heat shock factor 1 (HSF1)
and then HSF1 bound to the heat shock promoter element to induce
HSP47 synthesis (20), suggesting
that another signaling pathway TGF-β1/HSF1/HSP47 also affects
collagen deposition. It is well established that HSP47 is important
in the synthesis, processing and secretion of procollagen (21) and thus is important in the
pathogenesis of pulmonary fibrosis. In the current study, RT-PCR,
immunohistochemistry and western blotting confirmed that HSP47
expression was increased by bleomycin treatment, which is
consistent with a previous study in a bleomycin model of rat
pulmonary fibrosis (2). In this
study, the authors found that HSP47 protein in the lung was
increased, collagen accumulation and disease progression were
associated with the level of HSP47 protein expression and a
decrease in HSP47 expression was in line with the attenuation of
fibrotic lesions and collagen expression (2). Another study revealed that the
upregulated signaling pathway of TGF-β1/HSP47/collagen I may be
associated with the pathogenesis of fibrosis in rats (22). α-SMA, a downstream molecule of
TGF-β1, is known to be critical for pulmonary fibrosis (5,6).
HSP47 mRNA was found to be localized in α-SMA-positive
myofibroblasts in the active fibrotic areas (23), however, the precise association
between HSP47 and α-SMA remains to be elucidated.
Taken together, it was hypothesized that bleomycin
affects collagen deposition through the TGFβ1→Smad3 (or HSF1)→HSP47
(or α-SMA)→collagen I signaling pathway. As confirmed in the
present study, bleomycin treatment induced these increases in
pro-fibrotic molecules. Notably, artesunate could inhibit
bleomycin-induced pro-fibrotic molecule enhancement. Therefore, the
anti-fibrotic effect of artesunate may be associated with
alterations in these pro-fibrotic molecules. Thus, targeting these
pro-fibrotic molecules presents a promising method for the therapy
of pulmonary fibrosis. To the best of our knowledge, the present
study is the first experimental study investigating the effects of
artesunate in pulmonary fibrosis. The optimal effective and
tolerable dosage of artesunate in pulmonary fibrosis remains to be
elucidated and requires investigation in future studies. Our
findings demonstrated that artesunate inhibits pulmonary fibrosis
induced by bleomycin and its anti-fibrotic effects are possibly
associated with the attenuation of certain pro-fibrotic proteins,
including TGF-β1, Smad3, HSP47, α-SMA and collagen type I. However,
it is noteworthy that the mechanisms by which artesunate offers
protection against pulmonary fibrosis are not fully understood.
Although the present study confirmed that artesunate is a promising
novel drug to treat pulmonary fibrosis, clinical studies should be
performed to further examine the therapeutic value of artesunate in
lung fibrosis.
Acknowledgments
This study was supported by the Natural Science
Foundation of Guangxi (grant no. 2011GXNSFA018220).
References
|
1
|
Gharaee-Kermani M and Phan SH: Molecular
mechanisms of and possible treatment strategies for idiopathic
pulmonary fibrosis. Curr Pharm Des. 11:3943–3971. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hagiwara S, Iwasaka H, Matsumoto S and
Noguchi T: Antisense oligonucleotide inhibition of heat shock
protein (HSP) 47 improves bleomycin-induced pulmonary fibrosis in
rats. Respir Res. 8:372007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohashi S, Abe H, Takahashi T, Yamamoto Y,
Takeuchi M, Arai H, Nagata K, Kita T, Okamoto H, Yamamoto H and Doi
T: Advanced glycation end products increase collagen-specific
chaperone protein in mouse diabetic nephropathy. J Biol Chem.
279:19816–19823. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Wang T, Wang X, Sun BB, Li JQ, Liu
DS, Zhang SF, Liu L, Xu D, Chen YJ and Wen FQ: Blockade of advanced
glycation end product formation attenuates bleomycin-induced
pulmonary fibrosis in rats. Respir Res. 10:552009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang T, Chen M and Sun T: Simvastatin
attenuates TGF-beta1-induced epithelial-mesenchymal transition in
human alveolar epithelial cells. Cell Physiol Biochem. 31:863–874.
2013. View Article : Google Scholar
|
|
6
|
Ou XM, Feng YL, Wen FQ, Huang XY, Xiao J,
Wang K and Wang T: Simvastatin attenuates bleomycin-induced
pulmonary fibrosis in mice. Chin Med J (Engl). 121:1821–1829.
2008.
|
|
7
|
Zhu T, Zhang W, Xiao M, Chen H and Jin H:
Protective role of andrographolide in bleomycin-induced pulmonary
fibrosis in mice. Int J Mol Sci. 14:23581–23596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tzouvelekis A, Paspaliaris V, Koliakos G,
et al: A prospective, non-randomized, no placebo-controlled, phase
Ib clinical trial to study the safety of the adipose derived
stromal cells-stromal vascular fraction in idiopathic pulmonary
fibrosis. J Transl Med. 11:1712013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Woodcock HV, Molyneaux PL and Maher TM:
Reducing lung function decline in patients with idiopathic
pulmonary fibrosis: potential of nintedanib. Drug Des Devel Ther.
7:503–510. 2013.PubMed/NCBI
|
|
10
|
Raghu G, Collard HR, Egan JJ, et al: An
official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis:
evidence-based guidelines for diagnosis and management. Am J Respir
Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Atemnkeng MA, Chimanuka B, Dejaegher B,
Heyden YV and Plaizier-Vercammen J: Evaluation of Artemisia annua
infusion efficacy for the treatment of malaria in Plasmodium
chabaudi chabaudi infected mice. Exp Parasitol. 122:344–348. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nealon C, Dzeing A, Müller-Römer U,
Planche T, Sinou V, Kombila M, Kremsner PG, Parzy D and Krishna S:
Intramuscular bioavailability and clinical efficacy of artesunate
in gabonese children with severe malaria. Antimicrob Agents
Chemother. 46:3933–3939. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Batty KT, Le AT, Ilett KF, Nguyen PT,
Powell SM, Nguyen CH, Truong XM, Vuong VC, Huynh VT, Tran QB,
Nguyen VM and Davis TM: A pharmacokinetic and pharmacodynamic study
of artesunate for vivax malaria. Am J Trop Med Hyg. 59:823–827.
1998.PubMed/NCBI
|
|
14
|
Li H, Gu C, Ren Y, et al: The efficacy of
NP11-4-derived immunotoxin scFv-artesunate in reducing hepatic
fibrosis induced by Schistosoma japonicum in mice. J Biomed Res.
25:148–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ashcroft T, Simpson JM and Timbrell V:
Simple method of estimating severity of pulmonary fibrosis on a
numerical scale. J Clin Pathol. 41:467–470. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Willis BC, Liebler JM, Luby-Phelps K,
Nicholson AG, Crandall ED, du Bois RM and Borok Z: Induction of
epithelial-mesenchymal transition in alveolar epithelial cells by
transforming growth factor-beta1: potential role in idiopathic
pulmonary fibrosis. Am J Pathol. 166:1321–1332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cu A, Ye Q, Sarria R, Nakamura S, Guzman J
and Costabel U: N-acetylcysteine inhibits TNF-alpha, sTNFR and
TGF-beta1 release by alveolar macrophages in idiopathic pulmonary
fibrosis in vitro. Sarcoidosis Vasc Diffuse Lung Dis. 26:147–154.
2009.
|
|
18
|
Bartram U and Speer CP: The role of
transforming growth factor beta in lung development and disease.
Chest. 125:754–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leask A and Abraham DJ: TGF-beta signaling
and the fibrotic response. FASEB J. 18:816–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasaki H, Sato T, Yamauchi N, et al:
Induction of heat shock protein 47 synthesis by TGF-beta and IL-1
beta via enhancement of the heat shock element binding activity of
heat shock transcription factor 1. J Immunol. 168:5178–5183. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koide T, Asada S and Nagata K: Substrate
recognition of collagen-specific molecular chaperone HSP47.
Structural requirements and binding regulation. J Biol Chem.
274:34523–34526. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inoue K, Naito Y, Takagi T, et al:
Daikenchuto, a Kampo medicine, regulates intestinal fibrosis
associated with decreasing expression of heat shock protein 47 and
collagen content in a rat colitis model. Biol Pharm Bull.
34:1659–1665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kakugawa T, Mukae H, Hishikawa Y, Ishii H,
Sakamoto N, Ishimatsu Y, Fujii T, Koji T and Kohno S: Localization
of HSP47 mRNA in murine bleomycin-induced pulmonary fibrosis.
Virchows Arch. 456:309–315. 2010. View Article : Google Scholar : PubMed/NCBI
|