Introduction
Periprosthetic osteolysis is the predominant reason
causing of loosening of artificial hip joints (1,2).
Wear particles induce monocytes-macrophages, fibroblasts and
synovial cells in the tissue surrounding the joints to release
inflammatory cytokines, including tumor necrosis factor (TNF)-α,
interleukin (IL)-6 and monocyte chemoattractant protein (MCP)-1
(3,4). These inflammatory cytokines activate
osteoclasts by osteoprotegerin/receptor activator of nuclear factor
(NF)-κB ligand and the NF-κB signaling pathway, resulting in loss
of bone surrounding the prosthesis (5–7).
TNF-like weak inducer of apoptosis (TWEAK) is a
novel member of the TNF superfamily of ligands (8). TWEAK is involved in a variety of
biological processes by binding to its receptor, releasing
pro-inflammatory cytokines, regulating the immune response and
stimulating apoptosis, and is involved in tissue repair and
regeneration (9). The
pro-inflammatory effect of TWEAK has been observed in various cell
types, including synovial cells, macrophages, monocytes and
osteoclasts (10). p38 mitogen
activated protein kinase (p38 MAPK) is one member of the MAPK
family and is involved in regulating cellular responses to external
stimuli by intracellular signal transmission (11). The activation of the p38 MAPK
pathway activates NF-κB, which is involved in inflammation, by
regulating the secretion of pro-inflammatory factors (12,13).
However, few investigations have been performed on
the effects of the p38 MAPK signaling pathway in particle-induced
osteolysis, the specific function and mechanism of TWEAK in the
pathogenesis of prosthetic aseptic loosening or its correlation
with p38 MAPK. Previous studies have demonstrated that TWEAK
exhibits pathogenic importance by activating the p38 MAPK signaling
pathway in lupus nephritis and myositis diseases (14,15).
The present study investigated the expression levels
of TWEAK and p38 MAPK in periprosthetic interface membranes and in
RAW264.7 monocyte/macrophage cells treated with titanium (Ti)
particles to examine the effect of the TWEAK-p38 MAPK signaling
pathway in particle-induced inflammatory osteolysis.
Materials and methods
Patients and samples
All experimental procedures were approved by the
ethics committee of Southern Medical University and Jinling
Hospital (Nanjing, China). Informed consent was obtained from all
patients.
The synovium was obtained from patients who
underwent surgical procedures for plica syndrome or discoid
meniscus (2 males, 1 female; 12–41 years old), whereas
osteoarthritic synovium was obtained from patients who underwent
joint replacement for osteoarthritis (2 males, 1 female; 71–82
years old). Periprosthetic interface membranes were obtained from
patients undergoing surgical procedures for aseptic loosening (2
females, 1 male; 60–74 years old). The samples were obtained
between May and June 2013 at Jinling Hospital.
Particle preparation
TiAl6V4 particles were obtained from the College of
Materials Science and Engineering of Nanjing University of
Technology (Nanjing, China). The TiAl6V4 particles had a mean
diameter of 51.7 nm. Stock solutions of TiPS were prepared at a
concentration of 20 mg/ml in phosphate-buffered saline (PBS; Gibco
Life Technologies, Carlsbad, CA, USA), and the particles were
dispersed well by sonication for 10 min using Shumei KQ218
Ultrasonic Cleaning equipment (Kunshan Ultrasonic Instruments Co.,
Ltd., Jiangsu, China). The particles were autoclaved for 15 min at
121°C and 15 psi using a high-pressure steam sterilizer
(AMSCO® Evolution® Steam Sterilizer HC900;
STERIS Corporation, Mentor, OH, USA).
Cell culture
The RAW264.7 mouse macrophage-like cell line, was
obtained from China Center for Type Culture Collection (Shanghai,
China) and cultured in RPMI-1640 medium (Gibco Life Technologies)
supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis,
MO, USA). The cell density was adjusted to a concentration of
2×106 cells/ml, and 1 ml cell suspension was added into
each well of 24-well culture plates (BD Biosciences, Franklin
Lakes, NJ, USA) for culture in a 5% CO2 incubator at
37°C. The cells were divided into four groups: Group A, macrophage
cells; Group B, macrophage cells + p38 MAPK inhibitor (10
μmol/l SB203580; AdooQ Bioscience, Irvine, CA, USA); Group
C, macrophage cells + Ti particles (0.1 mg/ml TiPs); Group D,
macrophage cells + Ti particles + p38 MAPK inhibitor. The cells
were centrifuged at 300 × g for 10 min at 4°C, and the cells and
supernatant were harvested following 48 h culture in a 5%
CO2 incubator at 37°C, then centrifuged at 300 × g for
10 min at 4°C.
Histological evaluation
The harvested tissues were prepared for histological
examination in order to evaluate the inflammatory response. The
specimens were fixed in buffered 10% formalin (Sigma-Aldrich) in
PBS for 4–6 h at room temperature, embedded in paraffin
(Sigma-Aldrich) and then cut into 5 μm tissue sections. The
tissue sections were then stained with hematoxylin and eosin
(Sigma-Aldrich). The expression levels of TWEAK and phosphorylated
(p)-p38 MAPK were detected using a mouse anti-human TWEAK (cat. no.
CABT-47857MH; Creative Biomart, Shirley, NY, USA), or rabbit
polyclonal p-p38 MAPK monoclonal antibody (cat. no. sc-101758;
1:150 in PBS; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
and a horseradish peroxidase-conjugated anti-mouse secondary
antibody (1:300 in PBS; Santa Cruz Biotechnology, Inc.) at room
temperature for 4 h, followed by color development using
diaminobenzidine tetrahydrochloride (Santa Cruz Biotechnology,
Inc.) (16). The cells were
observed under a MX61A microscope (Olympus Corporation, Tokyo,
Japan).
Gene expression of TWEAK and p38
MAPK
The total RNA was extracted from each specimen using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) and cDNA was obtained by stereverse transcription (RT)
using SuperScript First-Strand Synthesis sym for PCR (Invitrogen
Life Technologies), as described previously (17). RT-quantitative polymerase chain
reaction (qPCR) was performed to evaluate the expression levels of
TWEAK and p38 MAPK. The primers used are listed in Table I; the primer sequences were
retrieved from PrimerBank (http://pga.mgh.harvard.edu/primerbank/) and the
primers were prepared by MGH DNA Core facility (https://dnacore.mgh.harvard.edu/synthesis/index.shtml).
The expression of GAPDH was quantified as an internal control. The
PCR was conducted using SYBR Green PCR master mix (Applied
Biosystems, Foster City, CA, USA), and an ABI Prism 7000 Sequence
Detection system with ABI Prism 7000 software (Applied Biosystems).
The PCR cycle conditions were: 95°C for 15 min, 35 cycles of 94°C
for 1 min; 59°C for 1 min, 72°C for 1 min; and 72°C for 10 min. The
specificity of the amplification of the expected DNA fragments was
confirmed using 2% agarose gel electrophoresis and by analysis of
the melting curves. An amplification reaction control with no RT
enzyme was performed in order to assess the interference of
potential genomic DNA in the RNA solution. The relative gene
expression was calculated using the following equation: 2ΔCT; (ΔCT
= CTGAPDH - CTtarget).
 | Table IGene primer sequences. |
Table I
Gene primer sequences.
| Gene | Forward primer
(5′–3′) | Reverse primer
(5′–3′) | Product (bp) |
|---|
| TWEAK |
CCTCGCAGAAGTGCACCTAA |
ACACCATCCACCAGCAAGTC | 287 |
| p38MAPK |
GCATAATGGCCGAGCTGTTG |
TCATGGCTTGGCATCCTGTT | 130 |
| GAPDH |
GCTGGTCATCAACGGGAAA |
ACGCCAGTAGACTCCACGACA | 105 |
Western blotting
Western blot analysis was used to determine the
protein expression levels of TWEAK, p38 MAPK and p-p38 MAPK, as
described previously (18).
Periprosthetic interface membranes, osteoarthritic synovial
tissues, normal synovial tissues and RAW264.7 cells were used for
western blot analysis. The total cellular proteins were extracted
using radioimmunoprecipitation lysis buffer (Sigma-Aldrich),
containing 1 mM phenylmethylsulfonyl fluoride (Beyotime Institute
of Biotechnology, Lianyungang, China). The protein samples were
separated by 8% SDS-PAGE and were transferred to polyvinylidene
fluoride membranes (Shanghai Biyuntian Bio-Technology Co., Ltd.,
Shanghai, China). After blocking with 5% blocking buffer [3% bovine
serum albumin in Tris-buffered saline with Tween-20 (Sigma-Aldrich,
St. Louis, MO, USA)] the membranes were incubated with the
following antibodies at 4°C overnight: Mouse monoclonal anti-p38
MAPK (cat. no. sc-271120; Santa Cruz Biotechnology, Inc.), mouse
monoclonal anti-p-p38 MAPK (cat. no. sc-7973; Santa Cruz
Biotechnology, Inc.), goat polyclonal anti-TWEAK (cat. no.
sc-12405; Santa Cruz Biotechnology, Inc.) and mouse monoclonal
anti-β-actin (cat. no. sc-47778; Santa Cruz Biotechnology, Inc.),
as described previously (19). The
membranes were then incubated with alkaline phosphatase-conjugated
secondary antibodies (cat. no. sc-2033; donkey anti-goat IgG; Santa
Cruz Biotechnology, Inc.) at 37°C for 1 h.. Visualization of the
bound antibody was achieved using film (Kodak, Rochester, NY, USA)
and 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium
(Beyotime Institute of Biotechnology) at various time points (2
min, 10 min and 2 h). The relative density of each protein band was
determined using an Odyssey infrared imaging system (Li-Cor
Biosciences, Lincoln, NE, USA).
Cytokine detection in the
supernatant
The expression levels of IL-6 and MCP-1 in each
group were quantified using ELISA kits (BD Biosciences Pharmingen,
San Diego, CA, USA), according to the manufacturer’s instructions.
The experiments were performed in triplicate.
Statistical analysis
The data are expressed as the mean ± standard
deviation. The data from each group were analyzed by one-way
analysis of variance, and statistical analyses were conducted using
SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistical significant difference.
Results
Histological evaluation
The osteoarthritic synovial tissue contained more
fibrocytes and synovial cells compared with the normal synovial
tissue (Fig. 1) and little
inflammatory cell infiltration. Marked inflammatory cell
infiltration and hyperplasia of synovial cells were observed in the
interface membrane.
 | Figure 1Histological evaluation of (A) normal
synovial tissue, (B) osteoarthritic synovial tissue and (C)
interface membrane. The osteoarthritic synovial tissue contained a
large number of fibrocytes and synovial cells compared with the
normal synovial tissue. The osteoarthritic synovial tissue
exhibited little inflammatory cell infiltration, while a marked
inflammatory cell infiltration and hyperplasia was detected in
synovial cells in interface membrane. These findings were further
supported by the immunohistochemical analysis. TWEAK
immunohistochemical staining in (D) normal synovial tissue, (E)
osteoarthritic tissue and (F) interface membrane. P-p-38 MAPK
immunohistochemical staining in (G) normal synovial tissue, (H)
osteoarthritic synovial tissue and (I) interface membrane. A large
quantity of inflammatory cells with positive staining of TWEAK and
p-p38 MAPK were observed in the interface membrane, whereas fewer
inflammatory cells were observed in the osteoarthritic synovial
tissue. No positive staining of TWEAK and p-p38 MAPK was detected,
indicating that no inflammatory response had occurred in the normal
synovial tissue. HE, hematoxylin and eosin; TWEAK, tumor necrosis
factor-like weak inducer of apoptosis; MAPK, mitogen-activated
protein kinase; p-, phosphorylated. |
These findings were further supported by the results
of the immunohistochemical analyses. Increased levels of
inflammatory cells with positive staining of TWEAK and p-p38 MAPK
were observed in the interface membrane, whereas fewer inflammatory
cells were observed in the osteoarthritic synovial tissue. In
addition, no positive staining of TWEAK or p-p38 MAPK was detected,
indicating that no inflammatory response had occurred in the normal
synovial tissue.
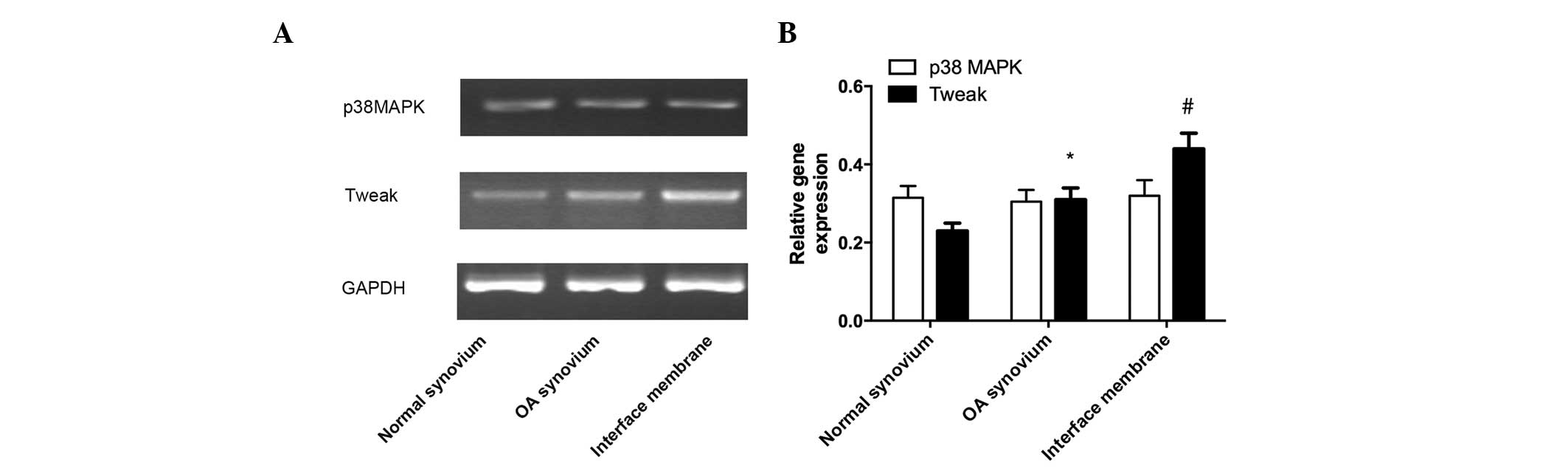
Gene expression of TWEAK and p38
MAPK
The mRNA expression of TWEAK in the interface
membrane was markedly higher in the normal tissue compared with the
osteoarthritic synovial tissue, whereas the mRNA expression of
TWEAK in the osteoarthritic synovial tissue was markedly increased
compared with the normal synovial tissue. No significant difference
in the mRNA expression of p38 MAPK was observed between the three
groups (Fig. 2).
Protein expression levels of TWEAK, p-p38
MAPK and p38 MAPK in the tissue sections
The expression levels of TWEAK and p-p38 MAPK were
increased in the interface membrane compared with the
osteoarthritic synovial tissue, whereas the mRNA expression levels
of TWEAK and p38 MAPK in the osteoarthritic synovial tissue were
higher compared with the normal synovial tissue. No significant
difference in the protein expression of p38 MAPK was observed
between the three groups (Fig.
3)
Effect of the SB203580 p38 MAPK inhibitor
on the protein expression levels of TWEAK, p38 MAPK and p-p38 MAPK
in RAW246.7 cells
As shown in Fig. 4,
the protein expression of TWEAK in group C was higher compared with
the other groups (P<0.05) and the protein expression of TWEAK in
group D was higher compared with those in groups A and B
(P<0.05), which indicated that the Ti particles stimulated the
upregulation of the expression of TWEAK. No significant difference
was observed between groups A and B (P>0.05).
The protein expression of p-p38 MAPK in group C was
higher compared with the other groups (P<0.05) and the protein
expression of p-p38 MAPK in group A was higher compared with groups
B and D (P<0.05). No significant difference in the protein
expression of p-p38 MAPK was observed between groups B and D
(P>0.05). No differences were observed in the protein expression
of p38 MAPK in the four groups (P>0.05).
Levels of IL-6 and MCP-1 in the
supernatant of cultured RAW246.7 cells
The levels of IL-6 and MCP-1 in group C were
significantly higher compared with the other groups (P<0.05;
Fig. 5). No significant difference
was observed between groups A and B (P>0.05).
Discussion
Aseptic loosening caused by periprosthetic
osteolysis is the most serious complication following total hip
arthroplasty (20). It is reported
that debris particle-induced inflammation is the main cause of
osteolysis, therefore, limiting the inflammatory response of the
membrane, induced by debris particles, has become an area of
interest (21). Several studies
have demonstrated that macrophages and foreign body giant cells
surrounding the prosthetic wear debris are the central effector
cells and regulatory cells of inflammation, which secret various
cytokines, including TNF-α, IL-1, IL-6 and MCP-1 (22,23).
Ingham and Fisher reported that the level of osteolysis is
significantly decreased following the neutralization of TNF-α,
which indicates that TNF-α is involved in the osteolytic process,
either directly or indirectly (22).
The MAPK cascade is an important intracellular
signal transduction system. p38 MAPK is a classical pathway of the
MAPK family, which is involved in the inflammatory process
(24). The p38 MAPK pathway is
activated by a variety of physical and chemical factors,
inflammatory factors, stress stimuli and Gram-positive bacterial
cell wall components, which causes effector cells to secrete a
variety of inflammatory cytokines involved in the inflammatory
response following injury (14).
It has been reported the p38 MAPK pathway is important in
periprosthetic osteolysis, which may be regulated by TWEAK
(14,25).
TWEAK is a novel member of the TNF ligand family and
is a type II membrane protein, which is expressed in a variety of
human tissues, including cartilage, skeletal muscle, macrophages
and peripheral blood lymphocytes (26). TWEAK is important in immune
system-mediated tissue damage, and the physiology and pathology of
the disease, which includes the secretion of growth factors, cell
proliferation and apoptosis (27).
In the present study, the interface membrane tissue,
normal synovial tissue and osteoarthritic synovial tissue were
compared. The results of histological staining demonstrated that
cell proliferation was significantly increased and accompanied by
focal inflammatory cell infiltration in the interface membrane
group compared with the other two groups. The existence of
inflammatory cells in the surrounding tissue of the prosthetic
loosening demonstrated that inflammation was important in
particle-induced osteolysis.
Several studies using an animal model of
particle-induced osteolysis have suggested that p38 MAPK is
important in inflammatory osteolysis (10,28).
The present study performed quantitative detection of the p-p38
MAPK protein in the three tissue groups and demonstrated that the
protein expression of p-p38 MAPK in the interface membrane tissue
was higher compared with the normal synovial tissue and
osteoarthritic synovial tissue, which confirmed that p38 MAPK was
important in prosthetic loosening. Additionally, the findings
demonstrated that the protein expression of p-p38 MAPK was higher
in the osteoarthritic synovial tissue compared with the normal
synovial tissue, which indicated that p-p38 MAPK exerted
pro-inflammatory effects in acute and chronic inflammation.
In the present study, the detection of TWEAK by
RT-qPCR and western blotting revealed similar results. The gene
expression levels of TWEAK in the three tissue groups were
consistent with the protein expression levels in the three groups,
which suggested that the gene and protein expression levels in the
interface membrane tissue were higher compared with the normal
synovial tissue and osteoarthritic synovial tissue. The expression
of TWEAK in the arthritis synovial group was higher compared with
the normal synovial group, which was consistent with the results of
van Kuijk et al (29),
which confirmed the inflammatory effect of TWEAK. In addition, the
data also suggested that there was a positive correlation between
the expression of TWEAK and the expression of p-p38 MAPK in the
three groups. According to the above results, it was hypothesized
that TWEAK was involved in the pathological process of
particle-induced inflammatory osteolysis in the loosening of
prostheses.
It has been suggested that there is a clear positive
correlation between TWEAK and p38 MAPK, and that TWEAK is involved
in the secretion of proinflammatory cytokines and chemokines, cell
proliferation and other biological effects (14,30).
Therefore, the present study hypothesized that the increased
expression of TWEAK may promote inflammatory osteolysis and aimed
to examine the molecular mechanisms of TWEAK in inflammatory
osteolysis and determine whether this protein is important for the
prevention of aseptic loosening of artificial joints
Previous studies have demonstrated that macrophages
and foreign body giant cells are stimulated by prosthetic wear
particles, and that these cells are the central effector cells and
regulatory cells in inflammation (21,22,31).
Therefore, macrophages (RAW246.7) in the interface membrane were
selected as study models, and Ti particles were used to stimulate
the cells, which were simultaneously treated with the SB203580 p38
MAPK inhibitor to detect the secretion of IL-6 and MCP-1. The
results demonstrated that the protein levels of TWEAK and p-p38
MAPK and the IL-6 and MCP-1 content of the supernatant in group C
was higher compared with groups A and B (P<0.05), which
indicated that macrophages under particle stimulation produced a
significant inflammatory response. The expression levels of p-p38
MAPK, IL-6 and MCP-1 in group D were lower compared with group C
(P<0.05), which indicated that the macrophage inflammatory
response was suppressed by the inhibition of p-p38 MAPK.
No significant differences in the expression of
TWEAK or in the concentrations of IL-6 and MCP-1 in the supernatant
were observed between groups A and B, however, the expression of
p-p38 MAPK was suppressed significantly. The expression of TWEAK in
group D was higher compared with groups A and B, whereas no
significant difference were observed in the secretion of IL-6 or
MCP-1 in the supernatant between groups A, B and D. These findings
indicated that Ti particles stimulated macrophages and promoted the
expression levels of TWEAK and p-p38 MAPK. TWEAK exerted its
inflammatory effects dependently and independently of the p38 MAPK
signaling pathway, and was only stimulated by the Ti particles. The
expression levels of IL-6 and MCP-1 were significantly dependent on
the p38 MAPK signaling pathway.
In conclusion, the present study hypothesized that
wear particles surrounding the prosthesis stimulate macrophages to
secrete TWEAK and simultaneously activate the p38 MAPK pathway.
TWEAK activated the p38 MAPK pathway by binding its receptor and
induced the secretion of inflammatory cytokines, including IL-6 and
MCP-1, which caused periprosthetic inflammatory osteolysis. In
addition, MCP-1 promoted the aggregation of the monocyte-macrophage
cells, further exacerbating the periprosthetic inflammatory
osteolytic response. This indicated that TWEAK/p38 MAPK, IL-6 and
MCP-1 signaling are important in particle-induced inflammatory
osteolysis. Therefore, further investigation into the mechanism
underlying p38 MAPK signaling pathways in periprosthetic
inflammatory osteolysis may provide a novel mechanism to inhibit
osteolysis and is important for the prevention and treatment of
aseptic loosening of artificial joints.
Acknowledgments
The present study was supported by the Jiangsu
Clinical Science and Technology project (grant no. BL2012002).
References
|
1
|
Moro T, Kyomoto M, Ishihara K, et al:
Grafting of poly(2-methacryloyloxyethyl phosphorylcholine) on
polyethylene liner in artificial hip joints reduces production of
wear particles. J Mech Behav Biomed Mater. 31:100–106. 2014.
View Article : Google Scholar
|
|
2
|
Fujii J, Yasunaga Y, Yamasaki A and Ochi
M: Wear debris stimulates bone-resorbing factor expression in the
fibroblasts and osteoblasts. Hip Int. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Antonios JK, Yao Z, Li C, Rao AJ and
Goodman SB: Macrophage polarization in response to wear particles
in vitro. Cell Mol Immunol. 10:471–482. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nich C, Takakubo Y, Pajarinen J, et al:
Macrophages-Key cells in the response to wear debris from joint
replacements. J Biomed Mater Res A. 101:3033–3045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimamura M, Na kagami H, Osa ko MK, et
al: OPG/RANKL/RANK axis is a critical inflammatory signaling system
in ischemic brain in mice. Proc Natl Acad Sci USA. 111:8191–8196.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma B, Zhang Q, Wu D, et al: Strontium
fructose 1,6-diphosphate prevents bone loss in a rat model of
postmenopausal osteoporosis via the OPG/RANKL/RANK pathway. Acta
Pharmacol Sin. 33:479–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Paun A, Claudio E, Wang H and
Siebenlist U: The tumor promoter and NF-κB modulator Bcl-3
regulates splenic B cell development. J Immunol. 191:5984–5992.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dwyer BJ, Olynyk JK, Ramm GA and
Tirnitz-Parker JE: TWEAK and LTβ signaling during chronic liver
disease. Front Immunol. 5:392014. View Article : Google Scholar
|
|
9
|
Martin P, Mora I, Cortes A, et al:
Relevant role of cGMP-dependent protein kinase (PKG) in the
progression of fibrosis induced by TNF-like weak inducer of
apoptosis (TWEAK). Am J Physiol Renal Physiol. 307:F75–F85. 2014.
View Article : Google Scholar
|
|
10
|
Winkles JA: The TWEAK-Fn14
cytokine-receptor axis: discovery, biology and therapeutic
targeting. Nat Rev Drug Discov. 7:411–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Q, Xu Y, Feng G, et al: p38 MAPK
signal pathway involved in anti-inflammatory effect of
Chaihu-Shugan-San and Shen-ling-bai-zhu-San on hepatocyte in
non-alcoholic steatohepatitis rats. Afr J Tradit Complement Altern
Med. 11:213–221. 2014.PubMed/NCBI
|
|
12
|
Moreira V, Lomonte B, Vinolo MA, Curi R,
Gutierrez JM and Teixeira C: An Asp49 phospholipase A2 from snake
venom induces cyclooxygenase-2 expression and prostaglandin E2
production via activation of NF-kappa B, p38MAPK, and PKC in
Macrophages. Mediators Inflamm. 2014:2014. View Article : Google Scholar
|
|
13
|
Wang X and Liu Y: Regulation of innate
immune response by MAP kinase phosphatase-1. Cell Signal.
19:1372–1382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhi-Chun L, Qiao-Ling Z, Zhi-Qin L,
Xiao-Zhao L, Xiao-xia Z and Rong T: Tumor necrosis factor-like weak
inducer of apoptosis (TWEAK) mediates p38 mitogen-activated protein
kinase activation and signal transduction in peripheral blood
mononuclear cells from patients with lupus nephritis. Inflammation.
35:935–943. 2012. View Article : Google Scholar
|
|
15
|
Li H, Mittal OK, Paul PK, et al: Tumor
necrosis factor-related weak inducer of apoptosis augments matrix
metalloproteinase 9 (MMP-9) production in skeletal muscle through
the activation of nuclear factor-kappaB-inducing kinase and p38
mitogen-activated protein kinase: A potential role of MMP-9 in
myopathy. J Biol Chem. 284:4439–4450. 2009. View Article : Google Scholar :
|
|
16
|
Wisniacki N, Amaravadi L, Galluppi GR, et
al: Safety, tolerability, pharmacokinetics, and pharmacodynamics of
anti-TWEAK monoclonal antibody in patients with rheumatoid
arthritis. Clin Ther. 35:1137–1149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xue K, Qi L, Zhou G and Liu K: A two-step
method of constructing mature cartilage using bone marrow-derived
mesenchymal stem cells. Cells Tissues Organs. 197:484–495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schultz DC, Bazel S, Wright LM, et al:
Western blotting and enzymatic activity analysis of cathepsin D in
breast tissue and sera of patients with breast cancer and benign
breast disease and of normal controls. Cancer Res. 54:48–54.
1994.PubMed/NCBI
|
|
19
|
Xiao W, Chen X and He M: Inhibition of the
Jagged/Notch pathway inhibits retinoblastoma cell proliferation via
suppressing the PI3K/Akt, Src, p38MAPK and Wnt/βcatenin signaling
pathways. Mol Med Rep. 10:453–458. 2014.PubMed/NCBI
|
|
20
|
Purdue PE, Koulouvaris P, Potter HG,
Nestor BJ and Sculco TP: The cellular and molecular biology of
periprosthetic osteolysis. Clin Orthop Relat Res. 454:251–261.
2007. View Article : Google Scholar
|
|
21
|
Gallo J, Goodman SB, Konttinen YT and
Raska M: Particle disease: biologic mechanisms of periprosthetic
osteolysis in total hip arthroplasty. Innate Immun. 19:213–224.
2013. View Article : Google Scholar :
|
|
22
|
Ingham E and Fisher J: The role of
macrophages in osteolysis of total joint replacement. Biomaterials.
26:1271–1286. 2005. View Article : Google Scholar
|
|
23
|
Pajarinen J, Kouri VP, Jamsen E, Li TF,
Mandelin J and Konttinen YT: The response of macrophages to
titanium particles is determined by macrophage polarization. Acta
Biomater. 9:9229–9240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goodman SB, Ma T, Spanogle J, et al:
Effects of a p38 MAP kinase inhibitor on bone ingrowth and tissue
differentiation in rabbit chambers. J Biomed Mater Res A.
81:310–316. 2007. View Article : Google Scholar
|
|
25
|
Tsirigotis M, Baldwin RM, Tang MY, Lorimer
IA and Gray DA: Activation of p38MAPK contributes to expanded
polyglutamine-induced cytotoxicity. PloS One. 3:e21302008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Desplat-Jégo S, Feuillet L, Creidy R, et
al: TWEAK is expressed at the cell surface of monocytes during
multiple sclerosis. J Leukoc Biol. 85:132–135. 2009. View Article : Google Scholar
|
|
27
|
Mao X, Pan X, Peng X, Cheng T and Zhang X:
Inhibition of titanium particle-induced inflammation by the
proteasome inhibitor bortezomib in murine macrophage-like RAW264.7
cells. Inflammation. 35:1411–1418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goodman SB, Gibon E, Pajarinen J, et al:
Novel biological strategies for treatment of wear particle-induced
periprosthetic osteolysis of orthopaedic implants for joint
replacement. J R Soc Interface. 11:201309622014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Kuijk AW, Wijbrandts CA, Vinkenoog M,
Zheng TS, Reedquist KA and Tak PP: TWEAK and its receptor Fn14 in
the synovium of patients with rheumatoid arthritis compared to
psoriatic arthritis and its response to tumour necrosis factor
blockade. Ann Rheum Dis. 69:301–304. 2010. View Article : Google Scholar
|
|
30
|
Perper SJ, Browning B, Burkly LC, et al:
TWEAK is a novel arthritogenic mediator. J Immunol. 177:2610–2620.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Neale SD and Athanasou NA: Cytokine
receptor profile of arthroplasty macrophages, foreign body giant
cells and mature osteoclasts. Acta Orthop Scand. 70:452–458. 1999.
View Article : Google Scholar
|