Introduction
Diabetic nephropathy (DN) is the leading cause of
chronic kidney failure and end-stage renal disease worldwide, and
the prevalence of this disease has progressively increased
(1,2). DN is characterized pathologically by
the progressive accumulation of extracellular matrix (ECM) proteins
in the basement membranes, glomerular mesangium and the peritubular
interstitium. DN may eventually lead to kidney scarring and
ultimately nephron dropout (3,4).
Although glomerulosclerosis is a defining feature of DN, it is the
extent of tubulointerstitial injury that fundamentally determines
the rate of decline in renal function (5). Data have suggested that
tubulointerstitial fibrosis also occurs at an early stage of
diabetic renal injury and correlates closely with the decline in
renal function observed in certain groups of patients (5–7).
Accumulating evidence has implicated the epithelial-to-mesenchymal
transition (EMT) of mature tubular epithelial cells in the kidney
as a contributing factor to the renal accumulation of matrix
proteins associated with DN. In addition, EMT is closely associated
with the progression of renal interstitial fibrosis, which is
characterized by a loss of the typical features of normal
epithelial cells and a gain in the characteristics of ECM-producing
myofibroblasts (8–10). Furthermore, blockade of certain
stages involved in EMT significantly reduces the formation of
fibrotic lesions in specific models of kidney fibrosis, suggesting
that EMT may be significant in the development of nephropathy
(11–13).
It has been hypothesized that oxidative stress may
contribute to the development of diabetic renal complications,
including the EMT of tubular epithelial cells, which are observed
in renal tissues even during the early stages of diabetes (14–16).
As a key feature of the intracellular antioxidant machinery,
nuclear factor (erythroid-derived 2)-like 2 (Nrf2) dissociates from
its cytosolic inhibitor Kelch-like erythropore concentrating
hormone-associated protein 1 (Keap1), translocates to the nucleus
and regulates the coordinated induction of a number of genes, which
encode numerous antioxidant and phase II detoxifying enzymes
(17–19). One important Nrf2 target gene, heme
oxygenase-1 (HO-1), is considered to be significant in the
degradation of pro-oxidant heme, which results in the production of
anti-inflammatory, antioxidant and anti-apoptotic metabolites
(20,21). The essential role of Nrf2 in
combating oxidative stress has been demonstrated by investigations
revealing the increased sensitivity of Nrf2−/− mice to
various types of insult, including high glucose(HG)-induced
oxidative damage (18,22). HO-1, which is induced by multiple
transcription factors, including Nrf2, to protect the kidney from
injury may aid in devising a therapeutic approach against the
development of DN (23,24). Previous studies have revealed that
the increased expression of HO-1 is able to attenuate cytokine- and
glucose-mediated cell growth arrest and apoptosis in vitro
and in vivo (25). In
addition, HO-1 deficiency has been demonstrated to be associated
with increased fibrosis, increased tubular transforming growth
factor (TGF)-β1 expression, inflammation and enhanced EMT in
obstructive kidney disease (26).
Curcumin (diferuloylmethane) is a commonly used
flavoring and coloring agent, and is a major component of the
yellow spice, turmeric, derived from the rhizomes of Curcuma
longa (27). Curcumin exhibits
a number of biological effects, including antioxidant,
anti-inflammatory and wound-healing properties (28–31).
In addition, previous studies have indicated that curcumin has
anti-fibrotic effects in the liver and the lungs, providing relief
from cystic fibrosis (32,33). In immortalized rat kidney
interstitial fibroblasts, curcumin has been observed to attenuate
TGF-β1-induced fibrosis through the downregulation of TGF-β
receptor II (34). In the
unilateral ureteral obstruction (UUO) rat kidney fibrosis model,
curcumin has been observed to inhibit inflammation and fibrosis of
the renal interstitium by inhibition of the NF-κB-dependent
signaling pathway (35).
Furthermore, the antioxidant properties of curcumin have been
observed to be effective in improving renal function in certain
diabetic animal models (36,37),
as well as in acute kidney failure induced by ischemia-reperfusion
(38). However, it remains to be
elucidated whether pretreatment with curcumin in tubular epithelial
cells leads to an increase in the Nrf2 protein level and alleviates
the EMT of tubular epithelial cells. Emerging evidence has
suggested that curcumin induces HO-1 mRNA and protein expression in
the proximal tubule cells through transcriptional mechanisms and
may also involve the NF-κB pathway (39) Notably, Gaedeke et al
(40) demonstrated that curcumin
treatment in nephritic animal models decreased fibrosis by inducing
the expression of Nrf2 and HO-1. Therefore, it was hypothesized
that administration of curcumin may increase the cellular
antioxidant defense capacity via activation of Nrf2 and HO-1
expression, thereby protecting NRK-52E cells from the effects of
HG-induced EMT.
Materials and methods
Cell culture
The NRK-52E normal rat kidney tubular epithelial
cell line was purchased from the American Type Culture Collection
(Manassas, VA, USA). The cells were cultured in a 5% CO2
atmosphere in complete Dulbecco’s modified Eagle’s medium (DMEM;
low glucose; Gibco Life Technologies, Grand Island, NY, USA), which
contained 10% fetal bovine serum (Gibco Life Technologies), 4 mM
L-glutamine (Boster Biological Technology Ltd., Wuahn, China) and
1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at a
density of 5×103 cells/well in six-well culture plates.
Once the cells were almost confluent, they were transferred to
serum-free DMEM for 24 h at 37°C to arrest and synchronize cell
growth. In the control groups, the cells were treated with fresh
serum-free DMEM only, which contained 5 mmol/l glucose. In the
experimental groups, the cells were subjected to pretreatment with
5, 10 or 20 μM curcumin (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and then cultured for 24 h at 37°C. Subsequently,
the medium was changed and the cells were treated for an additional
48 h with 30 mM HG at 37°C (Boster Biological Technology Ltd.). The
concentration of glucose was determined as previously described
(12) and with reference to
preliminary experiments by our group. In some experiments, the
cells were treated wuth 0, 5, 10 or 20 μM curcumin for 24 h,
or 10 μM curcumin for 0, 3, 6, 12, 24 or 48 h, and the
expression levels of Nrf2 were detected by western blotting. Cells
were also treated with 50 μM tin protoporphyrin (SnPP), a
known inhibitor of HO, in order to study changes to HO.
Experimental groups
The cells were divided into six groups, as follows:
Control group, treated with serum-free DMEM; siRNA group, subjected
to Nrf2-siRNA transfection; curcumin group, treated with 10
μM curcumin for 24 h; HG group, treated with 30 mM HG for 48
h; HG/curcumin group, pretreated with 10 μM curcumin for 24
h, followed by 30 mM HG treatment for 48 h; HG/curcumin/Nrf2-siRNA
group, 24 h post-transfection the cells were treated with 10
μM curcumin for 24 h and 30 mM HG for 48 h.
Assessment of cell viability
The cell viability was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, 10 μl MTT (500 μg/ml; Sigma-Aldrich)
was added to the medium and the sample was incubated for 3 h at
37°C following treatment. Subsequently, the MTT solution was
removed and 100 μl dimethyl sulfoxide (Sigma-Aldrich) was
added to the medium to dissolve the colored formazan crystals. The
absorbance of each aliquot at 540 nm was measured using a Sunrise
microplate reader (Tecan Group Ltd., Männedorf, Switzerland). The
cell viability was determined as the ratio of the signal between
the treated and control cultures.
Western blot analysis
The NRK-52E cells were pelleted by centrifugation at
125 × g at 4°C for 10 min and then washed once with
phosphate-buffered saline. The cells were then lysed using a
mixture of radioimmunoprecipitation assay buffer (Sigma-Aldrich)
and phenylmethylsulfonyl fluoride (1:100; Sigma-Aldrich), on ice
for 30 min with vortexing at intervals. The lysates were then
centrifuged at 8,000 × g for 5 min at 4°C. The total protein
concentration measurement was performed using the Bradford method
(15). The protein samples were
boiled for 5 min and 50 μg total protein was loaded into the
appropriate well for 10% SDS-PAGE (Beyotime Biotechnology,
Shanghai, China). The proteins on the gel were then transferred
onto a polyvinylidene difluoride membrane (EMD Millipore, Temecula,
CA, USA) using Bio-Rad apparatus (A101441, Bio-Rad Laboratories,
Inc., Hercules, CA, USA) for 2 h at 4°C and 100 V. The
protein-bound membranes were blocked and washed in Tris-buffered
saline (TBS)-Tween 20 (20%; Sigma-Aldrich). The membranes were
incubated overnight at 4°C with primary antibodies. The primary
antibodies used in the present study were as follows: Goat
polyclonal anti-α-smooth muscle actin (α-SMA; 1:400; cat. no.
sc-324317), mouse monoclonal anti-E-cadherin (1:400; cat. no.
sc-52327), mouse monoclonal anti-Nrf2 (1:400; cat. no. sc-365949),
mouse monoclonal anti-HO-1 (1:400; cat. no. sc-136961) and mouse
monoclonal anti-β-actin (1:400; cat. no. sc-47778) (all Santa Cruz
Biotechnology, Inc.). Following extensive washing in TBS-0.1% Tween
20, the membranes were then incubated with horseradish
peroxidase-conjugated secondary antibodies, including rabbit
anti-goat IgG (1:400; cat. no. sc-2922; Santa Cruz Biotechnology,
Inc.) and rabbit anti-mouse IgG (1:400; cat. no. sc-358920; Santa
Cruz Biotechnology, Inc.) overnight at 4°C. Subsequently, the
membranes were visualized using an enhanced chemiluminescence kit
(Walterson Biotechnology Inc., Beijing, China) using the ChemiDoc™
XRS system with Quantity One software version 4.6 (Bio-Rad
Laboratories, Inc.) and the G-BOX EF Chemi HR16 gel imaging system
(Syngene, Frederick MD, USA). Following development, the band
intensities were quantified using Image-Pro Plus 6.0 analysis
software (Media Cybernetics, Inc., Rockville, MD, USA). The blots
were repeated at least three times for each condition.
Transient transfection with Nrf2-small
interfering RNA (siRNA)
The cells were plated in six-well plates at a
density of 2×105 cells/well in 2 ml DMEM. The cells were
transfected with Nrf2-specific siRNA (sense,
5′-GCACGGUGGAGUUCAUGATT-3′ and antisense,
5′-UCAUUGAACUCCACCGUGCCT-3′) (Santa Cruz Biotechnology, Inc). The
target sequences of the Nrf2 siRNA and control Nrf2 siRNA were
aligned against the GenBank database using the Basic Local
Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Transient
transfections were performed according to the manufacturer’s
instructions using Lipofectamine® 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA) to attenuate Nrf2 expression. All
experiments were performed in six-well plates, with cells plated to
reach 50–60% confluence on the day of transfection. The cells were
incubated in growth medium with 10% fetal bovine serum for 24 h
after transfection. The knockdown of Nrf2 was determined using
western blot analysis.
Light microscopy
The cells (HG group) were cultured for 48 h with 30
mM HG at 37°C. The cells (curcumin/HG group) were cultured with 10
μM curcumin pretreatment for 24 h followed by 30 mM HG
treatment for 48 h at 37°C. The control cells were cultured for 48
h with 5 mM glucose and 0 μM curcumin at 37°C. Subsequently,
the cells were observed under a light microscope (Olympus
CKX41-A32PH, Olympus, Tokyo, Japan).
Statistical analysis
Continuous variables are expressed as the mean ±
standard error of the mean. One-way analysis of variance was used
to analyze the data. Tukey’s multiple comparison test was used.
P<0.05 was considered to indicate a statistically significant
difference.
Results
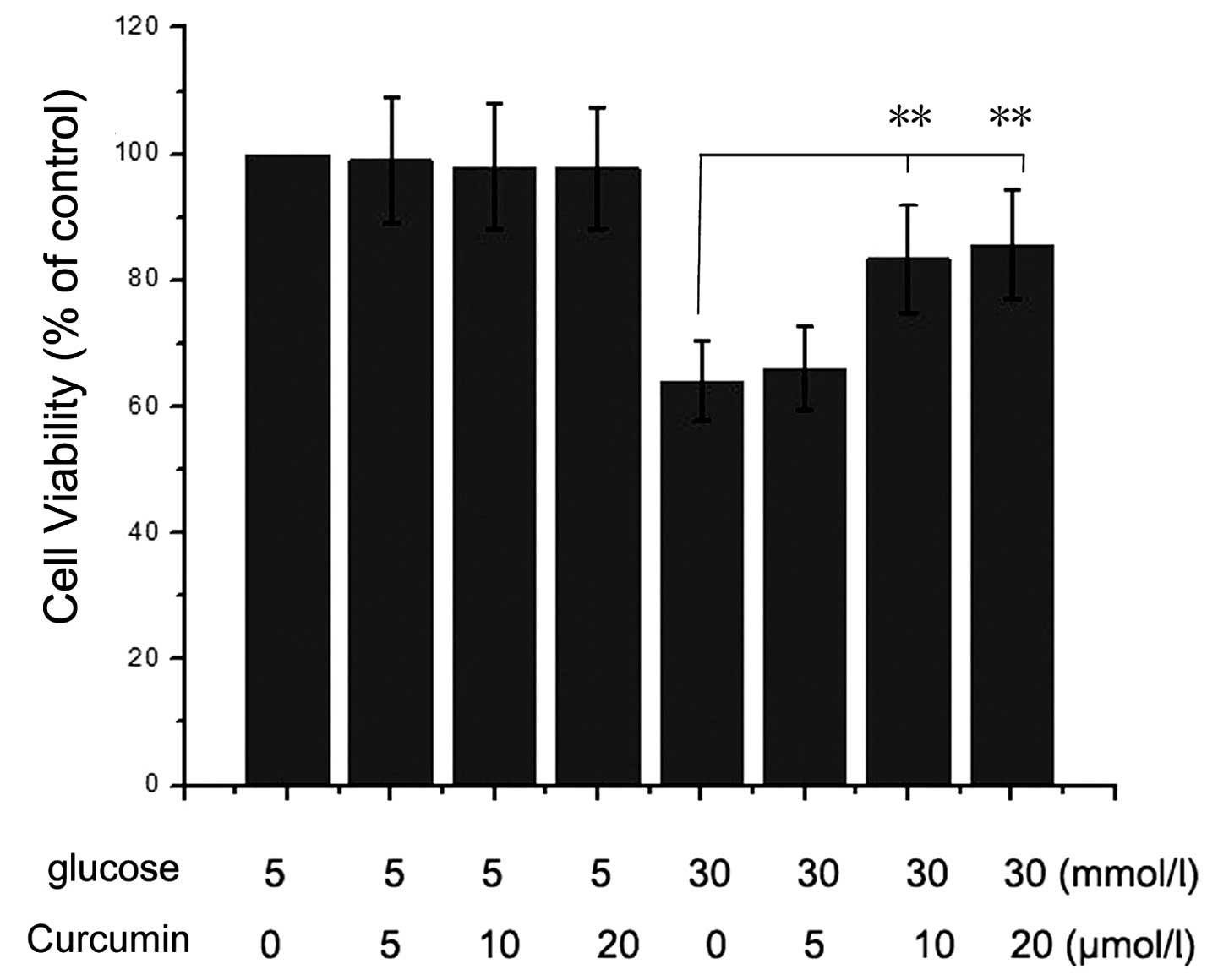
Curcumin rescues HG-induced inhibition of
cell viability
The cell viability of NRK-52E cells under HG (30 mM)
and curcumin (0–20 μM) conditions were assessed. The results
presented in Fig. 1 indicated that
the HG condition significantly inhibited NRK-52E cell viability
compared with that of the control group (5 mM glucose and 0
μM curcumin-treated cells). However, when the cells were
treated with 10 or 20 μM curcumin and HG, the viability of
the was cells increased. Therefore, it was identified that curcumin
had a protective effect on NRK-52E cells under HG conditions.
Curcumin decreases HG-induced EMT in
NRK-52E cells
The HG-induced EMT of NRK-52E cells following
curcumin treatment was assessed using light microscopy and western
blotting. NRK-52E cells cultured in medium alone for 48 h exhibited
typical cobblestone morphology under magnification. As shown in
Fig. 2A, a typical epithelial
cuboidal shape was observed in the NRK-52E cells cultured in DMEM
(5 mmol/l glucose), with the characteristic cobblestone morphology.
Following treatment with 30 mM HG, the cell morphology changed to a
fibroblast-like shape, with reduced adherence, and the cells lost
their apical-to-basal polarity. However, the cellular changes were
more noticeable in the cells exposed to 30 mM HG with 20 μM
curcumin for 48 h. A previous study revealed that HG conditions
were able to induce EMT in tubular epithelial cells (8). In fibroblasts, α-SMA and vimentin
proteins were detected; however, these proteins were not detected
in the NRK-52E cells (41).
E-cadherin, a Ca2+-dependent protein, is crucial in
modulating renal epithelial polarity and a decrease in the
expression of E-cadherin is considered to indicate EMT (12). In order to detect HG-induced EMT,
the levels of E-cadherin and α-SMA were assessed using western
blotting to analyze samples cultured under HG conditions (30 mM)
with or without curcumin pretreatment. A decrease was detected in
the levels of E-cadherin, accompanied by an increase in α-SMA
expression (Fig. 2B), which
suggested that these cells had undergone EMT in response to the HG
conditions. This reduction in E-cadherin protein expression in
response to HG conditions was also accompanied by an increase in
α-SMA protein expression in our preliminary experiments, confirming
that HG conditions promote EMT in NRK-52E cells. However, this
HG-induced EMT was attenuated by pre-treating the NRK-52E cells
with 10 or 20 μM curcumin, demonstrated by the reduced
upregulation of α-SMA and the ameliorated expression of E-cadherin
(Fig. 2B).
Curcumin increases Nrf2 expression in
NRK-52E cells
Previous studies revealed that Nrf2 was able to
regulate cytoprotective genes and cellular antioxidant proteins, as
well as allow cells to adapt to stress induced by electrophiles and
oxidants (42–44). To analyze the mechanism of action
of curcumin on HG-induced EMT in the NRK-52E cells in the present
study, the nuclear accumulation of Nrf2 protein in the
curcumin-treated NRK-52E cells was examined. The cells were
cultured with 0, 5, 10 or 20 μM curcumin for 24 h or 10
μM curcumin for 0, 3, 6, 12, 24 or 48 h and the expression
level of Nrf2 was detected using western blot analysis. The results
shown in Fig. 3A and B indicated
that the nuclear levels of Nrf2 were increased in a concentration
and time-dependent manner when cultured with curcumin, compared
with those of the control cells. It was therefore concluded that
curcumin was capable of effectively inducing the expression of Nrf2
in NRK-52E cells.
Curcumin promotes expression of HO-1 in
NRK-52E cells
A number of studies have revealed that HO-1 is able
to reduce apoptosis by inhibiting cellular oxidative stress
(20,45,46).
In various types of cell, including glomerular or endothelial
cells, the expression of HO-1 was demonstrated to be induced by
curcumin in previous studies (47,48).
To elucidate the role of curcumin in renal tubular epithelial
cells, HO-1 expression was assessed in NRK-52E cells cultured with
curcumin. As indicated in Fig. 4A and
B, curcumin was observed to upregulate HO-1 protein expression
in a dose- and time-dependent manner. Compared with the untreated
controls, curcumin treatment led to a significant increase in the
level of HO-1 protein expression. In order to determine whether
HO-1 exerted a cytoprotective effect against the HG-induced EMT, 50
μM SnPP, a known inhibitor of HO, was utilized. The
concentration of SnPP was determined as previously described
(49). As shown in Fig. 4C and D, SnPP treatment attenuated
the protective effects of curcumin against HG-induced EMT in renal
tubular epithelial cells. Notably, SnPP treatment alone did not
affect cell viability in the present study (data not shown). In
conclusion, curcumin was demonstrated to have a cytoprotective
role, which is mediated through the induction of HO-1
expression.
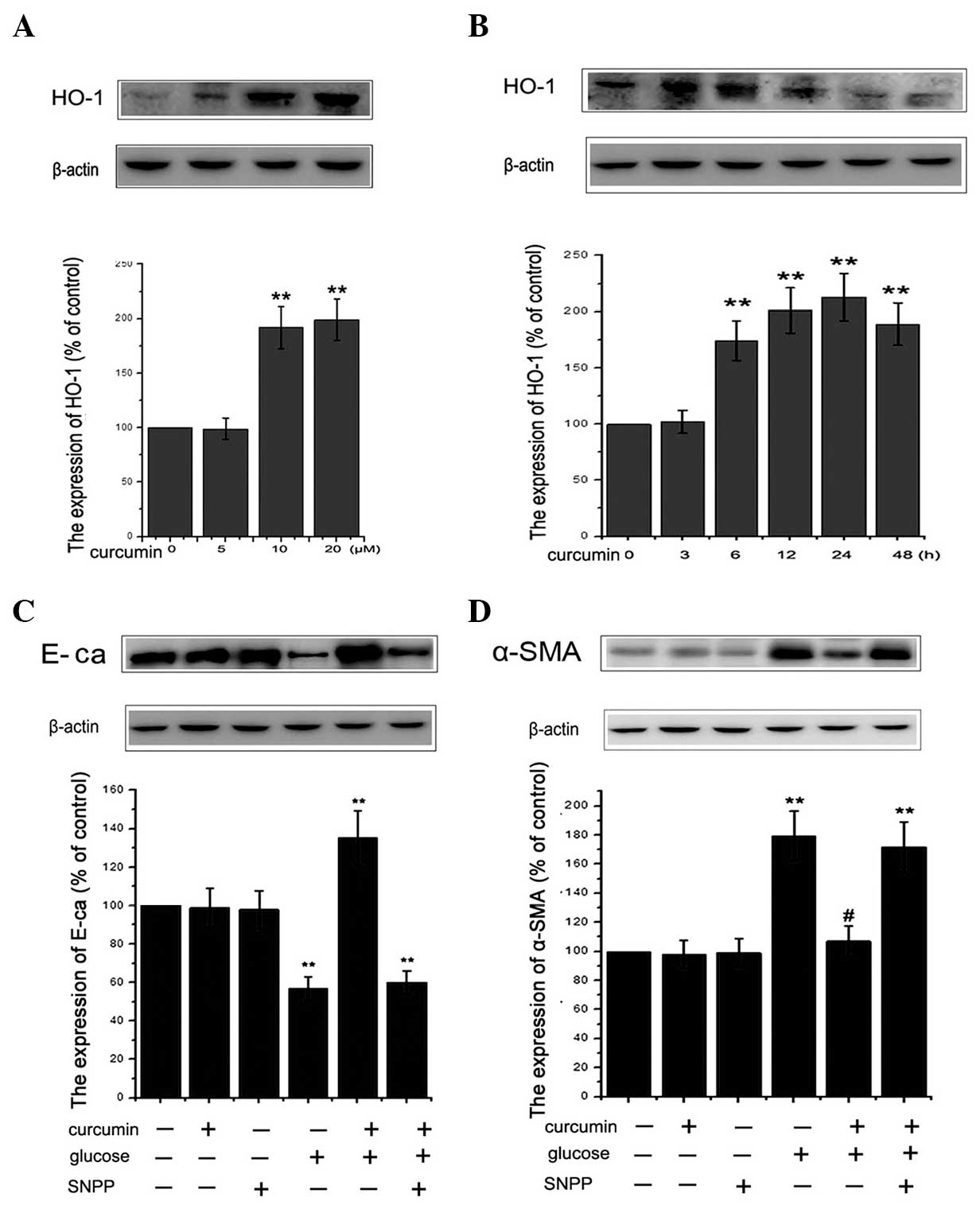 | Figure 4Induction of HO-1 protein expression
in NRK-52E cells by curcumin. HO-1 expression was analyzed using
western blotting following treatment of cells with (A) various
concentrations (0, 5, 10, 20 μM) of curcumin for 24 h or (B)
10 μM curcumin for 0, 3, 6, 12, 24 or 48 h. Results are
representative of three independent experiments. β-actin was used
as a loading control. (**P<0.01 vs. control). (C and
D) Cells were incubated with or without 50 μM SnPP for 12 h
and then administered 30 mM glucose for 48 h with or without 10
μM curcumin pretreatment for 24 h. The expression of
epithelial-mesenchymal transition proteins, E-cadherin and α-SMA
were assessed using western blot analysis. β-actin served as the
loading control. Quantitative analysis was performed by measuring
the fluorescence intensity relative to the control. Values are
expressed as the mean ± standard error of the mean (n=10). All
results were obtained from three independent experiments.
(**P<0.01 vs. control). α-SMA, α-smooth muscle actin;
SnPP, tin protoporphyrin; HO-1, heme oxygenase-1. |
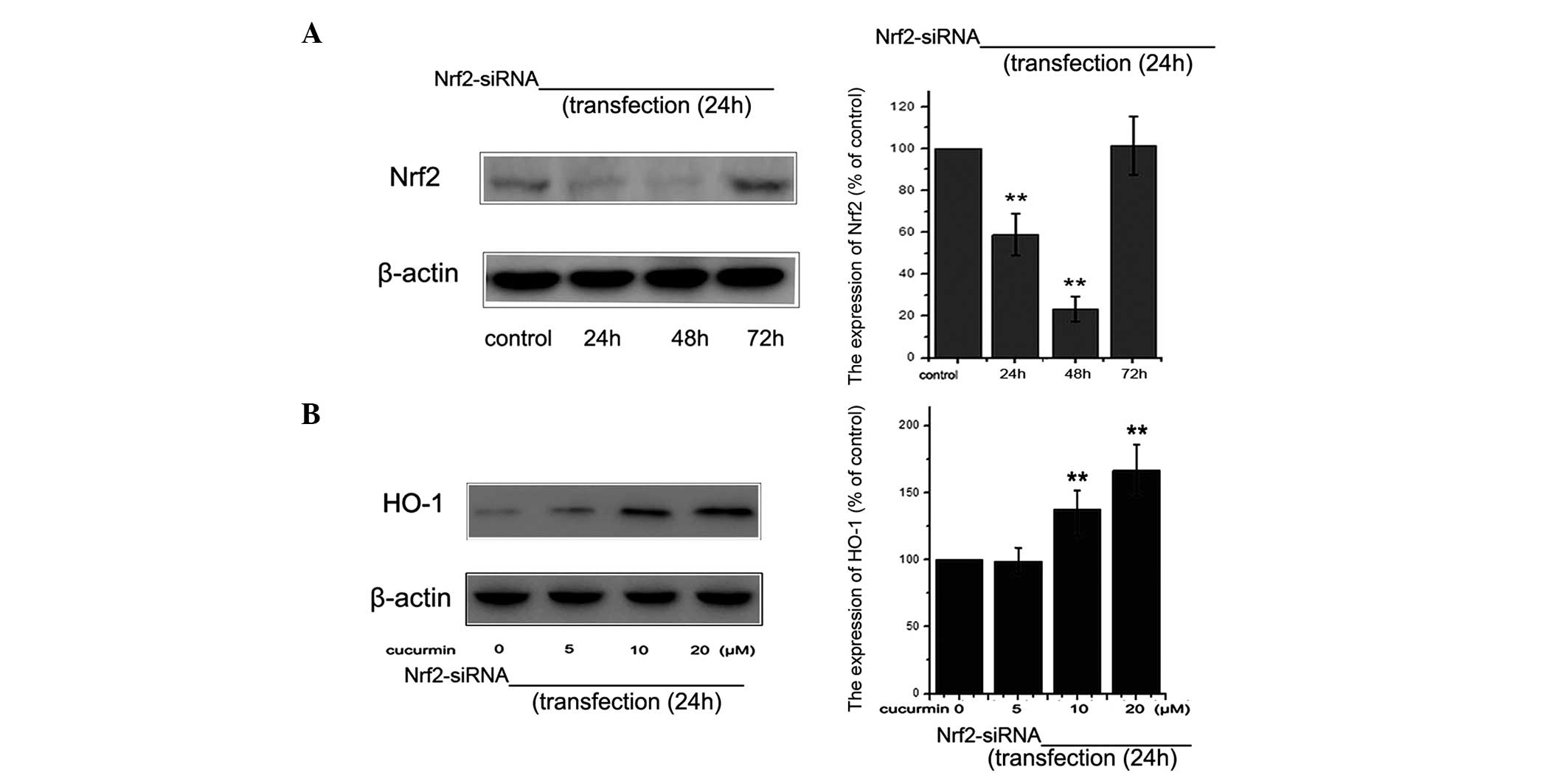
siRNA knockdown of Nrf2 abrogates
curcumin-induced HO-1 expression
In order to examine the role of Nrf2 in the
upregulation of HO-1 expression, siRNA knockdown of the Nrf2 gene
was used. The level of Nrf2 protein was detected using western blot
analysis at various time-points following siRNA-Nrf2 transfection
(Fig. 5A). siRNA-Nrf2
significantly reduced the HO-1 expression induced by curcumin
treatment (Fig. 5B). In
conclusion, the present findings supported the hypothesis that
curcumin promotes the expression of HO-1 through activation of Nrf2
in NRK-52E cells.
Anti-fibrotic effects of curcumin are
mediated by activation of Nrf2 signaling
To determine whether curcumin protects cells against
HG-induced EMT through the modulation of Nrf2 and HO-1 expression,
the role of Nrf2 in EMT was investigated via knockdown of Nrf2. The
cells were divided into six groups as follows: i) Control group;
ii) siRNA group, subjected to Nrf2-siRNA transfection; iii)
curcumin group, subjected to 10 μM curcumin treatment for 24
h; iv) HG group, 30 mM HG treatment; v) HG/curcumin group, 10
μM curcumin pretreatment for 24 h followed by 30 mM HG
treatment for 48 h; vi) HG/curcumin/Nrf2-siRNA group, following
transfection for 24 h, cells were treated with 10 μM
curcumin for 24 h and 30 mM HG for 48 h. The results revealed that
HG-induced EMT was partially prevented by curcumin pretreatment,
which resulted in a decrease in the HG-induced increase in α-SMA
expression and an increase in the expression of E-cadherin in
NRK-52E cells (Fig. 6). In
addition, Nrf2-siRNA alone did not induce EMT in the NRK-52E cells.
However, knockdown of Nrf2 with siRNA inhibited the
curcumin-induced anti-fibrotic effects (Fig. 6). These results suggested that
curcumin protects NRK-52E cells from HG-induced EMT processes
through activation of the Nrf2/antioxidant response signaling
pathway and subsequent targeting of gene expression.
Discussion
Multiple studies have demonstrated that curcumin is
capable of inhibiting fibrosis in certain chronic inflammatory
diseases, including cystic fibrosis, liver fibrosis and myocardial
fibrosis (32,33). In the present study, the protective
effect of curcumin on HG-induced EMT was analyzed in renal tubular
epithelial cells. The results of the present study revealed that
curcumin reduced HG-induced EMT in a dose- and time-dependent
manner, as indicated by the detected decrease in the upregulation
of α-SMA and the increase in E-cadherin expression. The mechanism
underlying this process may involve abrogation of HG-induced
oxidative stress via activation of Nrf2 and HO-1 in NRK-52E
cells.
EMT in mature tubular epithelial cells of the kidney
is currently considered to contribute to the renal accumulation of
matrix proteins associated with DN and is closely associated with
the progression of renal interstitial fibrosis (7,50).
In DN, EMT occurs in response to TGF-β1 activation under HG
conditions and contributes to the loss of tubular epithelial cells
and the accumulation of interstitial fibroblasts, which are
associated with a decline in renal excretory function (11,13).
Typical epithelial cell alterations, which are associated with EMT,
include reorganization of the actin cytoskeleton, de novo
acquisition of mesenchymal cytoskeletal markers and the
downregulation of epithelial adhesion molecules (51,52).
To the best of our knowledge, the anti-fibrotic effects of curcumin
have previously only been investigated in models of pulmonary or
liver fibrosis, and curcumin was found to be associated with a
reduction in the expression of inflammatory mediators, decreased
expression of the profibrotic cytokine TGF-β1 and a subsequent
decrease in the accumulation of collagen (32,33).
Curcumin was also found to inhibit renal interstitial inflammation
and fibrosis via inhibition of the NF-κB-dependent pathways in a
UUO rat model of kidney fibrosis (35). A recent study revealed that
curcumin inhibited TGF-β1-induced EMT in renal tubular epithelial
cells via the extracellular signal-regulated kinase-dependent and
peroxisome proliferator-activated receptor γ-dependent pathways
(53). In the present study, it
was confirmed that HG-induced changes in EMT markers were more
prominent than in the control and were accompanied by a decrease in
the expression of epithelial marker E-cadherin and an increase in
α-SMA. Curcumin pretreatment may provide effective protection
against HG-induced EMT, as evidenced by a decrease in the
upregulation of α-SMA and the amelioration of E-cadherin
expression, which was associated with the transition from the
epithelial to myofibroblastic phenotype in NRK-52E cells.
A number of previous studies have confirmed that EMT
in the tubular epithelial cells of patients with DN is generally
regarded to be the result of hyperglycemia-induced oxidative
stress; notably, antioxidants effectively reverse this induction of
EMT in tubular epithelial cells (6,13,16,54).
Nrf2-mediated transcriptional responses have been found to be
protective in a number of animal models of disease, including those
of oxidative lung injury and fibrosis, asthma and brain
ischemia-reperfusion (55,56). The induction of kidney ischemia
followed by reperfusion in wild-type mice was found to elevate Nrf2
levels and activate downstream target genes (57). By contrast, Nrf2 deficiency was
demonstrated to enhance the susceptibility of cells to ischemic and
nephrotoxic acute renal injury (58). Additionally, treatment of Nrf2
knockout mice with antioxidants, including N-acetyl-cysteine or
glutathione, is able to improve renal function (59). Furthermore, Nrf2 knockout mice with
streptozotocin-induced diabetes were found to exhibit progressively
increasing levels of nitric oxide metabolites in their urine,
eventually developing renal injury (19). Curcumin is able to stimulate the
dissociation of Nrf2 from Keap1, a cytosolic Nrf2 inhibitor, which
leads to increased Nrf2 binding to the antioxidant response element
in the promoters of target genes (33). Curcumin has also been demonstrated
to be a potent inducer of Nrf2-associated antioxidant enzymes and
an inhibitor of oxidant-induced NF-κB activation in lung epithelial
cells (60). Similarly, in mouse
alveolar macrophages in vitro and in the lungs in
vivo, curcumin has been observed to upregulate Nrf2 target
antioxidant gene expression (33).
In addition, in the present study, it was also confirmed that
curcumin induced Nrf2 activation in NRK-52E cells, which may
represent the mechanism responsible for the protective effects of
curcumin in cells subjected to HG-induced EMT.
To further examine the possible downstream
mechanisms through which Nrf2 may elicit protection against
HG-induced EMT, the expression of HO-1 was investigated in
HG-treated cells transfected with siRNA-Nrf2. HO-1 is a
rate-limiting enzyme involved in the degradation of heme to produce
equimolar quantities of CO, iron and biliverdin (23,26).
Growing evidence indicates that the HO-1 system is a regulator of
renal vascular integrity and responses to oxidative stress
(23). The induction of HO-1
expression by curcumin has been observed in several cell types,
including human renal tubular cells and renal fibroblasts (14). A previous study revealed that
curcumin exhibits an anti-fibrotic effect in a model of glomerular
fibrosis, and curcumin treatment in vitro and in vivo
may lead to the induction of HO-1 (47). The detailed mechanism through which
curcumin induces HO-1 has been investigated in cultured cells
(31). In human proximal tubular
cells, curcumin-induced HO-1 expression was reduced by co-treatment
with an NF-κB inhibitor, implicating this pathway in the modulation
of HO-1 in this cell type. Other studies have indicated that,
following curcumin treatment, there is an increase in HO-1 protein
expression levels in kidney tissue and this mechanism is essential
in the prevention of transplant-associated organ injury and
rejection (61) Consistent with
these results, the present data demonstrated that curcumin induced
a marked increase in HO-1 levels and that transfection with
siRNA-Nrf2 significantly attenuated this increase. Simultaneously,
the HG-induced reduction in E-cadherin and upregulation of α-SMA
were reversed by curcumin, whereas knockdown of Nrf2 with siRNA
inhibited the curcumin-induced anti-fibrotic effects. The present
results suggested that curcumin-mediated cell protection may occur
via the activation of Nrf2 and subsequently the key target gene
HO-1, thereby protecting NRK-52E cells from HG-induced EMT
processes.
In conclusion, the present study demonstrated that
curcumin exhibited inhibition of HG-induced EMT in NRK-52E cells
and that this effect was dependent on the activation of Nrf2 and
subsequent HO-1 induction. The present data suggested that curcumin
is a significant regulator of HG-induced EMT and may be beneficial
in the treatment of DN. However, the underlying mechanisms require
elucidation through further investigation.
Acknowledgments
The present study was supported by the National
Grand Fundamental Research 973 Program of China (grant no.
2012CB722405), the Natural Science Foundation of China (grant nos.
81170561 and 81170775) and the Shenyang City Science and Technology
program (grant no. F11-264-1-21).
Abbreviations:
|
NRK-52E cells
|
normal rat kidney tubular epithelial
cells
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
HG
|
high glucose
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
SMA
|
smooth muscle actin
|
|
RIPA
|
radioimmunoprecipitation assay
|
|
TGF
|
transforming growth factor
|
|
TBS
|
Tris-buffered saline
|
|
Nrf2
|
nuclear factor (erythroid-derived
2)-like 2
|
|
HO-1
|
heme oxygenase-1
|
|
MTT
|
3-(4,5-dimethylthiazol-2-y)-2,5-diphenyltetrazolium bromide
|
References
|
1
|
Schena FP and Gesualdo L: Pathogenetic
mechanisms of diabetic nephropathy. J Am Soc Nephrol. 16(Suppl 1):
S30–S33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lapice E, Pinelli M, Riccardi G and
Vaccaro O: Pro12Ala polymorphism in the PPARG gene contributes to
the development of diabetic nephropathy in Chinese type 2 diabetic
patients: comment on the study by Liu et al. Diabetes Care.
33:e114author reply. e1152010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ayodele OE, Alebiosu CO and Salako BL:
Diabetic nephropathy - a review of the natural history, burden,
risk factors and treatment. J Natl Med Assoc. 96:1445–1454.
2004.PubMed/NCBI
|
|
4
|
Yeh CH, Chang CK, Cheng KC, Li YX, Zhang
YW and Cheng JT: Role of bone morphogenetic proteins-7 (BMP-7) in
the renal improvement effect of DangGui (Angelica sinensis) in
type-1 diabetic rats. Evid Based Complement Alternat Med.
2011:7967232011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gilbert RE and Cooper ME: The
tubulointerstitium in progressive diabetic kidney disease: more
than an aftermath of glomerular injury? Kidney Int. 56:1627–1637.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simonson MS: Phenotypic transitions and
fibrosis in diabetic nephropathy. Kidney Int. 71:846–854. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang SC and Lai KN: The pathogenic role of
the renal proximal tubular cell in diabetic nephropathy. Nephrol
Dial Transplant. 27:3049–3056. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeisberg M and Kalluri R: The role of
epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med
Berl. 82:175–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iwano M, Plieth D, Danoff TM, Xue C, Okada
H and Neilson EG: Evidence that fibroblasts derive from epithelium
during tissue fibrosis. J Clin Invest. 110:341–350. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y: New insights into
epithelial-mesenchymal transition in kidney fibrosis. J Am Soc
Nephrol. 21:212–222. 2010. View Article : Google Scholar
|
|
11
|
Burns WC, Twigg SM, Forbes JM, et al:
Connective tissue growth factor plays an important role in advanced
glycation end product-induced tubular epithelial-to-mesenchymal
transition: implications for diabetic renal disease. J Am Soc
Nephrol. 17:2484–2494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lv ZM, Wang Q, Wan Q, et al: The role of
the p38 MAPK signaling pathway in high glucose-induced
epithelial-mesenchymal transition of cultured human renal tubular
epithelial cells. PLoS One. 6:e228062011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee YJ and Han HJ: Troglitazone
ameliorates high glucose-induced EMT and dysfunction of SGLTs
through PI3K/Akt, GSK-3β, Snail1, and β-catenin in renal proximal
tubule cells. Am J Physiol Renal Physiol. 298:F1263–F1275. 2010.
View Article : Google Scholar
|
|
14
|
Dogukan A, Sahin N, Tuzcu M, Juturu V,
Orhan C, Onderci M, et al: The effects of chromium histidinate on
mineral status of serum and tissue in fat-fed and
streptozotocin-treated type II diabetic rats. Biol Trace Elem Res.
131:124–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Simonian MH and Smith JA:
Spectrophotometric and colorimetric determination of protein
concentration. Curr Protoc Mol Biol. 10:Unit 10.1A. 2006.
View Article : Google Scholar
|
|
16
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen H, Zhang B, Yuan X, et al:
Isoliquiritigenin-induced effects on Nrf2 mediated antioxidant
defence in the HL-60 cell monocytic differentiation. Cell Biol Int.
37:1215–1224. 2013.PubMed/NCBI
|
|
18
|
Jiang T, Huang Z, Lin Y, Zhang Z, Fang D
and Zhang DD: The protective role of Nrf2 in streptozotocin-induced
diabetic nephropathy. Diabetes. 59:850–860. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoh K, Hirayama A, Ishizaki K, Yamada A,
Takeuchi M, Yamagishi S, et al: Hyperglycemia induces oxidative and
nitrosative stress and increases renal functional impairment in
Nrf2-deficient mice. Genes Cells. 13:1159–1170. 2008.PubMed/NCBI
|
|
20
|
Abraham NG and Kappas A: Pharmacological
and clinical aspects of heme oxygenase. Pharmacol Rev. 60:79–127.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sikorski EM, Hock T, Hill-Kapturczak N and
Agarwal A: The story so far: Molecular regulation of the heme
oxygenase-1 gene in renal injury. Am J Physiol Renal Physiol.
286:F425–F441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan K, Han XD and Kan YW: An important
function of Nrf2 in combating oxidative stress: detoxification of
acetaminophen. Proc Natl Acad Sci USA. 98:4611–4616. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abraham NG, Cao J, Sacerdoti D, Li X and
Drummond G: Heme oxygenase: the key to renal function regulation.
Am J Physiol Renal Physiol. 297:F1137–F1152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bolisetty S, Traylor A, Zarjou A, et al:
Mitochondria-targeted heme oxygenase-1 decreases oxidative stress
in renal epithelial cells. Am J Physiol Renal Physiol.
305:F255–F264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Quan S, Kaminski PM, Yang L, Morita T,
Inaba M, Ikehara S, et al: Heme oxygenase-1 prevents superoxide
anion-associated endothelial cell sloughing in diabetic rats.
Biochem Biophys Res Commun. 315:509–516. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kie JH, Kapturczak MH, Traylor A, Agarwal
A and Hill-Kapturczak N: Heme oxygenase-1 deficiency promotes
epithelial-mesenchymal transition and renal fibrosis. J Am Soc
Nephrol. 19:1681–1691. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soetikno V, Sari FR, Lakshmanan AP, et al:
Curcumin alleviates oxidative stress, inflammation, and renal
fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol Nutr
Food Res. 57:1649–1659. 2013. View Article : Google Scholar
|
|
28
|
Farhangkhoee H, Khan ZA, Chen S and
Chakrabarti S: Differential effects of curcumin on vasoactive
factors in the diabetic rat heart. Nutr Metab (Lond). 3:272006.
View Article : Google Scholar
|
|
29
|
Kowluru RA and Kanwar M: Effects of
curcumin on retinal oxidative stress and inflammation in diabetes.
Nutr Metab (Lond). 4:82007. View Article : Google Scholar
|
|
30
|
Maheshwari RK, Singh AK, Gaddipati J and
Srimal RC: Multiple biological activities of curcumin: a short
review. Life Sci. 78:2081–2087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sharma OP: Antioxidant activity of
curcumin and related compounds. Biochem Pharmacol. 25:1811–1812.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao QY, Xu BL, Wang JY, et al: Inhibition
by curcumin of multiple sites of the transforming growth
factor-beta1 signalling pathway ameliorates the progression of
liver fibrosis induced by carbon tetrachloride in rats. BMC
Complement Altern Med. 12:1562012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suzuki M, Betsuyaku T, Ito Y, et al:
Curcumin attenuates elastase- and cigarette smoke-induced pulmonary
emphysema in mice. Am J Physiol Lung Cell Mol Physiol.
296:L614–L623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gaedeke J, Noble NA and Border WA:
Curcumin blocks multiple sites of the TGF-beta signaling cascade in
renal cells. Kidney Int. 66:112–120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kuwabara N, Tamada S, Iwai T, et al:
Attenuation of renal fibrosis by curcumin in rat obstructive
nephropathy. Urology. 67:440–446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Murugan P and Pari L: Influence of
tetrahydrocurcumin on hepatic and renal functional markers and
protein levels in experimental type 2 diabetic rats. Basic Clin
Pharmacol Toxicol. 101:241–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sharma S, Kulkarni SK and Chopra K:
Curcumin, the active principle of turmeric (Curcuma longa),
ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol
Physiol. 33:940–945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bayrak O, Uz E, Bayrak R, Turgut F, Atmaca
AF, Sahin S, et al: Curcumin protects against ischemia/reperfusion
injury in rat kidneys. World J Urol. 26:285–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hill-Kapturczak N, Thamilselvan V, Liu F,
Nick HS and Agarwal A: Mechanism of heme oxygenase-1 gene induction
by curcumin in human renal proximal tubule cells. Am J Physiol
Renal Physiol. 281:F851–F859. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gaedeke J, Noble NA and Border WA:
Curcumin blocks fibrosis in anti-Thy 1 glomerulonephritis through
up-regulation of heme oxygenase 1. Kidney Int. 68:2042–2049. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang X, Zhao Y, Chu Q, Wang ZY, Li H and
Chi ZH: Zinc modulates high glucose-induced apoptosis by
suppressing oxidative stress in renal tubular epithelial cells.
Biol Trace Elem Res. 158:259–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Holmström TH and Eriksson JE:
Phosphorylation-based signaling in Fas receptor-mediated apoptosis.
Crit Rev Immunol. 20:121–152. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nishiura T and Abe K: Alpha1-adrenergic
receptor stimulation induces the expression of receptor activator
of nuclear factor kappaB ligand gene via protein kinase C and
extracellular signal-regulated kinase pathways in MC3T3-E1
osteoblast-like cells. Arch Oral Biol. 52:778–785. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang LY, Zhou YY, Chen F, et al: Taurine
inhibits serum deprivation-induced osteoblast apoptosis via the
taurine transporter/ERK signaling pathway. Braz J Med Biol Res.
44:618–623. 2011.PubMed/NCBI
|
|
45
|
Chen YC, Chow JM, Lin CW, Wu CY and Shen
SC: Baicalein inhibition of oxidative-stress-induced apoptosis via
modulation of ERKs activation and induction of HO-1 gene expression
in rat glioma cells C6. Toxicol Appl Pharmacol. 216:263–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Parfenova H, Basuroy S, Bhattacharya S,
Tcheranova D, Qu Y, Regan RF and Leffler CW: Glutamate induces
oxidative stress and apoptosis in cerebral vascular endothelial
cells: Contributions of HO-1 and HO-2 to cytoprotection. Am J
Physiol Cell Physiol. 290:C1399–C1410. 2006. View Article : Google Scholar
|
|
47
|
Yang C, Zhang X, Fan H and Liu Y: Curcumin
upregulates transcription factor Nrf2, HO-1 expression and protects
rat brains against focal ischemia. Brain Res. 1282:133–141. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sahin K, Orhan C, Tuzcu Z, Tuzcu M and
Sahin N: Curcumin ameliorates heat stress via inhibtion of
oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food
Chem Toxicol. 50:4035–4041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Uc A, Reszka KJ, Buettner GR and Stokes
JB: Tin protoporphyrin induces intestinal chloride secretion by
inducing light oxidation processes. Am J Physiol Cell Physiol.
292:C1906–C1914. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hills CE, Al-Rasheed N, Al-Rasheed N,
Willars GB and Brunskill NJ: C-peptide reverses TGF-beta1-induced
changes in renal proximal tubular cells: implications for treatment
of diabetic nephropathy. Am J Physiol Renal Physiol. 296:F614–F621.
2009. View Article : Google Scholar
|
|
51
|
Lan HY: Tubular epithelial-myofibroblast
transdifferentiation mechanisms in proximal tubule cells. Curr Opin
Nephrol Hypertens. 12:25–29. 2003. View Article : Google Scholar
|
|
52
|
Oldfield MD, Bach LA, Forbes JM,
Nikolic-Paterson D, McRobert A, Thallas V, et al: Advanced
glycation end products cause epithelial-myofibroblast
transdifferentiation via the receptor for advanced glycation end
products (RAGE). J Clin Invest. 108:1853–1863. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li R, Wang Y, Liu Y, et al: Curcumin
inhibits transforming growth factor-β1-induced EMT via PPARγ
pathway, not Smad pathway in renal tubular epithelial cells. PLoS
One. 8:e588482013. View Article : Google Scholar
|
|
54
|
Kosugi T and Sato W: Midkine and the
kidney: health and diseases. Nephrol Dial Transplant. 27:16–21.
2012. View Article : Google Scholar
|
|
55
|
Shih AY, Li P and Murphy TH: A
small-molecule-inducible Nrf2-mediated antioxidant response
provides effective prophylaxis against cerebral ischemia in vivo. J
Neurosci. 25:10321–10335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cho HY, Reddy SP, Yamamoto M and
Kleeberger SR: The transcription factor NRF2 protects against
pulmonary fibrosis. FASEB J. 18:1258–1260. 2004.PubMed/NCBI
|
|
57
|
Leonard MO, Kieran NE, Howell K, Burne MJ,
Varadarajan R, Dhakshinamoorthy S, et al: Reoxygenation-specific
activation of the antioxidant transcription factor Nrf2 mediates
cytoprotective gene expression in ischemia-reperfusion injury.
FASEB J. 20:2624–2626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu M, Grigoryev DN, Crow MT, Haas M,
Yamamoto M, Reddy SP and Rabb H: Transcription factor Nrf2 is
protective during ischemic and nephrotoxic acute kidney injury in
mice. Kidney Int. 76:277–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kanki K, Umemura T, Kitamura Y, et al: A
possible role of nrf2 in prevention of renal oxidative damage by
ferric nitrilotriacetate. Toxicol Pathol. 36:353–361. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Biswas SK, McClure D, Jimenez LA, Megson
IL and Rahman I: Curcumin induces glutathione biosynthesis and
inhibits NF-kappaB activation and interleukin-8 release in alveolar
epithelial cells: mechanism of free radical scavenging activity.
Antioxid Redox Signal. 7:32–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Balogun E, Foresti R, Green CJ and
Motterlini R: Changes in temperature modulate heme oxygenase-1
induction by curcumin in renal epithelial cells. Biochem Biophys
Res Commun. 308:950–955. 2003. View Article : Google Scholar : PubMed/NCBI
|