Introduction
Colorectal cancer is a cancer of the cells lining
the colorectal epithelium and has been identified as the third most
common type of cancer worldwide (1,2). It
is caused by a combination of a variety of factors, including diet,
genetic mutations in several different loci, and reproductive or
exogenous hormone use (3).
Colorectal cancer is characterized by high cell motility and
metastatic potential. Metastases from the cancer, rather than the
primary tumors, were observed to be the main causes of mortality
(4). To prevent further
deterioration, current therapies for colorectal cancer at different
stages concentrate on fluorouracil plus leucovorin based
conventional chemotherapeutics; however, these are accompanied by
negative side effects, high recurrence rates of >50% and high
costs (5). At present, the
underlying molecular mechanisms to suppress human colorectal cancer
development are poorly understood. Therefore, developing novel
genetic therapeutic targets for the potential treatment of
colorectal cancer is urgently required.
Reticulons (RTNs) have been identified as
endoplasmic reticulum integral membrane proteins involved in
multiple apoptotic signaling pathways (6). The family comprises four paralogs
termed RTN1, RTN2, RTN3 and RTN4 (7). Previous studies have revealed that
overexpression of RTN3 may induce cell apoptosis in normal HeLa
cells via ectopic overexpression of Bcl-2 (8). A transcript variant 3 of RTN4 termed
RTN4-C is highly expressed in the HEK293 human embryonic kidney
cell line and was observed to induce cell apoptosis through the
c-Jun N-terminal kinase-c-Jun signaling pathway (9). Additionally, RTN4-C inhibited
the growth of SMMC7721 hepatocellular carcinoma cells via inducing
apoptosis (10). However, the
functional role of RTN4-C in colorectal cancer remains to be
elucidated and requires further investigation.
To determine the role of RTN4-C in colorectal
cancer, and potentially identify a novel target for anti-tumor
therapy, the expression levels of RTN4-C were detected in
multiple colorectal cancer cell lines: SW480, SW620, RKO, DLD-1,
HCT116 and HT-29. Subsequently, lentivirus-mediated short hairpin
RNA (shRNA) was adopted to target RTN4-C in colorectal
cancer cells. Proliferation, colony formation and cell cycle assays
were also conducted.
Materials and methods
Cell culture
SW480, SW620, RKO, DLD-1, HCT116, HT-29 human
colorectal cancer cell lines and the HEK293T human embryonic kidney
cell line were purchased from the Cell Bank of the Chinese Academy
of Sciences (Shanghai, China). SW480, SW620, RKO and DLD-1 cells
were cultured in RPMI-1640 (Gibco-BRL, Grand Island, NY, USA)
containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA).
HCT116 and HT-29 cells were cultured in McCoys 5A medium
(Sigma-Aldrich, Poole, UK) supplemented with 10% FBS. HEK293T cells
were maintained in Dulbecco’s modified Eagle’s medium (Hyclone)
supplemented with 10% FBS. All cells were maintained in a
humidified incubator with 5% CO2.
Construction of RTN4-C shRNA-expressing
lentivirus
The cDNA sequence of RTN4-C was obtained from
NCBI (GenBank, NM_007008.2). Sequence-specific knockdown of
RTN4-C was induced with an shRNA with the following
sequence: 5′-CCGG GCTATATCTGAGGAGTTGGTTCTCGAGAACCAACTCC
TCAGATATAGCTTTTTTG-3′. The non-silencing shRNA had the following
sequence:
5′-CCGGCCAAGGAAGTGCAATTGCATACTCGAGTATGCAATTGCACTTCCTTGGTTTTTTG -3′
and was used as a control. The shRNAs were purchased from Shanghai
Hollybio (Shanghai, China). The two synthesized shRNAs were ligated
into the pFH-L vector (Shanghai Hollybio, Shanghai, China)
containing a green fluorescent protein (GFP) reporter driven by the
cauliflower mosaic virus 35S promoter. The generated plasmids were
transfected into HEK293T cells with the packaging vectors pVSVG-I
and pCMVΔR8.92 (Shanghai Hollybio) using Lipofectamine
2000® (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions. For lentivirus
transfection, RKO and DLD-1 cells were cultured in 6-well plates at
a density of 5×104 cells/well and transfected with
lentiviruses (shRTN4 or shControl) at a multiplicity of
transfection of 20, respectively. Transfection efficiency was
determined by counting GFP-expressing cells under a fluorescence
microscope (Olympus Corporation, Tokyo, Japan) 96 h after
transfection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The RT-qPCR experiment was conducted to elucidate
RTN4-C gene expression in the SW480, SW620, RKO, DLD-1,
HCT116 and HT-29 colorectal cancer cell lines. In addition, it was
performed to detect the knockdown efficiency of the RTN4-C
gene in cells transfected with shControl or shRTN4, RT-qPCR was
performed following transfection for 5 days. TRIzol reagent
(Invitrogen Life Technologies) was used to extract the total RNA of
the cultured cells. The primers sequences used were as follows:
Forward 5′-CTCCTCTGGTCTCGTCCTC-3′ and reverse
5′-GTCCTCGTCCTCCTCTTCC-3′ for RTN4-C; and forward
5′-GTGGACATCCGCAAAGAC-3′ and reverse 5′-AAAGGGTGTAACGCAACTA-3′ for
β-actin. Fold changes in expression were calculated using the
2−ΔΔCt method.
Western blot analysis
Following transfection for 5 days, RKO and DLD-1
cells were lysed in 2X SDS sample buffer (10 mM EDTA, 4% SDS and
10% Glycine in 100 mM Tris-HCl buffer, pH 6.8) for 1 h at 4°C.
Equal quantities of proteins (30 µg) were loaded and
separated on 10% SDS-PAGE gels and transferred onto polyvinylidene
difluoride membranes (Millipore, Bedford, MA, USA). Following
blocking with 5% skimmed milk, the membranes were exposed to the
primary antibodies RTN4-C (ab47085, 1:500 dilution; Abcam,
Cambridge, MA, USA) and mouse monoclonal GAPDH (sc-32233, 1:3,000;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at
4°C. Following incubation with goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (sc-2054, 1:5,000; Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature, the
expression of the target proteins were visualized with
chemiluminescence reagents (ECL kit; Amersham Pharmacia Biotech,
Amersham, UK). Bands were analyzed using the Imagequant
densitometric scanner (Molecular Dynamics, Sunnyvale, CA, USA).
MTT proliferation assay
To detect the effect on proliferation of RKO and
DLD-1 cells transfected by shRTN4 or shControl, an MTT assay was
conducted. Lentivirus-transduced RKO and DLD-1 cells were reseeded
in 96-well plates at a density of 2×103 cells/well,
respectively. Viable cell numbers were determined following seeding
for 1, 2, 3, 4 and 5 days. A total of 10 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
solution was added to each well. Following incubation for 3 h, 100
µl acidic isopropanol containing 10% SDS, 5% isopropanol and
0.01 mol/l HCl was added into each well to dissolve the formazan
crystals. Finally, the absorbance at 595 nm was recorded using the
Shimadzu UV-1603 spectrophotometer (Shimadzu, Kyoto, Japan).
Plate colony formation assay
Lentivirus-transduced RKO and DLD-1 cells were
cultured in 6-well plates at a density of 400 cells/well. The
medium was changed regularly. After 8 days of culture, the adherent
cells were washed twice with phosphate-buffered saline and fixed
with 4% paraformaldehyde for 30 min at room temperature. The fixed
cells were then stained with crystal violet (Beyotime Institute of
Biotechnology, Haimen, China). The number of colonies (>50
cells/colony) were observed and counted using a fluorescence
microscope (Olympus BX50; Olympus, Tokyo, Japan).
Cell cycle analysis
The RKO cells transfected with shRTN4 or shControl
were seeded at 5×104 cells/dish in 6-cm dishes and
incubated for 72 h. Cell cycle progression was subsequently
monitored using a flow cytometer (Navios; Beckman Coulter, Miami,
FL, USA) and cell cycle analysis kit (C1052; Beyotime Institute of
Biotechnology) according to the manufacturer’s instructions.
Statistical analysis
All data are expressed as the mean ± standard
deviation from three independent experiments. Student’s t-test was
performed for statistical analysis. Statistical analyses were
conducted using SPSS version 19.0 (IBM, Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Differential transcription and
translation of RTN4-C in six colorectal cancer cell lines
Firstly, RT-qPCR was used to analyze RTN4-C
expression at the transcriptional level in six colorectal cell
lines SW480, SW620, RKO, DLD-1, HCT116, and HT-29 (Fig. 1A). As a result, the SW620 cells
exhibited the highest expression level of RTN4-C among these
cells, while RKO cells exhibited the lowest. The SW480, DLD-1,
HCT116 and HT-29 cell lines exhibited relatively high expression
patterns of RTN4-C compared with RKO cells. The
translational levels of RTN4-C in these six cell lines were
detected using western blot analysis. As shown in Fig. 1B, SW480, DLD-1, HCT-116 and HT-29
cells exhibited high expression levels of RTN4-C protein. The RKO
cells, which exhibited the lowest expression levels of
RTN4-C mRNA expression also revealed the lowest
translational pattern. However, SW620 cells with the highest
transcriptional level of RTN4-C exhibited a relatively low
protein expression. To investigate the biological function of
RTN4-C in colorectal cancer, the DLD-1 and RKO cell lines
with relatively high and low RTN4-C expression patterns were
selected.
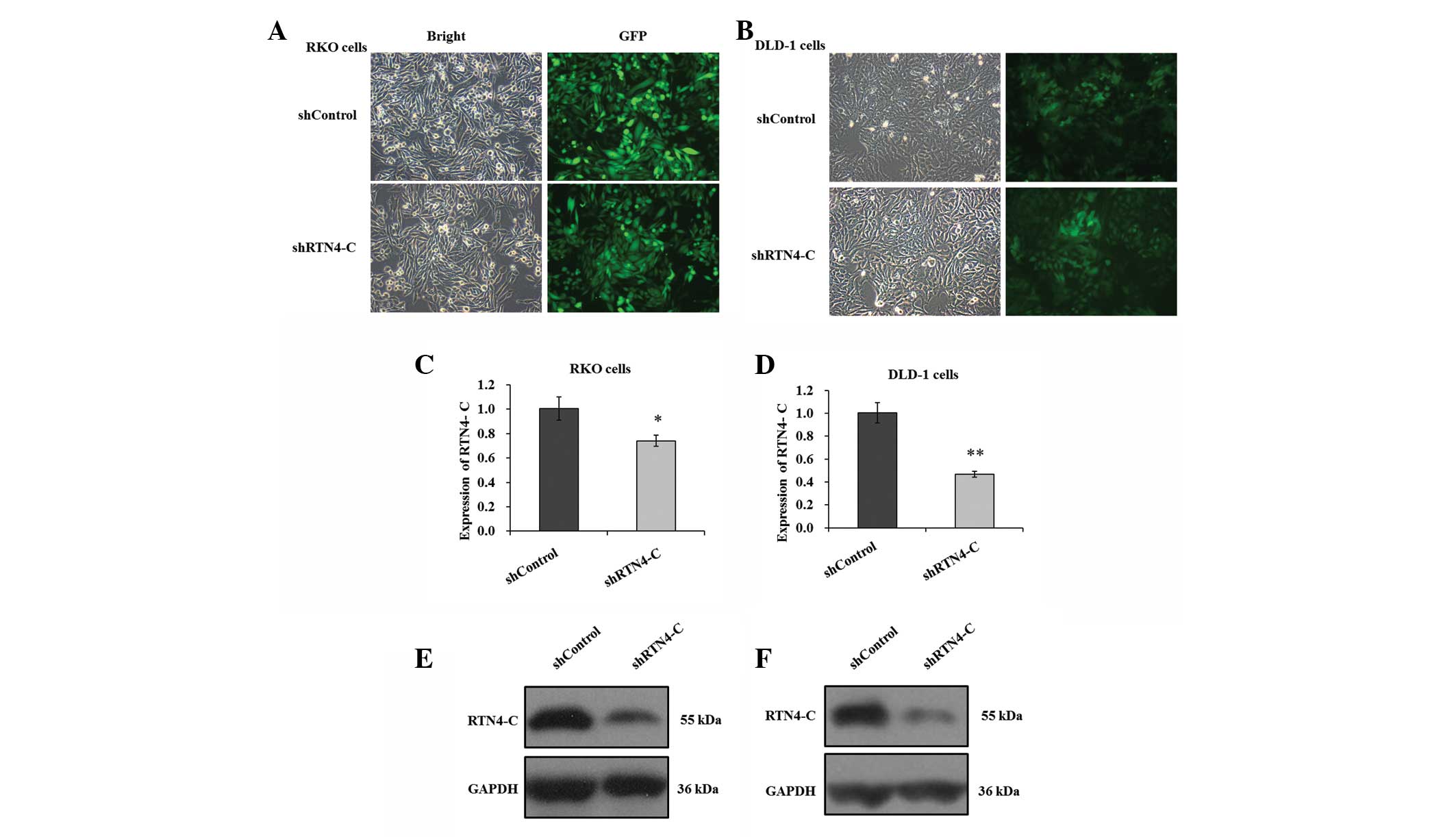
Successful depletion of RTN4-C in DLD-1
and RKO cells by lentivirus-derived RNA interference (RNAi)
RKO and DLD-1 cells transfected with recombinant
lentiviruses harboring shRTN4 or shControl were used to assess the
effects of RTN4-C knockdown on colorectal cancer growth. As shown
in Fig. 2A and B, the transfection
rates in RKO and DLD-1 cells were >80%, as detected by
GFP-fluorescence. The mRNA levels of RTN4-C were
significantly decreased in the two cell lines following shRTN4
transfection compared with shControl, as measured by RT-qPCR
(Fig. 2C and D). The knockdown
efficiency of RTN4-C was 26.2 and 53.2% in RKO and DLD-1
cells, respectively. The endogenous RTN4-C protein level was
estimated in the two cell lines using western blot analysis. This
revealed that the expression levels of RTN4-C were reduced markedly
in shRTN4-transfected RKO and DLD-1 cells in comparison with
shControl (Fig. 2E and F). In
conclusion, it was inferred that RTN4-C depletion was successfully
introduced in DLD-1 and RKO cells via shRTN4 transfection.
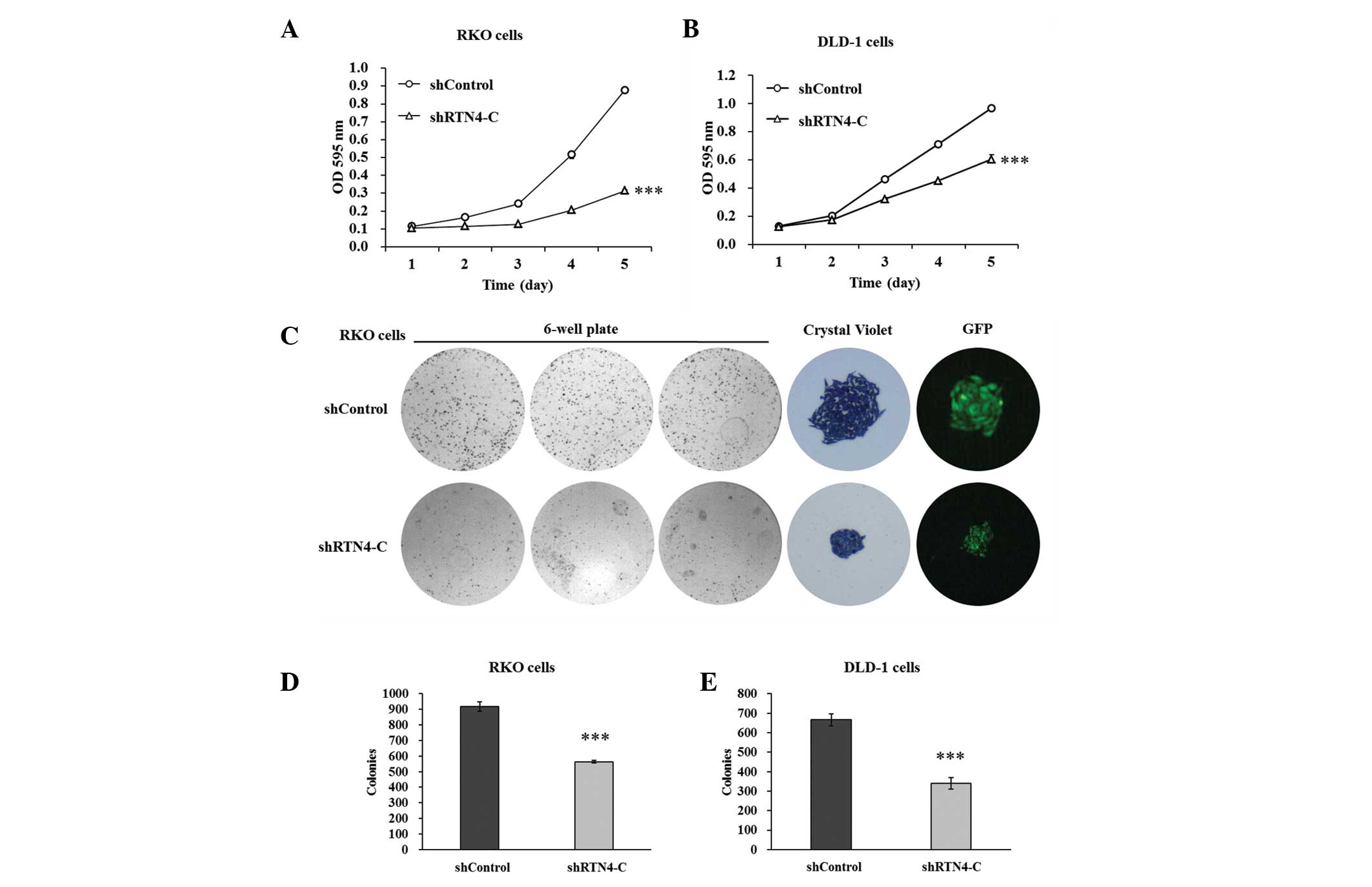
Cell proliferation and colony formation
of DLD-1 and RKO cells are inhibited by RTN4-C downregulation
To evaluate whether the proliferation of RKO and
DLD-1 cells was affected by RTN4-C downregulation, an MTT
assay was performed. Figure 3A and
B show that the proliferation rates were decreased markedly in
shRTN4-transfected RKO and DLD-1 cells relative to the shControl.
The proliferation of shRTN4-transfected RKO cells was suppressed by
60 and 64% at days 4 and 5 compared with the shControl. The
proliferation of DLD-1 cells was reduced by 36 and 37% at days 4
and 5, respectively, in the shRTN4 group compared with the
shControl group.
In addition, a colony formation assay was conducted
to determine the effect of RTN4-C on the in vitro
tumorigenicity of colorectal cancer cells. The number of colonies
was fewer and the size of each colony was markedly smaller in the
shRTN4 groups compared with the shControl groups (Fig. 3C). Total colony numbers were
reduced significantly as indicated in Fig. 3D and E. There were 564±28 colonies
formed of shRTN4-transfected RKO cells, while 917±10 colonies of
the shControl cells formed. There were 340±18 colonies formed of
shRTN4-transfected DLD-1 cells, while there were 666±30 colonies in
the shControl groups. These results demonstrated that RTN4-C
knockdown may inhibit colorectal cancer cell proliferation and
colony formation.
RTN4-C knockdown blocks cell cycle
progression in RKO cells
To examine the mechanisms of cell growth inhibition
by lentivirus-mediated RTN4-C knockdown, the cell cycle
progression of RKO cells was determined using flow cytometry
(Fig. 4A). As shown in Fig. 4B, cell numbers in shRTN4 groups
were significantly increased in the G0/G1 phase and the sub-G1
phase representing apoptotic cells, compared with those in the
shControl groups. The percentage of cells was increased by ~24 or
~92% in the G0/G1 phase or the sub-G1 phase, respectively,
following shRTN4 transfection. Whereas the percentage of cells was
decreased by 14 or 48% in the S phase or the G2/M phase following
shRTN4 transfection. Knockdown of RTN4-C may inhibit
colorectal cancer cell growth possibly via induction of cell cycle
arrest and apoptosis.
Discussion
Colorectal cancer is regarded as one of the most
common malignancies and it is difficult to treat (11,12).
Previous studies have indicated that >55,000 mortalities occur
due to metastatic colorectal cancer annually in the United States
alone (13). To gain insight into
the biological function of RTN4-C in colorectal cancer,
lentivirus-based RTN4-C knockdown in RKO and DLD-1
colorectal cancer cells were constructed and further investigated.
The results revealed that the cell proliferation and colony
formation were restrained in shRTN4 transfected RKO and DLD-1
cells.
Several previous studies have reported that changes
in cell cycle distribution contribute to cell proliferation
inhibition. Notably, microRNA-125b arrested the cell cycle at the
G1 to S transition to prevent cell proliferation and metastasis in
human liver cancer (14).
Nemo-like kinase expression knockdown blocks the G0/G1 phase to S
phase transition to inhibit human adenosquamous carcinoma cells
CAL-27 proliferation and colony formation (15). During the present study, knockdown
of RTN4 caused a decrease in the percentage of cells in S phase and
G2/M phase, but resulted in an increase of cells in G0/G1 phase, in
particular there was an increased number of apoptotic cells, as
indicated by an increase of cells in sub-G1 phase, which suggests
that RTN4-C may regulate G0/G1 to S phase transition and
inhibit cell apoptosis. Nevertheless, cell apoptosis was promoted
in SMMC7721 hepatocellular carcinoma cells with an increased level
of RTN4-C (10).
Additionally, Chen et al (9) reported that RTN4-C induced
HEK293 cell apoptosis through its involvement in the c-Jun
N-terminal kinase-c-Jun pathway. These studies suggest that
RTN4-C may function differentially in cancer cell growth and
apoptosis in various human organs. In the present study,
RTN4-C knockdown may have altered gene expression to
directly or indirectly activate the cell apoptosis pathway in
colorectal cancer cells. In conclusion, RTN4-C knockdown may
induce cell apoptosis and block cell cycle progression at the G0/G1
phase to suppress cell proliferation and colony formation in
colorectal cancer cells. However, the altered gene expression and
the activated apoptosis signaling pathway remain to be elucidated
and require further study.
It is well-established that tumor metastasis is
facilitated by cell proliferative activity (16). Therefore, controlling cell
proliferation may contribute to the prevention of tumor
development. The present study demonstrated that knockdown of
RTN4-C by RNAi resulted in a significant inhibition of
colorectal cancer cell growth via induction of G0/G1 phase cell
cycle arrest and apoptosis. The present study improves the
understanding of RTN4-C function in colorectal cancer and
may aid in the development of a novel therapeutic approach.
Acknowledgments
The present study was supported by the Research Fund
of the Science and Technology Commission of Shanghai Municipality
(grant no. 12ZR1418000).
References
|
1
|
Nasrallah A, Saykali B, Al Dimassi S,
Khoury N, Hanna S and El-Sibai M: Effect of StarD13 on colorectal
cancer proliferation, motility and invasion. Oncol Rep. 31:505–515.
2014.
|
|
2
|
Mathis KL and Nelson H: Controversies in
laparoscopy for colon and rectal cancer. Surg Oncol Clin N Am.
23:35–47. 2014. View Article : Google Scholar
|
|
3
|
Watson AJ and Collins PD: Colon cancer: a
civilization disorder. Dig Dis. 29:222–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chambers AF, Groom AC and MacDonald IC:
Metastasis: dissemination and growth of cancer cells in metastatic
sites. Nat Rev Cancer. 2:563–572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shakibaei M, Buhrmann C, Kraehe P, Shayan
P, Lueders C and Goel A: Curcumin chemosensitizes 5-fluorouracil
resistant mmr-deficient human colon cancer cells in high density
cultures. PLoS One. 9:e853972014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiurchiu V, Maccarrone M and Orlacchio A:
The role of reticulons in neurodegenerative diseases.
Neuromolecular Med. 16:3–15. 2014. View Article : Google Scholar :
|
|
7
|
Diekmann H, Klinger M, Oertle T, et al:
Analysis of the reticulon gene family demonstrates the absence of
the neurite growth inhibitor Nogo-A in fish. Mol Biol Evol.
22:1635–1648. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu L, Xiang R, Dong W, Liu Y and Qi Y:
Anti-apoptotic activity of Bcl-2 is enhanced by its interaction
with RTN3. Cell Biol Int. 31:825–830. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Tang X, Cao X, Chen H and Zhang X:
Human Nogo-C overexpression induces HEK293 cell apoptosis via a
mechanism that involves JNK-c-Jun pathway. Biochem Biophys Res
Commun. 348:923–928. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen YC, Lu DD, Cao XR and Zhang XR:
RTN4-C gene expression in hepatocellular carcinoma and its
influence on SMMC7721 cell growth and apoptosis. Yi Chuan Xue Bao.
32:891–897. 2005.PubMed/NCBI
|
|
11
|
Murray GI, Duncan ME, O’Neil P, Melvin WT
and Fothergill JE: Matrix metalloproteinase-1 is associated with
poor prognosis in colorectal cancer. Nat Med. 2:461–462. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saltz LB, Cox JV, Blanke C, et al:
Irinotecan plus fluorouracil and leucovorin for metastatic
colorectal cancer Irinotecan Study Group. N Engl J Med.
343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Halama N, Michel S, Kloor M, et al:
Localization and density of immune cells in the invasive margin of
human colorectal cancer liver metastases are prognostic for
response to chemotherapy. Cancer Res. 71:5670–5677. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang L, Wong CM, Ying Q, et al: MicroRNA.
125b suppressesed human liver cancer cell proliferation and
metastasis by directly targeting oncogene LIN28B2. Hepatology.
52:1731–1740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang B, Li KY, Chen HY, et al:
Lentivirus-based RNA silencing of nemo-like kinase (NLK) inhibits
the CAL 27 human adenosquamos carcinoma cells proliferation and
blocks G0/G1 phase to S phase. Int J Med Sci. 10:1301–1306. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao H, Ho PC, Lo YH, et al: Interaction
of proliferation cell nuclear antigen (PCNA) with c-Abl in cell
proliferation and response to DNA damages in breast cancer. PloS
One. 7:e294162012. View Article : Google Scholar : PubMed/NCBI
|