Introduction
Epithelial ovarian carcinoma (EOC) is the fifth
leading cause of cancer-associated mortality and remains one of the
most aggressive tumors of all types of gynecological malignancy in
Western countries (1). According
to Cancer Statistics 2012, there is an estimated 15,500 mortalities
due to this disease in the United States every year (2). The majority of patients with EOC have
advanced intraperitoneal metastases at diagnosis since this
carcinoma frequently remains clinically silent (3). Since the treatment strategy
consisting of maximum cytoreductive surgery followed by taxane plus
platinum chemotherapy was established, the short-term prognosis of
patients with EOC has largely improved (4). However, despite the comparatively
high-level sensitivity of EOC to paclitaxel, the prognosis of
advanced or recurrent cases remains poor as the majority of cases
of mortality are the result of metastasis that is refractory to
these chemotherapeutic agents. Although various additional
molecular-targeted therapies, including anti-angiogenic agents,
have been investigated in order to overcome paclitaxel resistance,
the effect of such treatment is not satisfactory (5,6).
Currently, numerous studies have been conducted in order to
identify new strategies and targets to treat this disease (7–9).
Recently, a growing emphasis has been placed on the
association between the nervous system and cancer as increasing
evidence supports the theory that there are common genetic
mechanisms in the development of cancer and in the progression of
neurodegenerative disease (10–12).
The nervous system may potentially affect the development of
cancer; environmental enrichment has been demonstrated to
significantly inhibit xenograft tumor growth, however, the
underlying mechanism remains to be elucidated (13). The neuropilin 1 (NRP1) and the
neuropilin 2 (NRP2) receptors were characterized as receptors for
axon guidance factors of the class-3 semaphorin (sema3) family
(14). It was subsequently
established that neuropilins are also expressed by endothelial
cells and by numerous types of cancer cells (15), and that they are involved in the
transduction of pro-angiogenic signals induced by angiogenic
factors, including vascular endothelial growth factor (VEGF) and
hepatocyte growth factor/scatter factor (16–18).
However, the role of NRP1 in EOC remains to be elucidated.
Therefore, the present study focused on the expression of NRP1 in
ovarian cancer in order to determine whether the expression of NRP1
is associated with ovarian cancer.
Patients and methods
Patient information and tissue
sampling
In total, 125 specimens of EOC from patients
diagnosed between 2000 and 2010 were obtained from surgery from the
Department of Pathology at the Affiliated Hospital of Nantong
University (Nantong, China). None of the patients had received any
form of tumor-specific therapy prior to surgery. Samples were
collected from patients aged between 33 and 82 years old with a
median age of 59 years. According to the classification 2009 of the
International Federation of Gynecology and Obstetrics (FIGO), there
were 20 cases of Stage I, 39 cases of Stage II, 35 cases of Stage
III and 37 cases of Stage IV. The histological grade of the tumor
was classified as GI (well differentiated) in 55 cases, GII
(moderately differentiated) in 32 cases and GIII (poorly
differentiated) in 38 cases. Of all the samples, there were 72
cases with lymph node metastases (median age 55 years; range 39–75
years), 58 cases with pelvic metastases (median age 53 years; range
42–79 years) and 53 cases with peritoneal metastases (median age
56; range 35–68 years). The follow-up period ranged between 2 and
60 months with an average cycle period of 29.7 months and a median
period of 20 months. A total of 15 cases of normal ovarian
epithelium specimens were obtained from preventive excision of the
uterus and adnexa uteri. All tissues were obtained with the consent
of the patients. The study protocol followed the guidelines of the
Helsinki Declaration and was approved by the Ethics Committee
(Institutional Review Board) of Nantong University.
Double-labeling immunofluorescence
staining and confocal microscopy
All specimens were embedded in optimum cutting
temperature and frozen in 2-methylbutane cooled by liquid nitrogen.
They were then sectioned into 20 μm thick sections using a
cryostat. Sections were fixed with cold acetone, blocked with 10%
bovine serum albumin (BSA) in phosphate-buffered saline (PBS)
containing 0.2% Triton X-100 and further permeabilized/blocked in
the blocking solution (5% BSA and 0.3% Triton X-100) for 1 h at
room temperature. Non-specific binding was blocked with 10% BSA for
30 min. Sections were then probed with a rabbit monoclonal NRP1
primary antibody (1:100; cat. no. ab81321; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C,
followed by fluorescein isothiocyanate-conjugated rabbit anti-goat
IgG (H&L) secondary antibody (1:100; Abnova Corporation, Tapei,
Taiwan; cat. no. PAB10575) for 2 h in a humidified chamber with
minimal exposure to light. 4′,6-diamidino-2-phenylindole (DAPI;
Sigma-Aldrich, St. Louis, MO, USA) was used to visualize nuclei and
sections were washed with 1X PBS. Images of the samples were
captured using a confocal microscope (Leica Microsystems, Wetzlar,
Germany). Sections were analyzed using a Leica SP5 high-speed
spectral confocal laser-scanning microscope (Leica Microsystems) or
a Zeiss LSM 710 confocal microscope (Carl Zeiss, Oberkochen,
Germany). Immunofluorescence staining for single or double
contractile markers was performed using randomly selected slides
(4–5 slides) containing four sections per slide and examined under
the confocal microscope. Specific fluorescence was captured by
confocal microscopy with exposure time kept constant across all the
images. Immunoreactivity was evaluated by semi-quantitative
evaluation using the immunofluorescence staining intensity score
and distribution score.
Evaluation of immunoreactivity
Immunoreactivity was evaluated by the quantification
and stereological counting procedure. Specific fluorescence from
tissue labeled in histological sections was captured by confocal
microscopy with a constant exposure time across all images. From
the quantification and stereological counting procedure, 16-bit
image sections were analyzed using NIH Image J software (National
Institutes of Health, Bethesda, MD, USA). Fluorescence intensity of
pixel gray values in eight separate regions of interest per region
of the normal and tumor tissues was calculated and averaged across
each tissue region. This was performed separately for each label
(NRP1 and DAPI). The fluorescence intensity for NRP1 in normal and
tumor tissues was then compared using analysis of variance and
Tukey’s and Sidak’s comparison tests.
Western blot analysis
Protein lysates were derived from the tumor
specimens and normal ovarian epithelial specimens via lysis in
buffer containing protease inhibitors (Promega Corporation,
Madison, WI, USA). Equal quantities of protein were separated by
10% sulfate polyacrylamide gel electrophoresis and then transferred
onto a polyvinylidene fluoride membrane. Membranes were blocked
with 5% non-fat milk in Tris-buffered saline containing 0.1%
Tween-20 (TBST) for 2 h to avoid nonspecific binding. Following
incubation with the primary antibodies overnight at 4°C (rabbit
monoclonal NRP1 primary antibody; 1:200; Santa Cruz Biotechnology,
Inc.) or rabbit polyclonal anti-β-actin (1:2,000; cat. no. A2668;
Sigma-Aldrich), membranes were washed three times in TBST for 5 min
and subsequently incubated with horseradish peroxidase-conjugated
goat polyclonal anti-rabbit secondary antibody (1:1,000 dilution;
cat. no. A0545; Sigma-Aldrich) for 2 h at room temperature. Signals
were detected using enhanced chemiluminescence (GE Healthcare,
Piscataway, NJ, USA) followed by film development.
Expression analysis by reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
The mRNA expression of NRP1 was analyzed by qPCR.
Total RNAs were extracted using TRIzol (Gibco-BRL, Carlsbad, CA,
USA). qPCR was performed using Hot Start-IT SYBR Green qPCR Master
mix (2X; USB Corporation, Cleveland, OH, USA). According to the
manufacturer’s instructions of the Hot Start-IT, 2 μg of RNA
was used as a template and RT-PCR was performed with 25 μl
Master mix. RT-PCR experiments were performed in a Light Cycler 480
system (Roche Applied Sciences, Indianapolis, IN, USA). Cycling
parameters were set as follows: Hot start at 95°C for 10 min, 40
cycles of amplification/quantification at 95°C for 10 sec, 60°C for
30 sec and 72°C for 30 sec. Melting curve analysis was performed
using continuous fluorescence acquisition from 65-97°C. These
cycling parameters generated single amplicons for the two primer
sets used according to the presence of a single melt peak. GAPDH
was selected as the internal reference. All qPCRs were repeated
three times for each gene and each sample was performed in
triplicate. The primer sequences were as follows: NRP1, forward
5′-AAAACGGTGCCATCCCT-3′ and reverse 5′-AAGAAGCAGAGTGGGTCGTT-3′. The
relative alterations in gene expression data were analyzed by the
2−ΔΔCT method. Triplicates were run for each sample in
three independent experiments.
Clinicopathological analysis and
postoperative follow up
Pathological analysis was performed by the
Department of Pathology of Nantong University and validated by
qualified experts assigned to the study. During the follow-up
period, overall survival time was measured from diagnosis to
mortality or to the last follow up (at 5 years). At the time of
analysis, 86 patients (68.8%) had succumbed to the disease, 37
patients (29.6%) were alive and 2 patients were not identified at
follow up. The estimated median survival time for all patients was
28 months and the calculated survival rates were 72.8% at 1 year,
48.0% at 2 years and 29.6% at 5 years. Following surgery, each
patient was scheduled for a follow-up examination, including
physical examination, complete blood count, tumor marker tests as
well as an ultrasound scan of the pelvis every 3 months in the
first year, semi-annually in the second year and annually after 3
years. More frequent examinations were scheduled if clinically
indicated. The cause of mortality was registered and classified as
mortality due to this cancer, other causes or unknown causes.
During the follow-up period, overall survival was measured from
diagnosis to mortality or to the last follow-up (at 5 years).
Patient mortality was ascertained by reporting from the family and
verified by a review of public records. For overall survival, the
median follow-up of the surviving patients was 28 months (range
2–60 months).
Statistical analysis
Tukey’s and Sidak’s comparison tests were used to
compare the fluorescence intensity. SPSS 19.0 statistical software
(SPSS, Inc., Chicago, IL, USA) was adopted for data analysis. The
χ2 test was used for comparisons between groups.
Survival analysis was calculated by means of the Kaplan-Meier
method and significant levels were assessed by means of the
log-rank test. The results are expressed as the mean ± standard
deviation of at least three independent experiments. For all
statistical analyses, P<0.05 was considered to indicate a
statistically significant difference.
Results
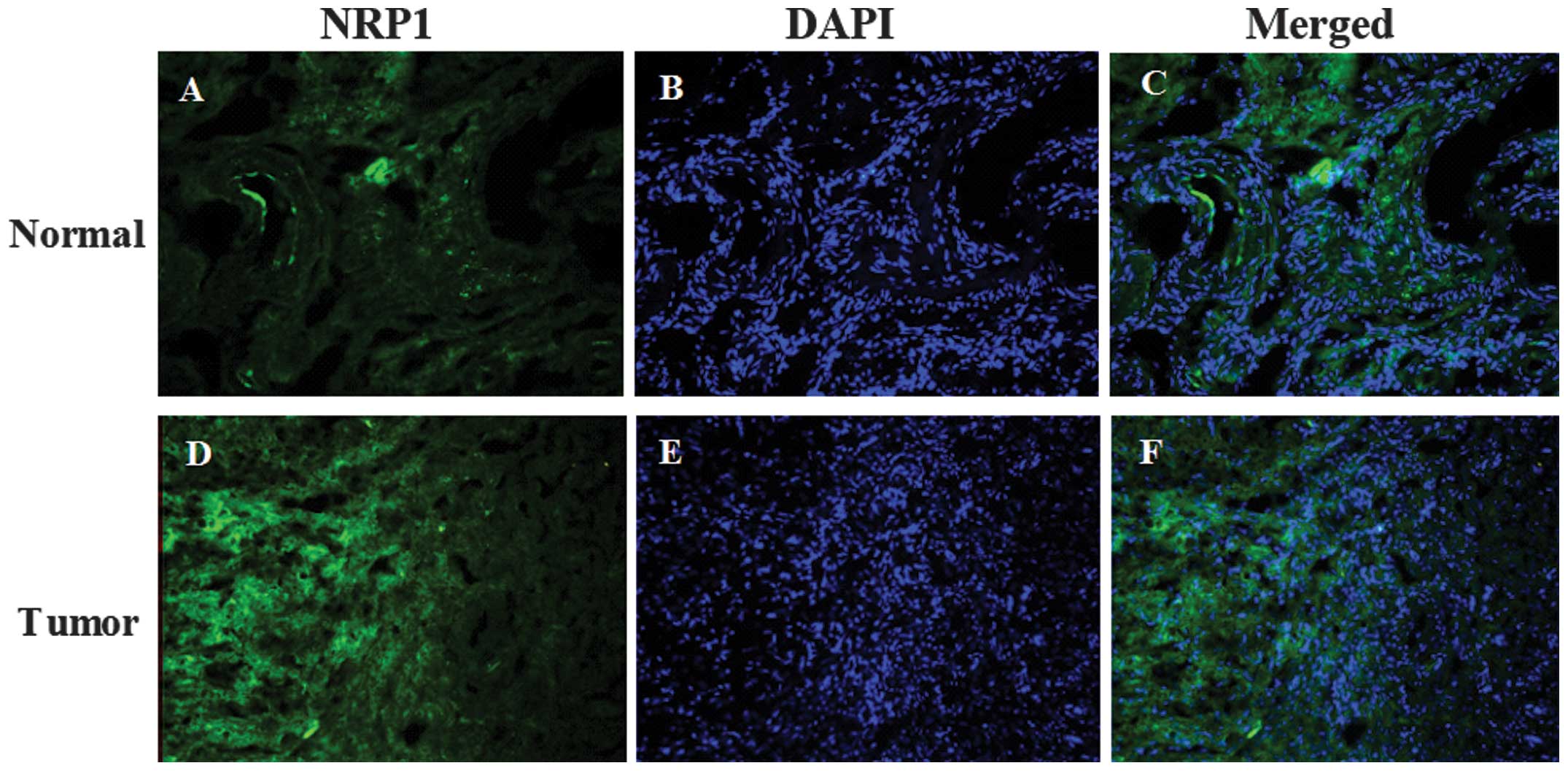
Immunofluorescence staining shows that
Nrp1 protein is present at a higher level in tumor tissue than in
normal ovarian epithelium
NRP1 was detected primarily in the nucleus and
cytoplasm of the normal ovarian epithelium (Fig. 1A and C). Compared with the normal
ovarian epithelium, the EOC specimens demonstrated a stronger
fluorescence intensity (Fig. 1).
In addition, the quantitative fluorescence intensity of NRP1 was
higher in tumor tissue than in normal specimens (Fig. 2). The expression of NRP1 was
significantly upregulated in the tumor tissues compared with the
normal tissues (P<0.001; Figs.
1 and 2).
Nrp1 protein is present at a higher level
in tumor tissue than in normal ovarian epithelium as shown by the
results of the western blotting
The protein expression of NRP1 in ovarian tumor
epithelium and normal ovarian epithelium was examined by western
blot analysis. The relative quantity of NRP1 protein expression was
normalized to β-actin. Five pairs of carcinoma and normal tissues
were randomly selected and presented in Fig. 3A. The results demonstrated a band
for NRP1 at 130 kDa (Fig. 3A). The
protein expression intensities of NRP1 were measured by
densitometry (Fig. 3B). It was
identified that the protein expression of NRP1 was upregulated in
the majority of the ovarian tumor samples compared with normal
tissues (Fig. 3A), while in a
small number of cases (4# in Fig.
3) the expression of NRP1 in the tumor tissues was similar to
that found in the normal tissue (Fig.
3A). The average protein level of NRP1 in EOC was significantly
higher than that in normal ovarian epithelial tissues (P<0.05;
Fig. 3B).
NRP1 mRNA level is higher in cancerous
tissue compared with in normal epithelium
RT-qPCR was also performed to detect the mRNA
expression of NRP1 in EOC and normal ovarian epithelial tissues to
determine whether there is also an upregulation at the mRNA level.
As shown in Table I, the mRNA
expression of NRP1 was significantly higher in ovarian cancerous
samples (median 474 copies/μl, range between 193 and 841
copies/μl) compared with that in normal samples (median 232
copies/μl, range between 102 and 314 copies/μl;
P=0.008). Quantification of the mRNA expression of NRP1 evaluated a
2.3-fold increase in cancerous compared with normal tissues
(Fig. 4).
 | Table ImRNA expression of NRP1 in normal
ovarian epithelium and epithelial ovarian carcinoma. |
Table I
mRNA expression of NRP1 in normal
ovarian epithelium and epithelial ovarian carcinoma.
| Gene | Normal
(copies/μl median) | Tumor
(copies/μl median) | P-value |
|---|
| NRP1 | 232 (102–314) | 474 (193–841) | 0.008 |
Expression of NRP1 is associated with
pathological features of ovarian tumors
Correlations between RT-qPCR results of NRP1
expression in ovarian tumor tissues and various clinicopathological
characteristics of patients were analyzed by χ2 test and
listed in Table II. Using the
quartile limits of mRNA expression to divide patient population
into negative and positive producers allowed us to set the
interquartile range (IQR) as a cut-off and to establish a
significant correlation between mRNA expression and
clinicopathological features. The median expression of NRP1 in
cancerous tissues was 474 copies/μl. The samples were
divided into the following two groups: The negative expression
group of NRP1 (≤474 copies/μl) and the positive expression
group of NRP1 (>474 copies/μl). The upregulation of NRP1
significantly correlated with FIGO stage, histological grade,
lymphatic metastasis and distant metastasis (P<0.05,
respectively). However, no significant correlation between NRP1
expression and age, tumor size and histological type was identified
(P>0.05; Table II).
 | Table IIAssociation between NRP1 expression
levels in epithelial ovarian carcinoma and clinicopathological
features. |
Table II
Association between NRP1 expression
levels in epithelial ovarian carcinoma and clinicopathological
features.
| Characteristic | No. | NRP1
|
|---|
| N | P | P-value |
|---|
| Age (years) | | | | 0.102 |
| ≤59 | 63 | 23 | 40 | |
| >59 | 62 | 18 | 44 | |
| Tumor size (cm) | | | | 0.105 |
| ≤2 | 45 | 17 | 28 | |
| >2 | 80 | 24 | 56 | |
| FIGO stage | | | | 0.025 |
| I/II | 59 | 16 | 43 | |
| III/IV | 72 | 31 | 41 | |
| Histological
grade | | | | 0.049 |
| Well
differentiated | 55 | 22 | 33 | |
| Poorly
differentiated | 70 | 19 | 51 | |
| Histotype | | | | 0.395 |
| Serous | 58 | 18 | 40 | |
| Endometrioid | 49 | 15 | 34 | |
| Clear cell | 13 | 5 | 8 | |
| Mucinous | 3 | 2 | 1 | |
|
Undifferentiated | 1 | 0 | 1 | |
| Other | 1 | 1 | 0 | |
| Lymphatic
metastasis | | | | 0.006 |
| Negative | 53 | 24 | 29 | |
| Positive | 72 | 17 | 55 | |
| Distant
metastasis | | | | 0.024 |
| Negative | 67 | 23 | 44 | |
| Positive | 58 | 18 | 40 | |
NRP1 expression is associated with
patient survival rate
The prognostic effect of NRP1 on the overall
survival rate of ovarian carcinoma patients was investigated by
comparing the 5-year survival rate of patients with tumors
expressing NRP1 using Kaplan-Meier survival curves and the log-rank
test. There were 70 cases in the positive NRP1 expression group
(>474 copies/μl), of which 58 cases succumbed to ovarian
carcinoma, two cases were not identified at follow up and the
5-year overall survival rate was 14.3%. For the negative NRP1
expression group (<474 copies/μl), there were 55 cases,
of which 28 cases succumbed to the disease and the 5-year overall
survival rate was 49.1%. It was found that the overall survival
time of the positive NRP1 expression group was significantly
shorter than that of the negative NRP1 expression group
(P<0.001; Fig. 5).
Discussion
In the present study, NRP1 was found to be
extensively upregulated in EOC compared with normal ovarian
epithelium. Immunofluorescence, western blot analysis and RT-qPCR
were used to detect the expression of NRP1 in ovarian cancerous
surgical specimens and normal ovarian epithelial tissues. The mRNA
and protein expression of NRP1 in ovarian epithelial cancer tissues
was significantly higher than that in normal ovarian epithelial
tissues. It was found that the overall survival time of the high
NRP1 expression group was significantly shorter than that of the
low NRP1 expression group (P<0.05). The changes in NRP1
expression in ovarian cancer in the present study indicates an
important role of NRP1 in the development of EOC. In addition, a
higher expression of NRP1 correlated with a poor prognosis in
ovarian tumors, demonstrating that NRP1 may act as a promoter in
ovarian epithelial cancer.
NRP1 is a transmembrane glycoprotein that acts as a
co-receptor for a number of extracellular ligands, including class
III/IV semaphorins, certain isoforms of VEGF and transforming
growth factor-β. NRP1 is expressed at high levels in several tumor
cells, where it has been implicated in cell migration and survival
(19,20). An exact understanding of the role
of NRP1 in the immune system has been obscured by the differences
in NRP1 expression observed between mice and humans. In mice, NRP1
is selectively expressed on thymus-derived T regulatory cells
(Tregs) and markedly enhances immunosuppressive functions. In
humans, NRP1 is expressed on plasmacytoid dendritic cells (pDCs)
where it aids in priming immune responses and on a subset of Tregs
isolated from secondary lymph nodes. Preliminary studies show that
NRP1 expression on T cells confers enhanced immunosuppressive
activity (21,22). However, the underlying mechanism
remains to be elucidated. The expression of NRP1 has also been
identified in activated T cells and Tregs isolated from
inflammatory microenvironments, suggesting that NRP1 may be a novel
T cell activation marker (23). Of
clinical interest, NRP1 may enhance tumor infiltration of Tregs and
a decrease in NRP1+Tregs correlates with successful chemotherapy,
suggesting a specific role for NRP1 in cancer pathology (21). As a therapeutic target, NRP1 allows
simultaneous targeting of NRP1-expressing tumor vasculature,
NRP1+Tregs and pDCs. With the development of anti-NRP1 monoclonal
antibodies and cell-penetrating peptides, NRP1 represents a
promising new target for cancer therapies (24).
NRP1 has been implicated as a tumor suppressor in
other types of cancer (25). NRP1
and NRP2 are transmembrane glycoproteins, which interact with VEGF
to prevent tumor cell apoptosis and regulate angiogenesis. For
example, in colorectal cancer, an increased expression of NRP1 and
NRP2 in epithelium as well as an increased expression of NRP1 in
vessels may be associated with the progression of colorectal cancer
(19). A more recent study on
miRNAs demonstrated that the downregulation of miR-320, which
regulates NRP1, in blood vessels is inversely correlated with
vascularity in oral squamous cell carcinoma (OSCC) tissues
(26). By administering either
miR-320 precursor or antagonist, miR-320 suppressed the migration,
adhesion and tube formation of vascular endothelial cells.
Knockdown of NRP1 reduced antagomiR-320-induced cell migration
(26). Additionally, miR-320
expression was demonstrated to be regulated by hypoxia in growth
factor-deficient conditions by hypoxia-inducible factor-1α
(27). Furthermore, a lentivirus
carrying the miR-320 precursor suppressed the tumorigenicity of
OSCC cells and tumor angiogenesis in vivo (28). These data show that miR-320 may
regulate the function of vascular endothelial cells by targeting
NRP1 and have the potential to be developed as an anti-angiogenic
or anti-cancer drug. These results taken together with the results
of the present study demonstrate that NRP1 may be associated with
cancer progression in EOC.
Although the present study provided valuable results
regarding the role of NRP1 in EOC, a limitation of the study was
that only 15 cases of normal tissues were used. A larger number of
cases should be used in future studies. The present study attempted
to use double-labeling immunofluorescence staining and confocal
microscopy instead of immunohistochemistry. It was difficult to
control the chromogenic time in immunohistochemistry and the
sensitivity of staining was lower than that of immunofluorescence.
In addition, the immunohistochemistry results could be affected by
numerous artificial factors (24,25).
By contrast, fluorescence could objectively reflect the expression
of the protein. The main advantages of this technology included its
strong specificity, high sensitivity and time efficiency.
In the present study, quantitative mRNA expression
was used to analyze the association of NRP1 with the
clinicopathological factors of EOC as well as the overall survival
rate. Using the quartile limits of mRNA expression to divide the
patient population into low and high producers, this allowed us to
set IQR as a cut-off and to establish a significant correlation
between mRNA expression and clinicopathological factors as well as
survival rate, which may more accurately reflect the real
situation.
In conclusion, in the present study, NRP1 was found
to be overexpressed in human EOC. A higher expression of NRP1 was
strongly associated with a poorer patient survival time. This may
provide a novel prognostic method and a promising treatment
strategy for EOC.
References
|
1
|
Paes MF, Daltoé RD, Madeira KP, et al: A
retrospective analysis of clinicopathological and prognostic
characteristics of ovarian tumors in the state of Espírito Santo,
Brazil. J Ovarian Res. 4:142011. View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hudson LG, Zeineldin R and Stack MS:
Phenotypic plasticity of neoplastic ovarian epithelium: unique
cadherin profiles in tumor progression. Clin Exp Metastasis.
25:643–655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Domenighetti G, Moccia A and Gayer R:
Observational case-control study of non-invasive ventilation in
patients with ARDS. Monaldi Arch Chest Dis. 69:5–10.
2008.PubMed/NCBI
|
|
5
|
Teoh D and Secord AA: Antiangiogenic
agents in combination with chemotherapy for the treatment of
epithelial ovarian cancer. Int J Gynecol Cancer. 22:348–359. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Usha L, Sill MW, Darcy KM, et al: A
Gynecologic Oncology Group phase II trial of the protein kinase
C-beta inhibitor, enzastaurin and evaluation of markers with
potential predictive and prognostic value in persistent or
recurrent epithelial ovarian and primary peritoneal malignancies.
Gynecol Oncol. 121:455–461. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu H, Yao L, Mei J and Li F: Development
of synthetic of peptide-functionalized liposome for enhanced
targeted ovarian carcinoma therapy. Int J Clin Exp Pathol.
8:207–216. 2015.PubMed/NCBI
|
|
8
|
Kannan K, Coarfa C, Chao PW, et al:
Recurrent BCAM-AKT2 fusion gene leads to a constitutively activated
AKT2 fusion kinase in high-grade serous ovarian carcinoma. Proc
Natl Acad Sci USA. 112:E1272–E1277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gurler H, Yu Y, Choi J, Kajdacsy-Balla AA
and Barbolina MV: Three-dimensional collagen type I matrix
up-regulates nuclear isoforms of the microtubule associated protein
tau implicated in resistance to paclitaxel therapy in ovarian
carcinoma. Int J Mol Sci. 16:3419–3433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morris LG, Veeriah S and Chan TA: Genetic
determinants at the interface of cancer and neurodegenerative
disease. Oncogene. 29:3453–3464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gan GN, Weickhardt AJ, Scheier B, et al:
Stereotactic radiation therapy can safely and durably control sites
of extra-central nervous system oligoprogressive disease in
anaplastic lymphoma kinase-positive lung cancer patients receiving
crizotinib. Int J Radiat Oncol Biol Phys. 88:892–898. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Catalano A, Caprari P, Rodilossi S, et al:
Cross-talk between vascular endothelial growth factor and
semaphorin-3A pathway in the regulation of normal and malignant
mesothelial cell proliferation. FASEB J. 18:358–360. 2004.
|
|
13
|
Cao L, Liu X, Lin EJ, et al: Environmental
and genetic activation of a brain-adipocyte BDNF/leptin axis causes
cancer remission and inhibition. Cell. 142:52–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Casazza A, Fu X, Johansson I, et al:
Systemic and targeted delivery of semaphorin 3A inhibits tumor
angiogenesis and progression in mouse tumor models. Arterioscler
Thromb Vasc Biol. 31:741–749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maione F, Molla F, Meda C, et al:
Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks
tumor growth and normalizes tumor vasculature in transgenic mouse
models. J Clin Invest. 119:3356–3372. 2009.PubMed/NCBI
|
|
16
|
Sakurai A, Gavard J, Annas-Linhares Y, et
al: Semaphorin 3E initiates antiangiogenic signaling through plexin
D1 by regulating Arf6 and R-Ras. Mol Cell Biol. 30:3086–3098. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Varshavsky A, Kessler O, Abramovitch S, et
al: Semaphorin-3B is an angiogenesis inhibitor that is inactivated
by furin-like pro-protein convertases. Cancer Res. 68:6922–6931.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng C, Zhou Q, Wu F, et al: Semaphorin3F
down-regulates the expression of integrin alpha(v)beta3 and
sensitizes multicellular tumor spheroids to chemotherapy via the
neuropilin-2 receptor in vitro. Chemotherapy. 55:344–352. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Staton CA, Koay I, Wu JM, Hoh L, Reed MW
and Brown NJ: Neuropilin-1 and neuropilin-2 expression in the
adenoma-carcinoma sequence of colorectal cancer. Histopathology.
62:908–915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia H, Cheng L, Tickner M, Bagherzadeh A,
Selwood D and Zachary I: Neuropilin-1 antagonism in human carcinoma
cells inhibits migration and enhances chemosensitivity. Br J
Cancer. 102:541–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chaudhary B, Khaled YS, Ammori BJ and
Elkord E: Neuropilin 1: function and therapeutic potential in
cancer. Cancer Immunol Immunother. 63:81–99. 2014. View Article : Google Scholar
|
|
22
|
Bruder D, Probst-Kepper M, Westendorf AM,
et al: Neuropilin-1: A surface marker of regulatory T cells. Eur J
Immunol. 34:623–630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Renand A, Milpied P, Rossignol J, et al:
Neuropilin-1 expression characterizes T follicular helper (Tfh)
cells activated during B cell differentiation in human secondary
lymphoid organs. PLoS One. 8:e855892013. View Article : Google Scholar
|
|
24
|
Chaudhary B, Khaled YS, Ammori BJ and
Elkord E: Neuropilin 1: function and therapeutic potential in
cancer. Cancer Immunol Immunother. 63:81–99. 2014. View Article : Google Scholar
|
|
25
|
Kamiya T, Kawakami T, Abe Y, et al: The
preserved expression of neuropilin (NRP) 1 contributes to a better
prognosis in colon cancer. Oncol Rep. 15:369–373. 2006.PubMed/NCBI
|
|
26
|
Wu YY, Chen YL, Jao YC, et al: miR-320
regulates tumor angiogenesis driven by vascular endothelial cells
in oral cancer by silencing neuropilin 1. Angiogenesis. 17:247–260.
2014. View Article : Google Scholar
|
|
27
|
Wan LY, Deng J, Xiang XJ, Zhang L, et al:
miR-320 enhances the sensitivity of human colon cancer cells to
chemoradiotherapy in vitro by targeting FOXM1. Biochem Biophys Res
Commun. 457:125–32. 2015. View Article : Google Scholar
|
|
28
|
Wu YY, Chen YL, Jao YC, Hsieh IS, Chang KC
and Hong TM: miR-320 regulates tumor angiogenesis driven by
vascular endothelial cells in oral cancer by silencing neuropilin
1. Angiogenesis. 17:247–260. 2014. View Article : Google Scholar
|