Introduction
Biliary tract cancer (BTC) is a highly aggressive
and heterogeneous type of cancer, originating in the bile ducts,
with high rates of relapse and poor clinical outcome (1). Despite current treatment strategies,
including surgery, liver transplantation, chemotherapy and
photodynamic therapy, the 5-year survival rate of only ~5% remains
low (2,3). For advanced, inoperable BTC,
palliative chemotherapy with cisplatin and gemcitabine results in a
median survival rate of ~1 year (4,5).
Chemokines are chemoattracting proteins, which bind
to their respective receptors and thereby activate them. Chemokine
receptor 4 (CXCR4) is a chemokine receptor, which specifically
binds to chemokine ligand 12 (CXCL12). First identified on
leukocytes, CXCR4 is expressed by several different cell types
(6). It is important in
organogenesis, and in tissue repair and regeneration in adults.
Additionally, the expression of CXCR4 was identified in
hematopoietic and non-hematopoietic tissue-committed stem cells
(6–8).
The CXCR4/CXCL12 axis is important in adhesion,
invasion, metastasis, migration and proliferation in various types
of cancer, including breast cancer (9–13),
small-cell lung cancer (14), head
and neck squamous cell carcinoma (15), neuroblastoma (16), hepatocellular carcinoma (17) and colon cancer (18), and is generally associated with
high aggressiveness and a poor prognosis. Several previous studies
have suggested a role for the CXCR4/CXCL12 axis in BTC (19–23).
Lee et al demonstrated that CXCL12 is associated with an
advanced histological grade and nodal metastasis in gallbladder
cancer, and observed increased anchorage-dependent, and
-independent growth of gallbladder cancer cells in vitro, in
a CXCR4-dependent manner (21).
Leelawat et al compared the gene expression levels of
cluster of differentiation (CD)24− and CD24+
BTC cells, and revealed an upregulation of CXCR4 in the
CD24+ subpopulation (19). AMD3100 (Plerixafor) is a
non-peptide antagonist of CXCR4, which inhibits CXCL12 binding and
is currently used for hematopoietic stem cell mobilization and
transplantation following chemotherapy of hematological
malignancies (24,25). The expression of CD24 is
accompanied by poor clinical outcome in BTC, and AMD3100 inhibits
the invasiveness of the CD24+ subpopulation (19), therefore suggesting CXCR4 as a
potential target for the treatment of BTC.
Taken together, CXCR4/CXCL12 are important in
several types of cancer, potentially including BTC. Therefore, the
aim of the present study was to examine the expression levels of
CXCR4 in a larger panel of BTC cell lines (n=8). Following
confirmation of the expression levels, the effects of the AMD3100
CXCR4 antagonist, alone and in combination with the standard
chemotherapeutic gemcitabine, on cell viability and
anchorage-independent growth pattern were investigated.
Materials and methods
Reagents and cell culture
AMD3100 (Mozobil™) was supplied by Sanofi-Aventis
(Paris, France) as a 20 mg/ml subcutaneous injection solution, and
was stored at room temperature. Gemcitabine was obtained from the
hospital pharmacy (Landesapotheke, Salzburger Landeskliniken), as a
stock solution of 152 mM in H2O, and stored in aliquots
at room temperature. Resazurin was purchased from Sigma-Aldrich
(Vienna, Austria), dissolved in Dulbecco’s phosphate-buffered
saline (Sigma-Aldrich) and sterile filtered using a 0.2 μm
filter (Sarstedt, Nüxsmbrecht, Germany). A total of five bile duct
carcinoma cell lines: CCSW-1 (G2), BDC (G4), EGI-1 (G3), SkChA-1
(G3), TFK-1 (G2); and three gallbladder cancer cell lines: MzChA-1
(G1), MzChA-2 (G2) and GBC (G1), were cultured in Dulbecco’s
modified Eagle’s medium (DMEM; PAA Laboratories, Pasching,
Austria), supplemented with 10% (v/v) fetal bovine serum (FBS; PAA
Laboratories) – see (26,27) for original references. Together,
these were referred to as BTC cell lines (1). Subsequent experiments were performed
using cells within 10 passages at seeding densities of
3.95×104 for BDC and MzChA-2, 4.74×104 for
CCSW-1 and GBC), 5.53×104 for SkChA-1,
6.32×104 for EGI-1 and TFK-1, and 7.11×104
for MzChA-1 (per cm2 of the culture dishes), in 10%
FBS-DMEM. Treatment with AMD3100 was performed in serum-free
(sf)DMEM to avoid interaction of the drug with the serum
ingredients.
Drug cytotoxicity
The dose- and cell line-dependent cytotoxicity of
AMD3100 was investigated on the cells in 96-well microplates
(Greiner Bio-One, Frickenhausen, Germany), using a resazurin assay
(Sigma-Aldrich) and an Infinite M200 microplate reader (Tecan,
Grödig, Austria) to assess the metabolic activity, as described
previously (27,28). For the cell line-dependent
analysis, 400 μg/ml AMD3100 was added to the cells in sfDMEM
for 72 h, and the cell viability was normalized against untreated
(sfDMEM only) samples. For dose-dependent analysis, a 1:2 dilution
series ranging between 0.8 and 400.0 μg/ml of AMD3100 were
added to SkCh-A1 cells in sfDMEM for 72 h, and the viability was
normalized against the untreated samples.
Anchorage-independent growth
The anchorage-independent growth of the SkChA-1
cells was assessed using the CytoSelect 96-well Cell Transformation
assay (Cell Biolabs, Inc., San Diego, CA, USA) and an Infinite M200
microplate reader. The SkChA-1 cells were seeded at a density of
1×105 cells/ml in a semi-solid agar, according to the
manufacturer’s instructions, and incubated with 100 μg/ml
AMD3100 in DMEM for 14 days at 37°C. Anchorage-independent growth
was monitored using a light microscope (Motic AE31; Nikon
Instruments, Melville, NY, USA) equipped with a CCD-1300B digital
camera (Allied Vision Technologies/VDS, Vosskühler, Stadtroda,
Germany), quantified using CyQuant GR dye and the growth was
normalized against the untreated (DMEM only) samples.
Analysis of the mRNA expression of
CXCR4
For mRNA expression analysis, the cells were grown
in 35 mm cell culture dishes in DMEM for 72 h. The total RNA was
isolated using the Direct-zol™ RNA MiniPrep kit (Zymo Research,
Irvine, CA, USA) with TRIzol Reagent (Ambion, Life Technologies,
Vienna, Austria). The cDNA synthesis was performed through reverse
transcription (RT), using 1 μg isolated RNA and an
ImProm-IITM Reverse Transcriptase system (Promega, Madison, WI,
USA), according to the manufacturer’s instructions. Quantification
of the cDNA was determined through quantitative polymerase chain
reaction (qPCR), using a GoTaq qPCR Master mix (Promega) and a
ViiA7 real time PCR system (Applied Biosystems, Invitrogen Life
Technologies, Carlsbad, CA, USA). cDNA (0.3 ng) was used for PCR
and the following cycling conditions were used: 95°C for 2 min and
45 cylces of 95°C for 3 sec and 60°C for 30 sec. The samples were
measured at least three times, and subsequent melting curve
analysis was performed for all the primers, to confirm the
specificity of the PCR products. The samples were normalized
against β-actin and the data were analyzed, according to the ΔΔCt
method (29). The primer sequences
used for RT-qPCR were as follows: β-actin, forward
GCACTCTTCCAGCCTTCCTTCC and reverse TCTTTGCGGATGTCCACGTCAC and CXCR4
forward GTCATCTACACAGTCAACCTCTACAGCAGT and reverse
AAGATGAAGTCGGGAATAGTCAGCAG.
Double treatments
The SkChA-1 cells were seeded into 96-well
microplates and incubated with different concentrations of either
AMD3100 (200, 100, 50, 25 or 12.5 μg/ml) or gemcitabine
(1000, 500, 250, 125 or 62.5 μM), or with combinations of
these concentrations (constant 25 μg/ml AMD3100 combined
with 1000, 500, 250, 125 and 62.5 μM gemcitabine or constant
gemcitabine (500 μM) combined with 200, 100, 50, 25 and 12.5
μg/ml AMD3100). Cell viability was measured using a
resazurin assay and an Infinite M200 microplate reader. The
obtained data were entered into CompuSyn software (version 1.0) to
calculate the combination index (CI), in which drug combinations
leading to a CI >1.1 were considered to be antagonistic, and CI
values <0.9 were considered to be synergistic (30).
Statistical analyses
Unless otherwise indicated, all data are presented
as the mean ± standard error of the mean, of at least three
biological replicates. An unpaired Student’s t-test was used for
calculation of the significance of differences in
anchorage-independent growth. A paired Student’s t-test was
performed for calculation of the significance of the effect of
AMD3100 on cell viability. All calculations were performed using
OriginPro 9.1 software (OriginLab, Northampton, MA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of CXCR4 in the BTC cell
lines
The present study confirmed the mRNA expression
levels of CXCR4 in eight BTC cell lines using RT-qPCR. The
expression values differed between the cell lines, with SkChA-1
exhibiting the highest expression levels of CXCR4 (Fig. 1A).
Effect of treatment with AMD3100 on
overall cell viability
The present study subsequently investigated whether
the addition of the CXCR4 antagonist, AMD3100, had an effect on the
cell viability of the CXCR4-expressing cell lines. The cells were
treated with 400 μg/ml AMD3100 for 72 h. The results
revealed that the addition of AMD3100 led to a reduction in cell
viability in seven of the cell lines, with no effect for CCSW-1.
Significant decreases of 20–30% were observed in the GBC, MzChA-1,
SkChA-1 and TFK-1 cell lines (Fig.
1B). Although no correlation was observed between the
expression levels of CXCR4 and the reduction in cell viability by
AMD3100 (data not shown), the most marked effect of AMD3100 on cell
viability was observed in the SkChA-1 cells which also exhibited
the highest mRNA expression levels of CXCR4.
To investigate the dose-dependent effects of AMD3100
on SkChA-1 cells, a dilution series ranging between 0.8 and 400.0
μg/ml was used, with cells incubated with AMD3100 for 72 h
at 37°C. As shown in Fig. 1C,
treatment with AMD3100 reduced the overall cell viability in a
dose-dependent manner, with a significant reduction of ~20% at
concentrations >100 μg/ml (Fig. 1C).
Taken together, treatment with AMD3100 reduced the
cell viability in seven of the eight BTC cell lines assessed. As
the most marked decline in cell viability was observed in the
SkChA-1 cells, this cell line was selected for use in the
subsequent experiments.
Combined treatment of AMD3100 and
gemcitabine
The present study subsequently investigated whether
the combination of AMD3100 with gemcitabine, a standard
chemotherapeutic drug, further reduced the viability of the SkChA-1
cells, and whether these drugs acted synergistically. The results
suggested that at least two combinations: 50 μg/ml AMD3100
with 500 μM gemcitabine, and 12.5 μg/ml AMD3100 with
500 μM gemcitabine, demonstrated a synergistic cytotoxic
effect on the SkChA-1 cells (Fig.
2). Experiments using a combination of AMD3100 and cisplatin
revealed no synergistic effect (data not shown).
Effect of AMD3100 on
anchorage-independent growth
Anchorage-independent growth is a hallmark of
increased tumor aggressiveness, metastatic potential and cancer
stem cell (CSC) characteristics (31). In order to assess the effect of
AMD3100 on anchorage-independent growth, the SkChA-1 cells were
seeded into semisolid agar and incubated for 14 days at 37°C, in
the presence or absence of AMD3100. Treatment with AMD3100
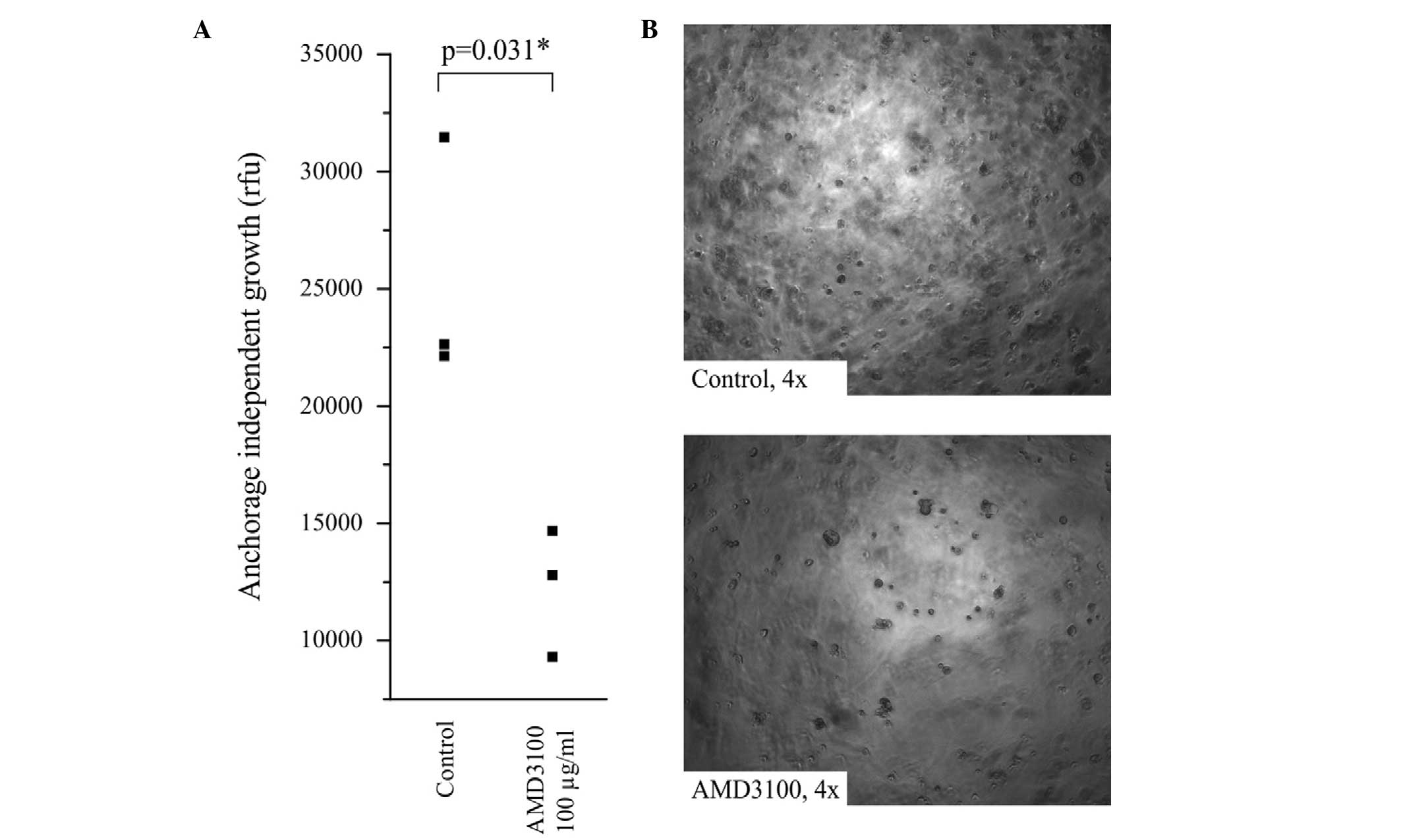
significantly inhibited anchorage-independent growth in the SkChA-1
cells, leading to a reduction of ~50% (Fig. 3A). Representative images of SkChA-1
cells with and without AMD3100 are shown in Fig. 3B and indicated a reduction in cell
aggregates.
Discussion
The high mortality rates and poor prognosis of BTC
underlines the requirement for novel therapeutic approaches. The
CXCR4 chemokine receptor and its ligand, CXCL12, are involved in
the regulation of tissue-committed stem cells (6–8).
Furthermore, these proteins are involved in cancer cell invasion,
metastasis, migration and proliferation (9–18).
CXCR4 is expressed in several types of cancer (7,8),
including BTC (19,22,23),
and its expression correlates with high levels of aggressiveness
and a poor prognosis (7). Previous
studies have demonstrated the involvement of CXCR4 signaling in the
migration and invasion of BTC cells, which can be reduced by the
pharmacological inhibition of CXCR4 using AMD3100 (19,22,23).
In accordance with these results, the present study
detected the mRNA expression of CXCR4 in eight BTC cell lines, to a
variable extent, indicating that this signaling axis exhibits
different activities in different cell lines. In the BDC and GBC
cell lines, the mRNA expression levels of CXCR4 were almost
undetectable, whereas those of CXCR4 were highest in the SkChA-1
cells, compared with the other cell lines.
Treatment of the cell lines with a high
concentration (400 μg/ml) of the AMD3100 CXCR4 antagonist
for 72 h reduced cell viability in seven cell lines, with no effect
in the CCSW-1 cells, of which significant reductions were observed
in the GBC, MzChA-1, SkChA-1 and TFK-1 cells. Since SkChA-1 not
only demonstrated the highest expression levels of CXCR4, but also
the most marked decline in cell viability following treatment with
AMD3100, the present study assessed the dose-dependent (0.78–400
μg/ml) effect of AMD3100 on the cell viability of the
SkChA-1 cell line. A dose-dependent reduction in viability
following 72 h AMD3100 treatment was confirmed, with significant
reductions observed at concentrations >100 μg/ml. These
effects were possibly caused by a slowdown of proliferation, rather
than actual cytotoxicity, as no previous reports have provided
evidence to suggest the induction of cell death following the
inhibition of CXCR4. For example, treatment of a cholangiocarcinoma
cell line with AMD3100 revealed no effect on cell viability
(19). Although the present study
used a higher concentration of AMD3100 compared with (19), it is also possible that, due to the
heterogeneity of BTC, cell lines respond differentially to the
inhibition of CXCR4. Previous studies using BTC cell lines
demonstrated an inhibitory effect on cell migration following the
inhibition of CXCR4 receptors, while the addition of the CXCL12,
SDF-1a ligand increases the proliferative capacity of BTC cells
(22,23). In the present study, no correlation
was observed between the expression of CXCR4 and the effect of
AMD3100 on cell viability.
A significant reduction of 50% was observed in the
anchorage-independent growth of the SkChA-1 cells following
treatment with AMD3100. This is particularly interesting, as this
assay is considered a functional assessment of stemness
characteristics in the CSC theory (31). Therefore, subsequent investigations
into the CSC-specific effects of the pharmacological inhibition of
CXCR4 are required. In line with this theory, CXCR4 is upregulated
in the CD24+ subpopulation, and the expression of this
surface molecule is associated with a poor clinical outcome
(19) and has been suggested as a
marker for putative CSCs in BTC (32).
Gemcitabine combined with cisplatin is the current
standard chemotherapy for patients with advanced BTC (4,5). The
present study examined the possible synergistic effects of each of
these drugs combined with AMD3100 on SkChA-1 cell viability.
Combination with cisplatin revealed no synergistic effect (data not
shown), whereas several combinations of gemcitabine with AMD3100
indicated a clear synergistic effect of those two drugs (30). Notably, at a constant gemcitabine
concentration (500 μM) the synergistic effect was more
pronounced as the concentrations of AMD3100 decreased, with the
lowest CI value being 12.5 μg/ml. This result suggested that
inhibition of CXCR4 combined with gemcitabine may be a suitable
treatment strategy, and is in line with a report by Singh et
al, which demonstrated that the inhibition of CXCR4 using
AMD3100 eliminated gemcitabine resistance in pancreatic cancer
cells (33).
In conclusion, the present study demonstrated that
CXCR4 was expressed in BTC cell lines and that the inhibition of
this receptor affected anchorage-dependent and
anchorage-independent growth. Furthermore, it was revealed for the
first time, to the best of our knowledge, that the combination of
AMD3100 with standard chemotherapeutic gemcitabine may be a
promising optional therapeutic approach for BTC.
Acknowledgments
This study was supported by funds from the
Oesterreichische Nationalbank (Anniversary fund no. 14842) and the
research fund of the Paracelsus Medical University Salzburg (no.
A12-02-006-KIE).
References
|
1
|
de Groen PC, Gores GJ, LaRusso NF,
Gunderson LL and Nagorney DM: Biliary tract cancers. N Engl J Med.
341:1368–1378. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braconi C and Patel T: Cholangiocarcinoma:
new insights into disease pathogenesis and biology. Infect Dis Clin
North Am. 24:871–884. vii2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malhi H and Gores GJ: Cholangiocarcinoma:
modern advances in understanding a deadly old disease. J Hepatol.
45:856–867. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valle J, Wasan H, Palmer DH, et al:
Cisplatin plus gemcitabine versus gemcitabine for biliary tract
cancer. N Engl J Med. 362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valle JW, Furuse J, Jitlal M, et al:
Cisplatin and gemcitabine for advanced biliary tract cancer: a
meta-analysis of two randomised trials. Ann Oncol. 25:391–398.
2014. View Article : Google Scholar
|
|
6
|
Furusato B, Mohamed A, Uhlén M and Rhim
JS: CXCR4 and cancer. Pathol Int. 60:497–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kucia M, Reca R, Miekus K, et al:
Trafficking of normal stem cells and metastasis of cancer stem
cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4
axis. Stem Cells. 23:879–894. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zlotnik A: Chemokines and cancer. Int J
Cancer. 119:2026–2029. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Allinen M, Beroukhim R, Cai L, et al:
Molecular characterization of the tumor microenvironment in breast
cancer. Cancer Cell. 6:17–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fernandis AZ, Prasad A, Band H, Klösel R
and Ganju RK: Regulation of CXCR4-mediated chemotaxis and
chemoinvasion of breast cancer cells. Oncogene. 23:157–167. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lapteva N, Yang AG, Sanders DE, Strube RW
and Chen SY: CXCR4 knockdown by small interfering RNA abrogates
breast tumor growth in vivo. Cancer Gene Ther. 12:84–89. 2005.
View Article : Google Scholar
|
|
12
|
Liang Z, Yoon Y, Votaw J, Goodman MM,
Williams L and Shim H: Silencing of CXCR4 blocks breast cancer
metastasis. Cancer Res. 65:967–971. 2005.PubMed/NCBI
|
|
13
|
Muller A, Homey B, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burger M, Glodek A, Hartmann T, et al:
Functional expression of CXCR4 (CD184) on small-cell lung cancer
cells mediates migration, integrin activation, and adhesion to
stromal cells. Oncogene. 22:8093–8101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Samara GJ, Lawrence DM, Chiarelli CJ, et
al: CXCR4-mediated adhesion and MMP-9 secretion in head and neck
squamous cell carcinoma. Cancer Lett. 214:231–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geminder H, Sagi-Assif O, Goldberg L, et
al: A possible role for CXCR4 and its ligand, the CXC chemokine
stromal cell-derived factor-1, in the development of bone marrow
metastases in neuroblastoma. J Immunol. 167:4747–4757. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schimanski CC, Bahre R, Gockel I, et al:
Dissemination of hepatocellular carcinoma is mediated via chemokine
receptor CXCR4. Br J Cancer. 95:210–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ottaiano A, di Palma A, Napolitano M, et
al: Inhibitory effects of anti-CXCR4 antibodies on human colon
cancer cells. Cancer Immunol Immunother. 54:781–791. 2005.
View Article : Google Scholar
|
|
19
|
Leelawat K, Keeratichamroen S, Leelawat S
and Tohtong R: CD24 induces the invasion of cholangiocarcinoma
cells by upregulating CXCR4 and increasing the phosphorylation of
ERK1/2. Oncol Lett. 6:1439–1446. 2013.PubMed/NCBI
|
|
20
|
Leelawat K, Leelawat S, Narong S and
Hongeng S: Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4
induced cholangiocarcinoma cell invasion. World J Gastroenterol.
13:1561–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HJ, Lee K, Lee DG, et al: Chemokine
(C-X-C motif) ligand 12 is associated with gallbladder carcinoma
progression and is a novel independent poor prognostic factor. Clin
Cancer Res. 18:3270–3280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gentilini A, Rombouts K, Galastri S, et
al: Role of the stromal-derived factor-1 (SDF-1)-CXCR4 axis in the
interaction between hepatic stellate cells and cholangiocarcinoma.
J Hepatol. 57:813–820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okamoto K, Tajima H, Nakanuma S, et al:
Angiotensin II enhances epithelial-to-mesenchymal transition
through the interaction between activated hepatic stellate cells
and the stromal cell-derived factor-1/CXCR4 axis in intrahepatic
cholangiocarcinoma. Int J Oncol. 41:573–582. 2012.PubMed/NCBI
|
|
24
|
Girbl T, Lunzer V, Greil R, Namberger K
and Hartmann TN: The CXCR4 and adhesion molecule expression of
CD34+ hematopoietic cells mobilized by ‘on-demand’ addition of
plerixafor to granulocyte-colony-stimulating factor. Transfusion.
54:2325–2335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fruehauf S: Current Clinical Indications
for Plerixafor. Transfusion medicine and hemotherapy: offizielles
Organ der Deutschen Gesellschaft fur Transfusionsmedizin und
Immunhamatologie. 40:246–250. 2013. View Article : Google Scholar :
|
|
26
|
Kiesslich T, Alinger B, Wolkersdörfer GW,
Ocker M, Neureiter D and Berr F: Active Wnt signalling is
associated with low differentiation and high proliferation in human
biliary tract cancer in vitro and in vivo and is sensitive to
pharmacological inhibition. Int J Oncol. 36:49–58. 2010.
|
|
27
|
Wachter J, Neureiter D, Alinger B, et al:
Influence of five potential anticancer drugs on wnt pathway and
cell survival in human biliary tract cancer cells. Int J Biol Sci.
8:15–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kiesslich T, Neureiter D, Alinger B, et
al: Uptake and photo-toxicity of meso-tetrahydroxyphenyl chlorine
are highly variable in human biliary tract cancer cell lines and
correlate with markers of differentiation and proliferation.
Photochem Photobiol Sci. 9:734–743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Charafe-Jauffret E, Ginestier C and
Birnbaum D: Breast cancer stem cells: tools and models to rely on.
BMC Cancer. 9:2022009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang M, Xiao J, Shen M, et al: Isolation
and characterization of tumorigenic extrahepatic cholangiocarcinoma
cells with stem cell-like properties. Int J Cancer. 128:72–81.
2011. View Article : Google Scholar
|
|
33
|
Singh S, Srivastava SK, Bhardwaj A, Owen
LB and Singh AP: CXCL12-CXCR4 signalling axis confers gemcitabine
resistance to pancreatic cancer cells: a novel target for therapy.
Br J Cancer. 103:1671–1679. 2010. View Article : Google Scholar : PubMed/NCBI
|