Introduction
Post-traumatic stress disorder (PTSD) is an anxiety
disorder that develops after exposure to a life-threatening
traumatic experience. It is characterized by symptoms that often
endure for years, including continuous re-experience of the
traumatic event, avoidance of stimuli associated with the trauma,
numbing of general responsiveness and increased arousal (1).
The endoplasmic reticulum (ER), a multifunctional
signaling organelle, has a vital role in a variety of cellular
functions, including post-translation modifications, synthesis and
folding of membrane and secretory proteins, calcium sequestration
for intracellular calcium homeostasis and apoptosis (2–4).
Physiological and pathological stimuli that disrupt ER homeostasis
can induce ER dysfunction or ER stress, including the accumulation
of unfolded or misfolded proteins, oxidative stress, perturbation
of calcium homeostasis and viral infection (5).
The 78-kDa glucose-regulated protein (GRP78),
identical to the immunoglobulin heavy chain-binding protein (BiP),
belongs to the heat shock protein 70 group and is located in the ER
lumen. GRP78 was one of the best characterized ER chaperone
proteins and its synthesis can be stimulated by a variety of
environmental and physiological stress conditions that perturb ER
function and homeostasis (6).
GRP78 is currently regarded as the master regulator of the
unfolding protein response (UPR) pathway (7), which may participate in mediate
neuronal cell death after traumatic brain injury (8).
Caspase-12 is localized to the ER and has been shown
to be activated by ER stress, including disruption of ER calcium
homeostasis and accumulation of excess proteins in the ER (9,10).
Caspase-12 is also the key molecule in ER-associated apoptosis and
activated caspase-12 can activate downstream apoptosis
executioners, such as caspase-3, leading to apoptosis (11).
Amygdala, one of the key regions in the limbic
system of the brain, has been recognized as a crucial brain
structure involved in fear, rage and emotional memory (12,13).
Amygdala is usually divided into three distinct nuclear subgroups:
Central nucleus, corticomedial nucleus and basolateral nucleus
(14). Among these different
nuclear sub-groups, the basolateral nucleus is the largest nucleus
of the amygdaloid complex (15),
which is a putative site of emotional memory and regulation of
anxiety (16,17). Thus, the present study focused on
changes of the basolateral nucleus.
Single prolonged stress (SPS) (18) was shown to induce enhanced
inhibition of the hypothalamo-pituitary-adrenal (HPA) axis, which
is a putative neuroendocrinological hallmark of PTSD (19–21).
Subsequently, SPS paradigms were extensively developed and employed
in the investigation of PTSD (22,23).
The present study investigated the effects of SPS on the function
of the ER by detecting GRP78 and caspase 12 in the amygdala of
rats, and aimed to examine whether there was a link between an
established rat model of PTSD and the ER of amygdala neurons. The
findings revealed part of the pathogenesis and provided novel
insight into the mechanism of how amygdala may participate in
PTSD.
Materials and methods
Experimental animals
Eighty male Wistar rats, aged 7 or 8 weeks at the
start of the study, weighing approximately 150–160 g, were supplied
by the Animal Experimental Center, China Medical University. Rats
were individually housed in an air-conditioned room (22±1°C and
55±5% humidity) on a 12-h light/dark schedule with free access to
food and water.
The rats were raised in the laboratory for at least
7 days prior to conducting the experiment. Experiments were
performed in accordance with the National Institute of Health Guide
for the care and use of laboratory animals, and approved by the
Welfare & Ethics Committee of Experimental Animals (China
Medical University, Shenyang, China). All efforts were made to
reduce the number of animals used and to minimize animal suffering
during the experiment.
Model establishment and grouping
Animals were divided randomly into four groups: 1)
Control group; 2) SPS 1 d (1 day) group; 3) SPS 7 d (7 day) group;
and 4) SPS 14 d (14-day) group. Control animals remained in their
home cages with no handling for 7 or 14 days and were sacrificed at
the same time as the SPS groups. SPS 1-, 7-and 14-day groups refer
to the time after exposure to SPS. SPS rats underwent the SPS
procedure on the first day. The SPS protocol was based on a
combined plural stress paradigm (24,25):
Immobilization (compression with plastic bags) for 2 h, forced
swimming in a clear acrylic cylinder for 20 min (24±1°C), rest for
15 min, followed by ether anesthesia (until consciousness was
lost).
Perfusion-based sections
Rats of the normal control and SPS groups were
prepared via left ventricle perfusion and fixation (26) with 200 ml of pre-cooled heparinized
0.9% saline, followed by 300 ml of 4% paraformaldehyde in 0.01 M
phosphate-buffered saline (PBS) (pH 7.2–7.4). Brains were rapidly
removed and post-fixed in the same fixative for 4–6 h at 4°C, and
were immersed in a 20% sucrose solution in 0.01 M phosphate buffer
(PB; pH 7.4) at 4°C thereafter. Samples were snap-frozen in liquid
nitrogen and 12-µm coronal sections were prepared for
morphological studies.
Immunofluorescence analysis of GRP78
The sections of the normal control and SPS groups
were treated with 5% bovine serum albumin (BSA; Roche Co.,
Shanghai, China) and 0.3% Triton X-100 (Sigma-Aldrich, St. Louis,
MO, USA) in PBS for 30 min to block non-specific staining at room
temperature (RT). Endogenous peroxidase was inactivated with 3%
H2O2 in double distilled H2O for 5
min at room temperature. The sections were then incubated with
GRP78 mouse monoclonal antibody (A-10) (1:200 dilution; cat. no.
sc-376768; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) in 2%
BSA-PBS overnight at 4°C. After being washed with PBS three times,
the sections were incubated with fluorescein isothiocyanate goat
anti-mouse monoclonal immunoglobulin G (IgG) (1:50 dilution; cat.
no. BA1101; Boster Biological Technology, Wuhan, China) for 2 h at
room temperature. To assess non-specific staining, several sections
in every experiment were incubated in buffer without primary
antibody. Slices were then mounted with glycerin and observed by
fluorescence microscopy (BX61+DP71; Olympus Corp., Tokyo,
Japan).
Fifteen slides were randomly selected from each
group. In each slide, five visual fields in the basolateral
amygdala were randomly selected (magnification, x400). The optical
density (OD) of GRP78-immunopositive cells in each field was
recorded to evaluate the average OD.
Western blot analysis of GRP 78 and
caspase 12
Rats of the normal control and SPS groups were
rapidly decapitated following inhalation anesthesia with ether, and
the brains were removed and immediately placed in a dish standing
on crushed ice. The basolateral amygdala was then dissected out
according to the atlas of rats (27) using a stereomicroscope (SZX12;
Olympus Corp.) and washed twice with 0.01 M PBS (pH 7.2–7.4) at
4°C. Then the samples were homogenized with a sample buffer
containing 200 mM tris-buffered saline (TBS), pH 7.5, 4% SDS, 20%
glycerol, 10% 2-mercaptoethanol and were denatured by boiling for 3
min. The protein fraction (50 µg/lane) prepared from each
sample was separated by 12% (w/v) gradient SDS-PAGE and
electroblotted to a polyvinylidene difluoride (PVDF) membrane
(Millipore, Bedford, MA, USA) from the gel using a semi-dry
blotting apparatus (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
The membrane was blocked with 5% skimmed milk powder
and 0.05% Tween-20 (KeyGen Biotech. Co., Ltd., Nanjing, China) in
TBS at room temperature for 2 h and incubated with GRP78 mouse
monoclonal antibody (A-10) (1:200 dilution; cat. no. sc-376768;
Santa Cruz Biotechnology, Inc.,) or caspase 12 rabbit polyclonal
antibody (1611) (1:500 dilution; cat. no. sc-21747; Santa Cruz
Biotechnology, Inc.) overnight at 4°C.
Blots were washed three times with TBST and then
incubated with horseradish peroxidase-conjugated goat anti-mouse
(1:400; cat. no. sc-2073) or goat anti-rabbit (1:400; cat. no.
sc-2040) IgG secondary antibodies for 2 h at room temperature and
washed with TBST. After the incubation, the PVDF membrane was
washed three times with TBST prior to visualization by enhanced
chemiluminescence (ECL; KeyGen Biotech. Co., Ltd.). To confirm
equal protein loading, the same blots were re-incubated with
antibodies specific for β-actin (1:100 dilution; mouse monoclonal;
cat. no. BM0627; Boster Biological Technology), which were detected
using ECL. The OD was analyzed using the Gel Image Analysis System
(Tanon 2500R; Tanon, Shanghai China). The relative expression
levels of GRP78 and caspase 12 were determined by calculating the
OD ratio of GRP78/β-actin and caspase 12/β-actin.
Semiquantitative reverse
transcription-polymerase chain reaction (RT-PCR) for detection of
GRP78 and caspase 12
Total mRNA of each group was extracted from the
basolateral amygdala according to the instructions of the TRIzol
kit (Invitrogen Life Technologies, Carlsbad, CA, USA) and 1
µg of total RNA was reverse transcribed into cDNA. cDNA was
amplified using an RNA PCR kit (AM Ver. 3.0; Takara Bio, Inc.,
Otsu, Japan). The primers were designed and synthesized by
Shenggong Biotech Company (Shanghai, China) according to the serial
number from GenBank and are shown in Table I. The reaction was started at 94°C
for 2 min, amplification comprised 30 cycles of 30 sec at 94°C, 30
sec at 56°C, 40 sec at 72°C (for GRP78) or 27 cycles of 30 sec at
94°C, 30 sec at 50°C, 45 sec at 72°C (for caspase-12) and ended
with 5 min extension at 72°C, using XP Thermal Cycler (Hangzhou
Bioer Technology Co., Ltd., Hangzhou, China). β-actin mRNA used as
an internal control and was co-amplified with GRP78- or caspase 12-
mRNA. The products were separated by electrophoresis on a 1.2%
agarose gel, and the density of each band was analyzed using the
Gel Image Analysis System (Tanon 2500R; Tanon). The levels of
GRP78- and caspase 12-mRNA were determined by calculating the
density ratio of GRP78 mRNA/β-actin mRNA or caspase 12 mRNA/β-actin
mRNA.
 | Table IPrimer sequences of GRP78, caspase 12
and β-actin. |
Table I
Primer sequences of GRP78, caspase 12
and β-actin.
| Name | Upstream primer | Downstream
primer | Product size
(bp) |
|---|
| GRP78 |
5′-TAATCAGCCCACCGTAACAATC-3′ |
5′-ACCTCCCAGCTTCTCTTTATCT-3′ | 385 |
| Caspase 12 |
5′-TGCCAATTCCGACAAACAGC-3′ |
5′-TGCCGTCCCACATAAAGACC-3′ | 512 |
| β-actin |
5′-ATCACCCACACTGTGCCCATC-3′ |
5′-ACAGAGTACTTGCGCTCAGGA-3′ | 542 |
Assessment of morphological changes of
the ER using transmission electron microscopy (TEM)
Rats of each group were perfused with pre-cold
heparinized 0.9% saline, followed by 0.01 M PBS (pH 7.2–7.4)
containing 4% paraformaldehyde and 2.5% glutaraldehyde. The brain
was removed and dissected on ice, followed by 4–6 h of
post-fixation in the same fixative at 4°C. The basolateral amygdala
was dissected by using a stereomicroscope and cut into blocks of ~1
mm3. The blocks were post-fixed in 1% osmium tetroxide
for 2 h at 4°C. They were rinsed in 0.01 M PBS (pH 7.4) several
times, dehydrated in a graded series (20–100%) of ethanol and then
in acetone, infused with Epon 812, and finally polymerized in pure
Epon 812 (Serva, New York, NY, USA) at 65°C for 72 h. The
basolateral amygdala was localized on semi-thin sections.
Ultra-thin sections were cut on an ultramicrotome, collected on
copper grids, and stained with 4% uranyl acetate and lead citrate.
A minimum of 5 sections comprising ~250 cells from each basolateral
amygdala were studied with transmission electron microscopy
(TEM-1200EX; 80KV; Jeol Ltd., Tokyo, Japan).
Statistical analysis
All values were expressed as the mean ± standard
error. Data among groups were analyzed by one-way analysis of
variance using SPSS 13.0 software (SPSS, Inc. Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
SPS increases GRP78 and caspase 12 levels
in the basolateral amygdale
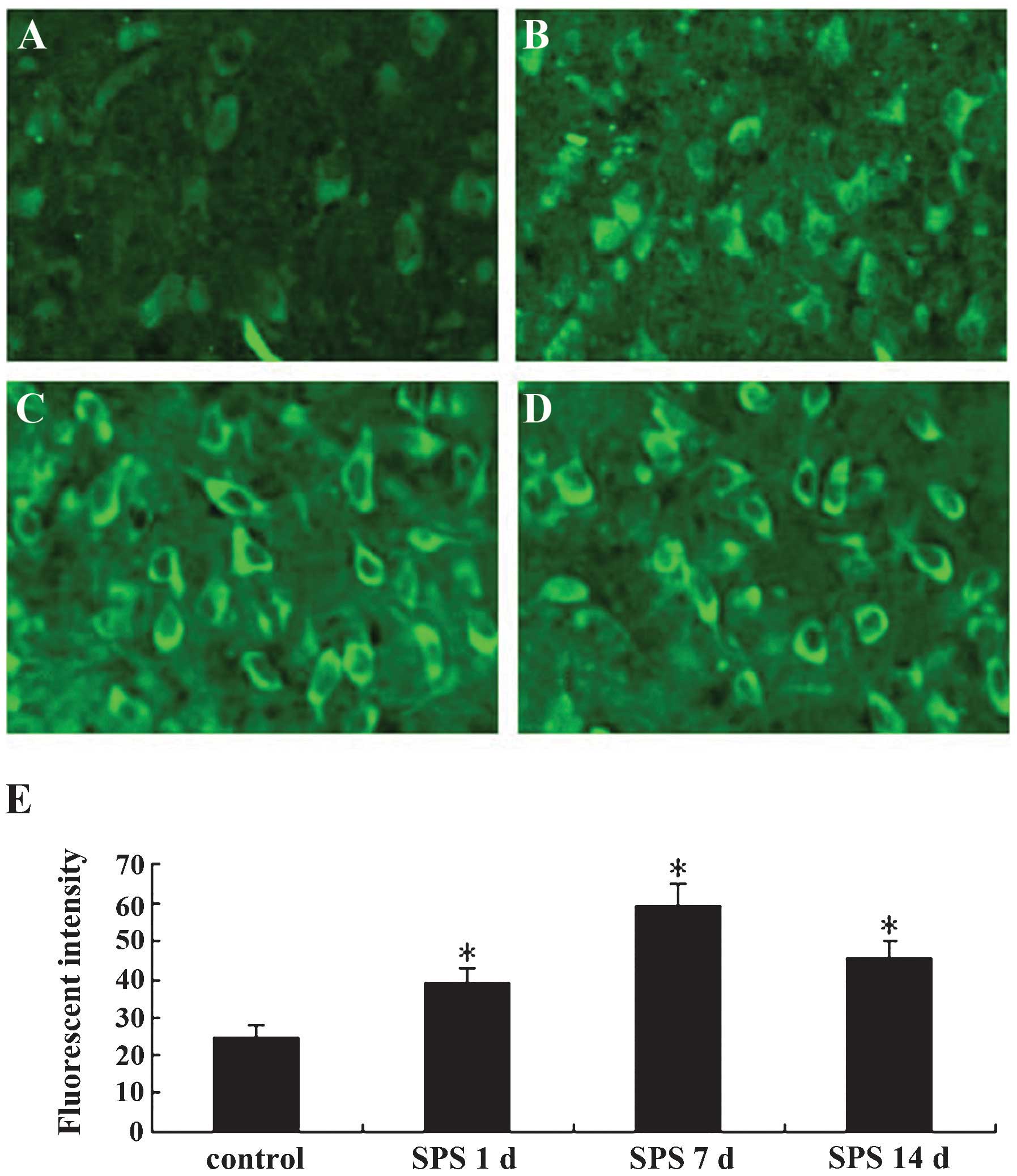
The immunofluorescence staining results are shown in
Fig. 1. The GRP78 protein was
located in the cytoplasm (Fig.
1A–D). In the normal control group, the fluorescent intensity
of GRP78-positive cells was low, while that in SPS rats was
significantly higher and was highest at 7 days after exposure to
SPS (Fig. 1E) (P<0.01).
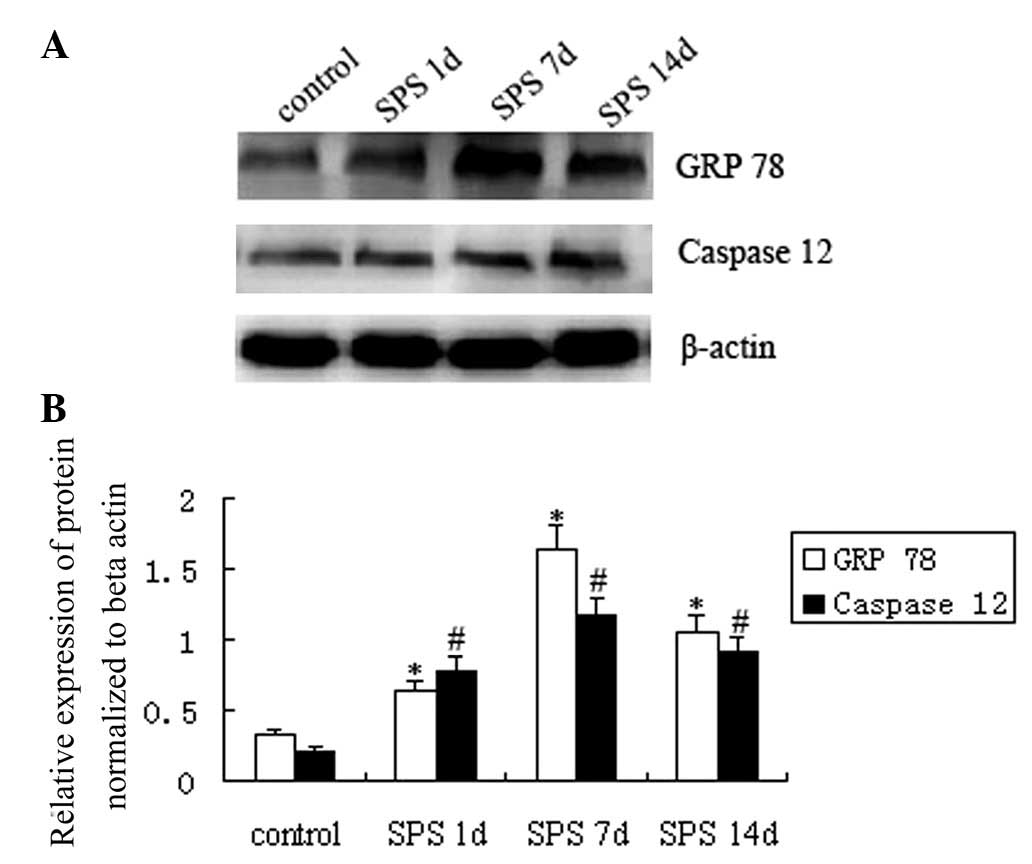
In the western blots, immunoreactive signals for
GRP78, caspase 12 and β-actin appeared at 78 kDa, 50 kDa and 42
kDa, respectively (data not shown), and the mean value of band
densities of the control group was set as 100%. Data were expressed
as normalized optical density. GRP78 and caspase 12 protein levels
in the basolateral amygdala region of the different groups are
presented in Fig. 2A. The results
showed that SPS exposure resulted in a significant change of GRP78
and caspase 12. GRP78 expression was upregulated 1 day after SPS
stimulation as compared with that in the control group, was highest
in the SPS 7 d group and was decreased thereafter in the SPS 14 d
group, while still being higher than that in the SPS 1 d group
(P<0.05). Similarly, caspase 12 protein expression in the SPS
model groups also showed a significant upregulation in comparison
with control rats, was highest in the SPS 7 d and then declined in
the SPS 14 d group (Fig. 2B)
(P<0.05).
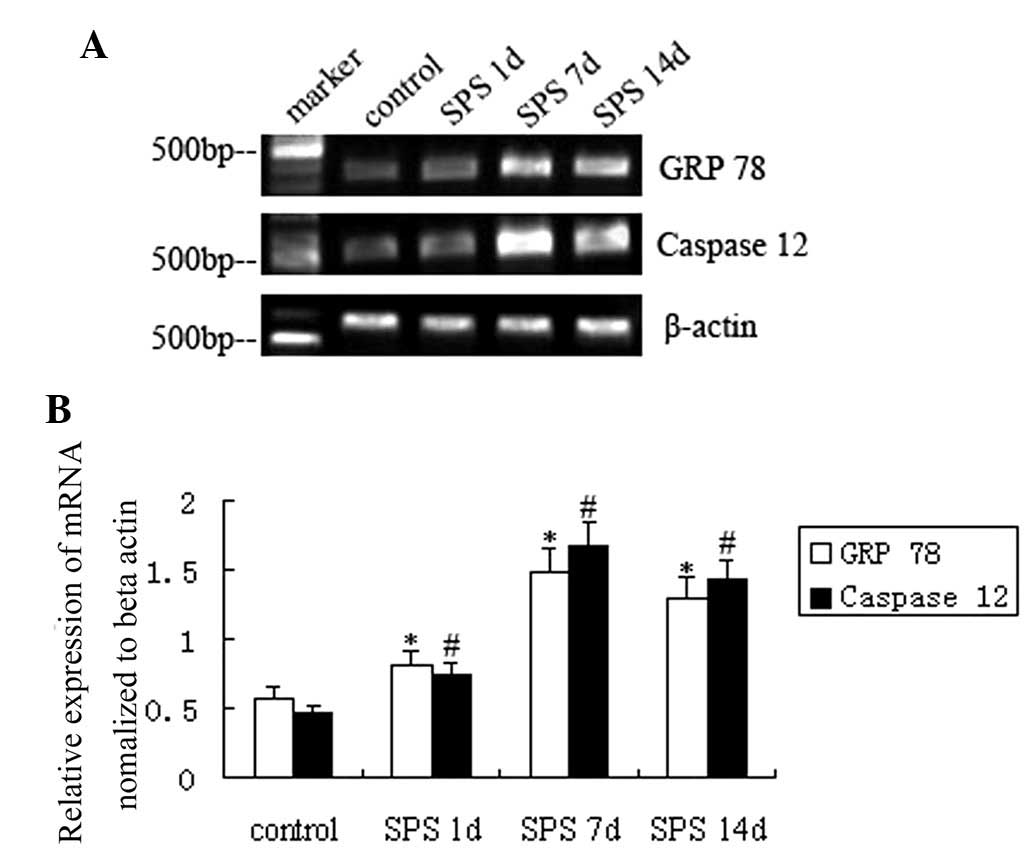
For semiquantitative PCR, levels of GRP 78 and
caspase-12 mRNA were normalized to β-actin mRNA levels. In analogy
with the protein levels, mRNA levels of GRP 78 and caspase-12
gradually increased after SPS stimulation compared with those in
the control group and were highest at SPS 7 d (P<0.01) (Fig. 3).
SPS causes morphological changes of the
ER of amygdala neurons
As shown in Fig.
4A, the intracellular ER of amygdala neurons exhibited a normal
structure in the control rats (Fig.
4A). Mild distension of the ER was observed in the SPS 1 d
group (Fig. 4B). A tumescent ER,
ER vacuolization and degranulation of ER were observed in the SPS 7
d group (Fig. 4C) (P<0.05).
Furthermore, as shown in Fig. 4D,
the ER of amygdala neurons also exhibited an abnormal structure in
the SPS 14 d group.
Discussion
PTSD is thought to involve a disfunction in response
to fear-associated stimuli. Four major types of characteristic
symptoms of PTSD are re-experiencing, avoidance, numbing and
hyperarousal (1). The specific
role of amygdala in the processing of threat-associated stimuli, in
particular anger and fear, has been well documented based on
investigations on animals and humans (28–30).
Numerous lines of evidence have implicated the basolateral amygdala
(BLA) as a substrate for stress-associated modulation of memory
(31). Therefore, the present
study focused on observing SPS-induced changes in the basolateral
nucleus.
A previous study has demonstrated that ER stress is
closely associated with several diseases, including neuronal cell
injury (32), Alzheimer’s disease
(33) and Parkinson’s disease
(34). In the present study,
changes in the levels of ER stress protein GRP78 and ER-resident
caspase-12 in the amygdala of rats were detected in order to
identify whether ER stress is involved in PTSD. GRP78, the master
regulator of the UPR pathway and a molecular chaperone in the ER
that provides cytoprotection in response to cellular stresses, was
significantly upregulated in the rats after exposure to SPS
stimuli, which may have resulted in dysfunction of the ER. Caspase
12 was also significantly upregulated after SPS stimulation. The
results of the morphological evaluation showed that tumescent ER,
ER vacuolization and degranulation of the ER were present in the
SPS groups. In conclusion, the results indicated that GRP78 and
caspase 12 were significantly upregulated and morphological changes
in the amygdala of rats were present after exposure to SPS. The
possible reason for this is that SPS stimuli induced the activation
of the UPR pathway, and the accumulation of unfolded or misfolded
proteins led to ER dysfunction of amygdala neurons, which may play
an important role in the pathobiological basis for the abnormality
of affect and behavior induced by PTSD.
One limitation of the present study is that it did
not examine whether apoptosis was induced via the ER pathway in the
amygdala, although a previous study by our group has demonstrated
that SPS induced more apoptotic cells and increased the apoptotic
rate in the amygdala of rats after exposure to SPS compared with
that in the normal control group (35). Further study will examine whether
apoptosis activated by ER stress participates in mechanisms of
PTSD. The results of the present study revealed increases in the
levels of ER stress protein GRP78 and ER-resident caspase-12;
however, their pathophysiological roles in PTSD remain elusive.
At present, the pathogenesis of PTSD is not yet
entirely clear. PTSD may cause a series of biochemical
abnormalities and dysfunction of the amygdala, which leads to
dysfunction of the brain (36).
The present study has shed light on the cellular mechanisms of ER
stress in the amygdala and their participation in the pathogenesis
of PTSD, which may lead to the development of novel treatments for
PTSD. Further investigation into the molecular mechanisms of how
the ER regulates neuronal function and the exact cellular pathway
should also be elucidated. Thus, the pathogenesis of PTSD requires
further investigation.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 31200772) and the
Doctoral Program Research Foundation of Higher Education of China
(no. 20132104110021). The authors would like to thank the anonymous
reviewers for their valuable comments on how to improve the quality
of the paper.
References
|
1
|
American Psychiatric Association:
Diagnostic and Statistical Manual of Mental Disorders. 4th ed.
(DSM-IV). American Psychiatric Press; Washington DC: 1994
|
|
2
|
Corbett EF and Michalak M: Calcium, a
signaling molecule in the endoplasmic reticulum. Trends Biochem
Sci. 25:307–311. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakamura K, Bossy-Wetzel E, Burns K, et
al: Changes in endoplasmic reticulum luminal environment affect
cell sensitivity to apoptosis. J Cell Biol. 150:731–740. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Su HL, Liao CL and Lin YL: Japanese
encephalitis virus infection initiates endoplasmic reticulum stress
and an unfolded protein response. J Virol. 76:4162–4171. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rao RV and Bredesen DE: Misfolded
proteins, endoplasmic reticulum stress and neurodegeneration. Curr
Opin Cell Biol. 16:653–662. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee AS: The ER chaperone and signaling
regulator GRP78/Bip as a monitor of endoplasmic reticulum stress.
Methods. 35:373–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Radley JJ, Arias CM and Sawchenko PE:
Regional differentiation of the medial prefrontal cortex in
regulating adaptive responses to acute emotional stress. J
Neurosci. 26:12967–12976. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martinez JA, Zhang Z, Svetlov SZ, et al:
Calpain and caspase processing of caspase-12 contribute to the ER
stress-induced cell death pathway in differentiated PC12 cells.
Apoptosis. 15:1480–1493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Momoi T: Caspases involved in ER
stress-mediated cell death. J Chem Neuroanat. 28:101–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakagawa T, Zhu H, Morishima N, et al:
Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and
cytotoxicity by amyloid-β. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu LQ, Fan ZQ, Tang YF and Ke ZJ: The
Resveratrol attenuates ethanol-induced hepatocyte apoptosis via
inhibiting ER-related caspase-12 activation and PDE activity in
vitro. Alcohol Clin Exp Res. 38:683–693. 2014. View Article : Google Scholar
|
|
12
|
McGaugh JL and Cahill L: Interaction of
neuromodulatory systems in modulating memory storage. Behav Brain
Res. 83:31–38. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
LeDoux JE: Emotion: clues from the brain.
Annua Rev Psychol. 46:209–235. 1995. View Article : Google Scholar
|
|
14
|
Harding AJ, Stimson E, Henderson JM, et
al: Clinical correlates of selective pathology in the amygdala of
patients with Parkinson’s disease. Brain. 125:2431–2445. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sims KS and Williams RS: The human
amygdaloid complex: a cytologic and histochemical atlas using
Nissl, myelin, acetyl-cholinesterase and nicotinamide adenine
dinucleotide phosphate diaphorase staining. Neuroscience.
36:449–472. 1990. View Article : Google Scholar
|
|
16
|
Davis M: The role of the amygdala in
emotional learning. Int Rev Neurobiol. 36:225–266. 1994.PubMed/NCBI
|
|
17
|
McGaugh JL, Mclntyre CK and Power AE:
Amygdala modulation of memory consolidation: interaction with other
brain systems. Neurobiol Learn Mem. 78:539–552. 2002. View Article : Google Scholar
|
|
18
|
Liberzon I, Krstov M and Young EA:
Stress-restress: effects on ACTH and fast feedback.
Psychoneuroendocrinology. 22:443–453. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stein MB, Yehuda R, Koverola C, et al:
Enhanced dexamethasone suppression of plasma cortisol in adult
women traumatized by childhood sexual abuse. Biol Psychiatry.
42:680–686. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yehuda R: Biology of posttraumatic stress
disorder. J Clin Psychiatry. 62:41–46. 2001.PubMed/NCBI
|
|
21
|
Yehuda R: Neuroendocrine aspects of PTSD.
Handb Exp Pharmacol. 169:371–403. 2005.
|
|
22
|
Khan S and Liberzon I: Topiramate
attenuated exaggerated acoustic startle in an animal model of PTSD.
Psychopharmacology (Berl). 172:225–229. 2004. View Article : Google Scholar
|
|
23
|
Iwamoto Y, Morinobu S, Takahashi T, et al:
Single prolonged stress increases contextual freezing and the
expression of glycine transporter 1 and vesicle-associated membrane
protein 2 mRNA in the hippocampus of rats. Prog
Neuropsychopharmacol Biol Psychiatry. 31:642–651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takahashi T, Morinobu S, Iwamoto Y, et al:
Effect of paroxetine on enhanced contextual fear induced by single
prolonged stress in rats. Psychopharmacology (Berl). 189:165–173.
2006. View Article : Google Scholar
|
|
25
|
Kohda K, Harada K, Kato K, et al:
Glucocorticoid receptor activation is involved in producing
abnormal phenotypes of single-prolonged stress rats: a putative
post-traumatic stress disorder model. Neuroscience. 148:22–33.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu HY: Technical operations and its
common problems of perfusion fixation in mice. Qiqihaer Yixueyuan
Xuebao. 27:13412006.
|
|
27
|
Paxinos G and Watson C: The Rat Brain in
Stereotaxic Coordinates. 4th edition. Academic Press; 1998
|
|
28
|
Derntl B, Windischberger C, Robinson S, et
al: Amygdala activity to fear and anger in healthy young males is
associated with testosterone. Psychoneuroendocrinology. 34:687–693.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McGauqh JL: The amygdala modulates the
consolidation of memories of emotionally arousing experience. Annu
Rev Neurosci. 27:1–28. 2004. View Article : Google Scholar
|
|
30
|
Cahill L and McGaugh JL: Mechanisms of
emotional arousal and lasting declarative memory. Trends Neurosci.
21:294–299. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chavez CM, McGaugh JL and Weinberger NM:
The basolateral amygdala modulates specific sensory memory
representations in the cerebral cortex. Neurobiol Learnand Mem.
91:382–392. 2009. View Article : Google Scholar
|
|
32
|
Paschen W and Frandsen A: Endoplasmic
reticulum dysfunction-a common denominator for cell injury in acute
and degenerative diseases of the brain. J Neurochem. 79:719–725.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Katayama T, Imaizumi K, Sato N, et al:
Presenilin-lmutations downregulate the signaling pathway of the
unfolded-protein response. Nat Cell Biol. 1:479–485. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Imai Y, Soda M, Inoue H, et al: An
unfolded putative trans-membrane polypeptide, which can lead to
endoplasmic reticulum stress, is a substrate of Parkin. Cell.
105:891–902. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ding JL, Han F and Shi YX:
Single-prolonged stress induces apoptosis in the amygdala in a rat
model of post-traumatic stress disorder. J Psychiatr Res. 44:48–55.
2010. View Article : Google Scholar
|
|
36
|
Xiao B, Han F and Shi YX: Dysfunction of
Ca2+/CaM kinase IIalpha cascades in the amygdala in
post-traumatic stress disorder. Int J Mol Med. 24:795–799.
2009.PubMed/NCBI
|