Introduction
Laryngeal squamous cell carcinoma (LSCC), one of the
most common malignancies of the head and neck region, accounts for
~2.4% of diagnosed malignancies worldwide every year (1,2).
Despite novel treatment modalities (including surgical and adjuvant
chemo- or radiotherapy) and their success in terms of overall
quality of life, survival rates for this disease have not improved
in the past 30 years (1). Regional
lymph node and distant metastasis and loco-regional recurrence are
the two major reasons of LSCC treatment failure (1,2).
At present, an evaluation of LSCC prognosis is
primarily based on the clinical tumor, nodes and metastasis (TNM)
staging; however, LSCC patients with the same clinical stage often
display considerable variability in survival, suggesting that TNM
staging is insufficient for precisely predicting the prognosis of
this disease (3). Therefore, the
discovery of specific biomarkers is important for appropriate
therapy and patient surveillance.
DJ-1, also known as Parkinson disease 7 (PARK7)
encoding a conserved protein belonging to the ThiJ/PfpI/DJ-1
superfamily (4), was firstly
discovered as an oncogene that was able to transform NIH-3T3 cells
in co-operation with Ras (5). DJ-1
is localized in the cytoplasm and nucleus, and can be translocated
from the former to the latter in the S phase of the cell cycle.
Studies found that the expression of DJ-1 is elevated in prostate
cancer and primary lung cancer compared with that in adjacent
non-cancerous tissues (6,7); furthermore, its expression is
positively correlated with the probability of recurrence in
patients with non-small cell lung carcinoma (8). In a previous study of LSCC (9), DJ-1 was identified as an independent
molecular marker for poor prognosis, and was correlated with the pT
status and tumor grading; this was further confirmed by another
study on LSCC (10). Collectively,
the above evidence indicated that the DJ-1 gene, which is
associated with progression of human malignant tumors and
prognosis, may be a potential anti-cancer target. However, the
detailed mechanism of the role of DJ-1 in tumorigenesis of
laryngeal cancer cells remains to be elucidated.
A recent study by our group reported that
overexpression of DJ-1 in LSCC is negatively correlated with the
expression of phosphatase and tensin homolog (PTEN), a
dual-specific phosphatase that controls a variety of processes
associated with cell survival, proliferation and invasion (11). Other studies showed that PTEN was
downregulated by DJ-1 in several cancer types, including breast and
ovarian cancer (8,12–14).
These evidences suggested that DJ-1-induced PTEN down-regulation
may be involved in LSCC progression and act as an activator of the
invasion process in LSCC.
In the present study, it was hypothesized that
DJ-1-induced PTEN downregulation may be involved in the
proliferation and invasion of LSCC. With increasing knowledge of
the molecular mechanisms of endogenous RNA interference in cancer
studies, small interfering RNAs (siRNAs) directed against the DJ-1
gene in the laryngeal cancer cell lines Hep-2 and SNU-899 was used
in the present study. The aim of the present study was to
investigate the underlying mechanisms of the involvement of DJ-1 in
the tumorigenesis of LSCC.
Materials and methods
Cell lines and cell culture
The human laryngeal cancer cell line Hep-2 was a
kind gift from Dr. Kai-Tao Feng (passage 5; Cell Bank of
Experimental Institute of Sun Yat-sen University Cancer Center,
Guangzhou, China). The human laryngeal cancer cell line SNU-899
(Passage 7; Korean Cell Line Bank of Cancer Research Institute,
Seoul, Korea) was a kind gift from Prof. Stanley Thian Sze Wong
(Department of Surgery, Hong Kong University, Hong Kong, China).
Hep-2 and SNU-899 cells were maintained in RPMI 1640 media
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 2 mM
L-glutamine and 10% fetal bovine serum (FBS; HyClone Laboratories,
Logan, UT, USA). The cultures were grown for a maximum of 10
passages prior to retrieving fresh cells from frozen stock.
RNA extraction and semi-quantitative
reverse-transcription polymerase chain reaction (RT-qPCR)
RNA extraction and RT-qPCR were performed as
previously described (9). Briefly,
total RNA was extracted using TRIzol reagent (Fermentas, Thermo
Fisher Scientific, Waltham, MA, USA) according to the
manufacturer’s instructions. The total RNA (2 µg) was
reverse transcribed using a RevertAid First Strand cDNA synthesis
kit (Fermentas) in a 20-µl reaction mixture containing 1X
reverse transcriptase (RT) reaction buffer (Fermentas), 0.5
µg oligo (dT) 18 primer and 1 µl RevertAid Moloney
murine leukemia virus (Fermentas) reverse transcriptase for 60 min
at 42°C, and then heated for 10 min at 70°C. After heat
inactivation of the RT at 94°C for 5 min, 1 µl of the RT
reaction mixture and 19 µl of the PCR mixture (11 µl
0.1% DEPC, 4 µl 5X buffer, 2 µl 10 mM dNTP, 1
µl RiboLock™ ribonuclease inhibitor (Fermentas), 1 µl
200 u/µl RevertAid™ M-MuLV) were mixed and then amplified by
PCR (PTC-200 PCR; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The PCR conditions were as follows: DJ-1, 94°C, 5 min for
denaturation, 94°C for 45 sec, 51°C for 45 sec and 72°C for 10 min,
for 29 cycles then 10 min for the final extension; PTEN, 94°C, 5
min, 94°C for 50 sec; 58°C for 50 sec and 72°C for 1 min, for 32
cycles, then 8 min for final extension. The RT-qPCR products were
then eletrophoresed on 1.5% agarose gel containing ethidium bromide
(Sigma-Aldrich) and gel imaging was performed using GelDoc™ XR
(Bio-Rad Laboratories, Inc.). The image was analyzed using
Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA). The following primer sequences were used: DJ-1 sense,
5′-GCCAGCCTTGAAGATGCAAA-3′ and antisense,
5′-GGCTTGTAAGAATCAGGCCGT-3′; PTEN sense,
5′-CCGAAAGGTTTTGCTACCATTCT-3′ and antisense,
5′-AAAATTATTTCCTTTCTGAGCATTCC-3′; S26 sense,
5′-CCGTGCCTCCAAGATGACAAAG-3′ and antisense,
5′-GTTCGGTCCTTGCGGGCTTCAC-3′. S26 served as the positive control,
and RT-negative samples served as the negative control.
Western blot analysis
Protein extracts of Hep-2 and SNU-899 cells were
prepared as previously described (9). Briefly, the cells were harvested and
washed with cold phosphate-buffered saline, and total proteins were
extracted using lysis buffer (Tris 50 mM pH 8.0, NaCL 150 mM,
Sodium deoxycholate 1%, SDS 0.1%, NP-40 1%, Phenylmethylsulfonyl
fluoride 1 mM, Aprotinin 10 µg/ml−1, Leupeptin 10
µg/ml−1). Immunoblotting experiments were
performed according to standard procedures. Protein concentration
was determined using the Bradford assay (Bio-Rad Laboratories,
Inc.). Equal amounts of protein (50 µg) were separated by
electrophoresis on 8–12% SDS/polyacrylamide gels, and were then
transferred onto Biodyne A Membrane (Pall Life Sciences, Ann Arbor,
MI, USA). The membranes were then incubated with DJ-1 goat
polyclonal antibody (1:1,000; cat. no. sc-27004; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and PTEN rabbit monoclonal
antibody (1:1,000; cat. no. 9559; Cell Signaling Technology,
Danvers, MA, USA) overnight at 4°C. GAPDH mouse monoclonal antibody
(1:4,000; cat. no. sc-365062; Santa Cruz Biotechnology, Inc.) was
used as an internal loading control. The secondary antibodies for
DJ-1, PTEN and GAPDH were rabbit anti-goat (1:2,000; cat. no.
SC-2768; Santa Cruz Biotechnology, Inc.), goat anti rabbit
(1:1,000; cat. no. 77671; Sigma-Aldrich) and goat anti-mouse
(1:2,000; cat. no. 7076; Cell Signaling Technology), respectively.
The integrated optical densities of each band were analyzed using
Image-Pro Plus 6.0 software.
RNA silencing
As previously described, cells were transfected with
the siRNAs using Lipofectamine™ 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA). Scrambled siRNA and small inhibitor duplex RNAs
targeting human DJ-1 were designed using an online siRNA selection
program (http://www.dharmacon.com/sidesign/default.aspx) and
were chemically synthesized by RiboBio Co., Ltd. (Guangzhou,
China). The sequences were as follows: DJ-1-specific siRNA,
targeting GATTAAGGTCACCGTTGCA (sense, 5′-GAUUAAGGUCACCGUUGCA-3′,
and antisense, 3′-CUAAUUCCAGUGGCAACGU-5′); and scrambled siRNA
targeting TTCTCCGAACGTGTCACGT (sense, 5′-UUCUCCGAACGUGUCACGU-3′ and
antisense, 3′-AAGAGGCUUGCACAGUGCA-5′), which was used as a
scrambled control (9).
MTT assay
Hep-2 and SNU-899 cells were seeded in a 96-well
plate at a concentration of 1×104 cells/well for 24 h
and transfected with DJ-1-specific siRNA or negative control siRNA
by Lipofectamine™ 2000 (Invitrogen Life Technologies). Cell
proliferation was assessed at 72 h after transfection using an MTT
assay, as reported previously (9,15).
Briefly, 15 µl MTT (5 mg/ml; Sigma-Aldrich) was added to
each well and incubated at 37°C for 4 hr, then 150 µl
dimethly sulfoxide (Sigma-Aldrich) was added to each well. The
absorbance (A) value was evaluated using an enzyme linked
immunosorbent assay reader (Model 680; Bio-Rad Laboratoties, Inc.).
All experiments were repeated three times, and the mean and
standard deviation were calculated.
Cell viability assay
At 48 h post-transfection, the cells were fixed with
fixation buffer (eBioscience, San Diego, CA, USA) for 1 h at room
temperature, and stained with X-gal (Sigma-Aldrich) at 37°C
overnight. Finally, surviving blue cells were counted in five
fields using a microscope (ECLIPSE Ti-E; Nikon Corporation, Tokyo,
Japan).
Transwell migration assay
The assay was performed as previously described by
using chambers with an 8 micron pore size polyethylene
terephthalate membrane and a thin layer of matrigel basement
membrane matrix (BD BioCoat™ Matrigel™ Invasion Chamber; BD
Biosciences, San Jose, CA, USA) (16). Forty-eight hours after transfection
with DJ-1 siRNA, cells and culture medium were harvested, and
2.5×104 cells in 0.5 ml harvest medium were placed in
the upper chamber. The scrambled siRNA-transfected cells were used
as a negative control. The lower chamber was filled with 10% FBS
medium (0.75 ml). After incubation of the cells for 22 h, the cells
in the upper chamber were removed with a cotton swab. The cells on
the lower side of the filter were permeabilized with
fixation/permeabilization buffer (eBioscience) for 1 h at room
temperature, and stained with eosin (Sigma-Aldrich). Finally, the
stained cells were counted in five fields using a microscope
(ECLIPSE Ti-E; Nikon Corporation).
Statistical analysis
Statistical analysis was performed using SPSS 13
software (SPSS, Inc., Chicago, IL, USA). Student’s t-test
was used for evaluation of differences of gene expression between
controls and experiments in vitro. P<0.05 was considered
to indicate a statistically significant difference.
Results
DJ-1 decreases the expression of PTEN at
the mRNA level in Hep-2 and SNU-899 cells
To investigate whether DJ-1 regulates the mRNA
expression levels of PTEN, Hep-2 and SNU-899 cells were then
transfected with DJ-1 siRNA or scrambled siRNA and then collected
to examine mRNA levels by RT-qPCR. The results showed that DJ-1
silencing significantly increased the mRNA expression levels of
PTEN in Hep-2 (P<0.001) and SNU-899 cells (P<0.05) compared
with those in the controls two days following transfection
(Fig. 1A and B).
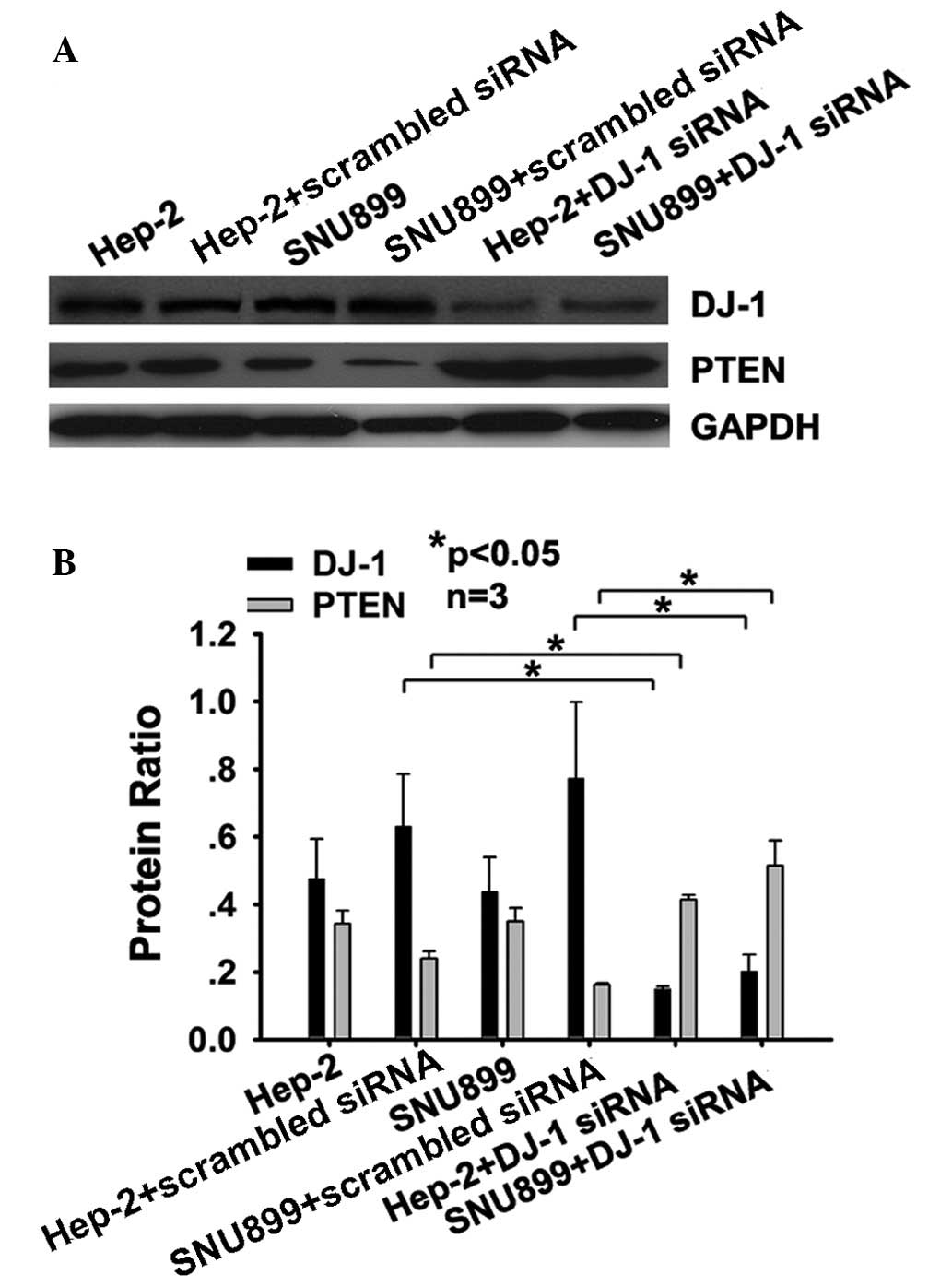
DJ-1 decreases the expression of PTEN at
the protein level in Hep-2 and SNU-899 cells
To determine whether DJ-1 has an influence on the
levels of PTEN protein, DJ-1 siRNA-transfected cells were assessed
for PTEN using western blot analysis. The results showed that three
days after DJ-1 siRNA transfection, the levels of PTEN protein in
Hep-2 (P<0.05) and SNU-899 cells (P<0.05) increased compared
with those in cells transfected with scrambled siRNA (Fig. 2A and B). Transfection with the DJ-1
siRNA also affected the protein expression levels of DJ-1 and PTEN,
due to the alterations in the mRNA expression levels of the two
genes.
Downregulation of DJ-1 decreases
proliferation and viability of Hep-2 and SNU-899 cells
Hep-2 and SNU-899 cells were transfected with DJ-1
siRNA for 72 h and their proliferation was assessed using an MTT
assay. The results showed that cell proliferation in the siRNA
group was inhibited (Hep-2 cells, 1.35±0.07 vs. 2.19±0.25,
P<0.05; SNU-899 cells, 1.08±0.10 vs. 1.68±0.17, P<0.05)
(Fig. 3A). Furthermore, at 48 h
post-transfection, cells were fixed and stained with X-gal, and
surviving blue cells were counted. Cell viability in the siRNA
group was lower than that in the scrambled siRNA-transfected group
(Hep-2 cells, 53.00±2.00 vs. 94.67±3.51%, P<0.05; SNU-899 cells,
51.33±2.52 vs. 95.67±3.21%, P<0.05) (Fig. 3B).
DJ-1 increases the invasiveness of Hep-2
and SNU-899 cells
As DJ-1 has a negative regulatory function in the
invasion in solid cancers (17,18),
the present study investigated the migratory activity of DJ-1
siRNA-transfected cells using a Transwell assay. After 22 h
incubation in Matrigel™ Invasion Chambers, significantly fewer
cells migrated through the filter after transfection with DJ-1
siRNA as indicated by reduced migration rates (Hep-2 cells,
0.143±0.012; SNU-899 cells, 0.135±0.012) compared with the
migration rate of cells transfected with scrambled siRNA (Hep-2
cells, 0.830±0.018; SNU-899 cells, 0.824±0.008; P<0.001)
(Fig. 4A and B). These results
demonstrated a potential association between the increased PTEN
levels and the low invasive capability of Hep-2 and SNU-899 cells.
These findings indicated that DJ-1 may be involved in the process
of tumor cell invasion via downregulation of PTEN in
vitro.
Discussion
Previous studies showed that DJ-1 has an important
role in the development of human malignancies (7,8). Le
Naour et al (19) showed
that DJ-1 protein levels were increased in the serum of patients
with breast cancer and suggested that this protein can be used as a
prognostic marker. Subsequent studies reported that DJ-1
overexpression is correlated with poor prognosis of patients with
cancer types including pancreatic and esophageal cancers (20,21).
In a previous study by our group, DJ-1 expression in tumor tissues
was firstly identified as a prognostic marker for patients with
laryngeal carcinoma (9), which was
further confirmed by another study on laryngeal carcinoma (10). Based on the clinical significance
of DJ-1 in human malignancies, its role in tumor progression was
assessed in numerous studies. Firstly, DJ-1 protein promoted the
proliferation and decreased apoptosis of tumor cells (6,8,22).
For instance, Hod (6) showed that
DJ-1 protein was able to regulate apoptosis of prostate cancer
cells. Kim et al (8) found
that DJ-1 protein was able to promote the proliferation of breast
cancer cells. Secondly, DJ-1 was associated with aggressive
behavior of tumor cells. He et al (17) showed that DJ-1 promoted the
invasion and metastasis of pancreatic cancer cells by activating
SRC/ERK/uPA signaling. Bai et al (18) found that DJ-1 contributed to the
metastasis of non-small cell lung cancer. Moreover, a recent study
by our group showed that overexpression of DJ-1 was linked to lymph
node metastasis in LSCC patients (11).
In the present study, siRNA directed against the
DJ-1 gene was transfected into the laryngeal carcinoma cell line
Hep-2 and SNU899 cells, respectively. MTT analysis showed that the
proliferation of these cells was significantly inhibited by
transfection with DJ-1-siRNA. Furthermore, a cell viability assay
showed that transfection with DJ-1-siRNA resulted in decreased cell
viability. Finally, the effect of DJ-1 on the invasive activity of
laryngeal cancer cells was evaluated using a Transwell migration
assay. The results showed that the invasive activity of tumor cells
was significantly inhibited following transfection with
DJ-1-siRNA.
Several studies showed that DJ-1-induced PTEN
down-regulation is associated with tumorigenesis of several cancer
types (8,12–14).
For example, DJ-1 was able to activate cell proliferation by
negatively regulating PTEN expression in breast cancer cells
(8). Moreover, overexpression of
DJ-1 and loss of PTEN are associated with invasive urothelial
carcinoma of the urinary bladder (13). A recent study by our group showed
that the inverse association between DJ-1 and PTEN was associated
with node metastasis of LSCC patients (11). All of the above results indicated
that DJ-1 promotes proliferation and invasiveness of laryngeal
cancer cells via downregulating the expression of PTEN.
To identify whether DJ-1 was able to downregulate
the expression of PTEN in LSCC, transient transfection with DJ-1
siRNA was performed in Hep-2 and SNU-899 cells, which were then
analyzed for PTEN expression. The results showed that the mRNA and
protein levels of PTEN were increased following DJ-1 siRNA
transfection. MTT analysis, viability assay and Transwell migration
assay were used to understand whether DJ-1 had a role in the
proliferation and invasion of the cells. As expected, the results
showed that the percentage of viable cells in the siRNA group was
lower than that in the control group, and cell proliferation in the
siRNA group was inhibited. Furthermore, the Transwell migration
assay showed that the number of cells migrating through the
membrane was significantly decreased after transfection with DJ-1
siRNA compared with that of scrambled siRNA-transfected cells.
These results indicated that DJ-1 may have an important role in the
proliferation and invasive activity of laryngeal cancer cells via
downregulation of PTEN.
In conclusion, the present study indicated, for the
first time, to the best of our knowledge, a link between the DJ-1
gene and the PTEN gene, and that DJ-1-induced PTEN down-regulation
may have an important role in the progression of LSCC. However,
there the present study had certin limitations; although PTEN was
shown to be regulated by DJ-1 the association between PTEN and cell
invasion and proliferation was not examined and will be the subject
of future studies. Collectively, the findings of the present study
provided important information which may be utilized for future
design of individualized therapeutic strategies for LSCC, for
example, selection of a more aggressive treatment regimen in
patients with tumors exhibiting high DJ-1 expression levels.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81271055/H1301).
References
|
1
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchese F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forastiere A, Koch W, Trotti A and
Sidransky D: Head and neck cancer. N Engl J Med. 345:1890–1900.
2001. View Article : Google Scholar
|
|
3
|
Kleinsasser O: Revision of classification
of laryngeal cancer; is it long overdue? (Proposals for an improved
TN-classification). J Laryngol Otol. 106:197–204. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Gehrke S, Haque ME, et al:
Inactivation of Drosophila DJ-1 leads to impairments of oxidative
stress response and phosphatidylinoditol 3-kinase⁄Akt signaling.
Proc Natl Acad Sci USA. 102:13670–13675. 2005. View Article : Google Scholar
|
|
5
|
Nagakubo D, Taira T, Kitaura H, Ikeda M,
Tamai K, Iguchi-Ariga SM and Ariga H: DJ-1, a novel oncogene which
transforms mouse NIH3T3 cells in cooperation with ras. Biochem
Biophys Res Commun. 231:509–513. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hod Y: Differential control of apoptosis
by DJ-1 in prostate benign and cancer cells. J Cell Biochem.
92:1221–1233. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
MacKeigan JP, Clements CM, Lich JD, Pope
RM, Hod Y and Ting JP: Proteomic profiling drug-induced apoptosis
in non-small cell lung carcinoma: identification of RS/DJ-1 and
RhoGDIalpha. Cancer Res. 63:6928–6934. 2003.PubMed/NCBI
|
|
8
|
Kim RH, Peters M, Jang Y, et al: DJ-1, a
novel regulator of the tumor suppressor PTEN. Cancer Cell.
7:263–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu XL, Wang ZF, Lei WB, Zhuang HW, Jiang
HY and Wen WP: DJ-1: a novel independent prognostic marker for
survival in glottic squamous cell carcinoma. Cancer Sci.
101:1320–1325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen Z, Ren Y, Ye D, Guo J, Kang C and
Ding H: Significance and relationship between DJ-1 gene and
surviving gene expression in laryngeal carcinoma. Eur J Histochem.
55:e92011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu XL, Wang ZF, Lei WB, Zhuang HW, Hou
WJ, Wen YH and Wen WP: Tumorigenesis role and clinical significance
of DJ-1, a negative regulator of PTEN, in supraglottic squamous
cell carcinoma. J Exp Clin Cancer Res. 31:942012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sitaram RT, Cairney CJ, Grabowski P, Keith
WN, Hallberg B, Ljungberg B and Roos G: The PTEN regulator DJ-1 is
associated with hTERT expression in clear cell renal cell
carcinoma. Int J Cancer. 125:783–790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee H, Choi SK and Ro JY: Overexpression
of DJ-1 and HSP90α and loss of PTEN associated with invasive
urothelial carcinoma of urinary bladder: Possible prognostic
markers. Oncol Lett. 3:507–512. 2012.PubMed/NCBI
|
|
14
|
Davidson B, Hadar R, Schlossberg A, et al:
Expression and clinical role of DJ-1, a negative regulator of PTEN,
in ovarian carcinoma. Hum Pathol. 39:87–95. 2008. View Article : Google Scholar
|
|
15
|
Weichert H, Blechschmidt I, Schröder S and
Ambrosius H: The MTT-assay as a rapid test for cell proliferation
and cell killing: application to human peripheral blood lymphocytes
(PBL). Allerg Immunol (Leipz). 37:139–144. 1991.
|
|
16
|
Sun W, Guo MM, Han P, et al: Id-1 and the
p65 subunit of NF-κB promote migration of nasopharyngeal carcinoma
cells and are correlated with poor prognosis. Carcinogenesis.
33:810–817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He X, Zheng Z, Li J, et al: DJ-1 promotes
invasion and metastasis of pancreatic cancer cells by activating
SRC/ERK/uPA. Carcinogenesis. 33:555–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bai J, Guo C, Sun W, et al: DJ-1 may
contribute to metastasis of non-small cell lung cancer. Mol Biol
Rep. 39:2697–2703. 2012. View Article : Google Scholar
|
|
19
|
Le Naour F, Misek DE, Krause MC, Deneux L,
Giordano TJ, Scholl S and Hanash SM: Proteomics-based
identification of RS/DJ-1 as a novel circulating tumor antigen in
breast cancer. Clin Cancer Res. 7:3328–3335. 2001.PubMed/NCBI
|
|
20
|
Yuen HF, Chan YP, Law S, Srivastava G,
El-Tanani M, Mak TW and Chan KW: DJ-1 could predict worse prognosis
in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers
Prev. 17:3593–3602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He XY, Liu BY, Yao WY, et al: Serum DJ-1
as a diagnostic marker and prognostic factor for pancreatic cancer.
J Dig Dis. 12:131–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Junn E, Taniguchi H, Jeong BS, Zhao X,
Ichijo H and Mouradian MM: Interaction of DJ-1 with Daxx inhibits
apoptosis signal-regulating kinase 1 activity and cell death. Proc
Natl Acad Sci USA. 102:9691–9696. 2005. View Article : Google Scholar : PubMed/NCBI
|