Introduction
Apoptosis-inducing factor (AIF) is a flavoprotein,
which usually performs a redox reaction in the electron transport
chain (1) and induces apoptosis
under conditions of injury. AIF is cleaved by calpain is and
released from the mitochondria into the cytoplasm, where it
relocates to the nucleus, cleaves DNA into fragments and induces
apoptosis (2,3). AIF is commonly expressed in cells,
however, apoptosis is not induced in all types of cell (4), only in neuronal cells and certain
tumour cells. Calpains belong to a family of calcium-dependent,
non-lysosomal cysteine proteases, which are expressed ubiquitously
in all cells (5). Regulated by the
amount of Ca2+, calpain has been previously reported to
promote apoptosis (5,6). Apoptosis is central in neural injury
disease, including spiral ganglion neuron (SGN) injury (7). SGNs, as the first afferent neuron in
the auditory pathway, have become a focus of investigation. SGNs
are susceptible to damage and the induction of apoptosis by noise,
ischemia and hypoxia, which can lead to noise-induced hearing loss,
age-associated hearing loss and even auditory neuropathy (8,9).
In the cochlea, SGN damage and apoptosis are
regulated by a complex signaling pathway. Fu et al perfused
ouabain into the cochlea to induce SGN apoptosis via the expression
of caspase (9). Schmutzhard
demonstrated that severe sepsis-induced hearing loss can be
attributed to apoptosis of the supporting cells, mediated by an
upregulation of caspase 3 (10).
However, inhibition of caspases results in incomplete or limited
protection, and AIF is concentrated in cochlea sensory cells. A
previous study observed that 122 dB white noise induces the
relocation of AIF into the nuclei (11).
Therefore, in the present study, Glu was directly
perfused into the cochlear tympanic canal (12) and SGNs were cultured in
vitro in order to examine the expression levels of AIF and
calpain in Glu-damaged SGNs.
Materials and methods
Animals and ethical statement
A total of 18 female Sprague-Dawley rats, aged 4–6
weeks and weighing between 150 and 180 g, were used for liquid
perfusion, and postnatal 0–3-day old rats were used for the in
vitro culture of SGNs. The rats were maintained in clean
conditions, with ad libitum access to food and water at 37°C
and 40% humidity. All animals were provided by and cared for by the
Institutional Animal Care and Use Committee of the Fourth Military
Medical University (Xi’an, China). The present study was approved
by the Ethics Committee of Xijing Hospital (Xi’an, China).
Liquid perfusion
The rats for the liquid perfusion analysis were
administered 2% sodium pentobarbital (0.25 ml/100 g; Sigma-Aldrich,
St. Louis, MO, USA) to induce anaesthesia, and placed on a board at
37°C to maintain their temperature. The skin and fascia surrounding
the ear were dissected, and the muscles were separated to expose
the otocyst. A power drill (XiChang Inc., Xi’an, China) was used to
drill a hole in the otocyst, revealing the cochlea and the artery
at the top of the hole. A hole (~0.2 mm in diameter) was then
opened on the wall of the scala tympani using a hand drill (XiChang
Inc.). A plastic tube connected to a Hamilton syringe pump (XiChang
Inc.) was used to inject different concentrations of Glu (Hexin
Chemical Industry, Shenzhen, China) for 30 min. Subsequently, the
wound was closed. All the animals were divided into six groups,
which received either injected saline water (control), 5, 10, 20 or
40 mM glutamate, or no treatment. In the control animals, the
normal saline was perfused into the perilymph via the same
procedure and in all rats, with the exception of the untreated
rats, surgery was performed on both ears.
Auditory brainstem response (ABR)
The auditory thresholds were determined by measuring
the ABR. A TDT III System Auditory Evoked Potential Workstation,
controlled using SigGenRZ and BioSigRZ software (Tucker-Davis
Technologies, Fort Lauderdale, FL, USA) was used to collect data.
The ABRs were elicited using tone bursts (4, 8, 16, 24 and 32 kHz;
0.5 ms rise/fall time; no plateau; alternating phase). The stimulus
was provided through an RZ6 D/A converter (Tucker-Davis
Technologies) and was presented using a high-frequency speaker (MF1
Multi-Field Magnetic Speakers; Tucker-Davis Technologies) placed ~2
cm in front of the ear being assessed. The stimulus was reduced in
5 dB steps until the response ceased. The differential potential
was sampled over 10 ms, filtered (low-pass, 4 kHz; high-pass, 100
Hz) and averaged (512 sweeps of alternating stimulus polarity) in
order to obtain the mean traces at each intensity. The rats were
assessed 2 days following the perfusion, and the resulting data
were analysed using SPSS version 17.0 software (SPSS, Inc.,
Chicago, IL, USA).
SGNs culture
Dissociated spiral ganglion cultures were prepared,
as described previously, from postnatal 0–3 day rats (13). In brief, the SGNs
(~1×104) were incubated in SGN culture medium:
Dulbecco’s modified Eagle’s medium with B27 (2 ml/ml;
Sigma-Aldrich), brain-derived neurotrophic factor (10 µg/ml;
Sigma-Aldrich), penicillin (100,000 U/l; 1%; Sigma-Aldrich).
Subsets of the cultures were maintained at 37°C in a humidified
incubator (Heraeus CO2; Bole Company, Beijing, China)
with 5% CO2, which was divided into four groups, in
which either 10, 20 or 40 mM Glu was added to cells for 48 h at
37°C.
Immunofluorescent staining
The slides were fixed by perfusing with 4%
paraformaldehyde (XiChang Inc.) for 1 day, following which, the
slides were immersed in 0.1 M phophate-buffered saline (PBS; Huamei
Biotech Co., Ltd.) for 20 min and Triton X-100 (0.3%;
Sigma-Aldrich) for 15 min. The slides were washed with 0.1 M PBS
for 5 min each time and were then incubated at 37°C in a 5%
goat-serum blocking solution (Huamei Biotech Co., Ltd., Wuhan,
China) for 30 min. The slides were incubated with primary
antibodies against AIF (polyclonal goat; 1:100; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and β-tubulin (poly-clonal
rabbit; 1:200; Abcam, Cambridge, MA, USA) at 4°C in a refrigerator
for 3 days. The slides were then washed with 0.1 M PBS and were
incubated with Alexa Fluor 488 (poly-clonal donkey anti-goat;
1:200; Invitrogen Life Technologies, Carlsbad, CA, USA) and Cy3
(polyclonal goat anti-rabbit; 1:100; Abcam) at 4°C for 1 day.
Subsequent to the addition of 0.1% DAPI (Sigma-Aldrich) to stain
the nuclei, glycerol (Huamei Biotech Co., Ltd.) was used to seal
the coverslips. The sections were observed using a laser scanning
confocal microscope (FV1000; Olympus Corporation, Tokyo,
Japan).
Transmission electron microscopy
(TEM)
The treated rats were fixed using a 2.5%
glutaraldehyde (Huamei Biotech Co., Ltd.) solution. The rats were
sacrificed by decapitation, and the cochlea was removed from the
head and immersed in the same fixative at 4°C overnight. The
specimens were then rinsed with 0.1 M PBS and immersed in a 10%
EDTA (Sigma-Aldrich) solution for 7 days for decalcification.
Subsequently, the cochlea was placed in a 1% osmic acid solution
(Abcam) for 2 h at 4°C. The entire cochlea was removed from the
bony shell, embedded and cut into ultrathin transverse sections
(70–100 nm) using an ultramicrotome (Reichert UltracutE, Leica,
USA). Counterstaining was then performed using uranyl acetate
(Huamei Biotech Co., Ltd.) for 50 min and lead citrate (Huamei
Biotech Co., Ltd.) for 10 min. Finally, the specimens were observed
using a TEM (JEM-1230; Olympus Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The RNA from the treated cochleae were used for
RT-qPCR, according to the QuantiTect Reverse Transcription (Qiagen,
Valencia, CA, USA) instructions. RT-qPCR analysis was performed
using a SYBR® Green Master Mix kit (Applied Biosystems
Life Technologies, Foster City, CA, USA) and RNase-free 96-well PCR
plates. The qPCR was performed on a CFX96 Touch™ Real-Time PCR
Detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The following forward and reverse primers (Genecopoeia, Rockford,
MD, USA) were used for the specific RNAs in RT-qPCR: R-AIF, forward
5′-TAGAACTCCAGATGGCAAGACA-3′; R-AIF, reverse
5′-AAGCCCACAATAAGGACTAACAC-3′; R-caspase 3, forward
5′-GAATGACTGGGAGTGGGGTAG-3′; R-caspase 3, reverse
5′-GACCTGGAACATCGGATTTGA-3′; R-calpain, forward
5′-CAAAGTGGACCCCTATGAACG-3′; R-calpain, reverse
5′-TAAGGGCGTCAGGTGTAAGGT-3′. The threshold cycles (Ct) value of the
genes under examination in each sample were normalised using the
value of the endogenous control gene, 18S. The relative fold
changes in gene expression levels were obtained by comparing the
2-ΔΔCt data of the different groups.
Data analysis
Wherever possible, the data are expressed as the
mean ± standard deviation. Statistical significance was determined
using analysis of variance followed by a multiple comparison
Dunnett’s test (using SPSS software). P<0.05 was considered to
indicate a statistically significant difference.
Results
ABR thresholds increase following Glu
perfusion
Subsequent to Glu perfusion, the ABR threshold was
markedly increased. No significant difference was observed among
the values for the control, 5 or 10 mM Glu groups (P>0.05),
however, a significant increase was observed in the 20 and 40 mM
Glu groups (P<0.05). In the control and 5 mM groups, there was a
7–8 dB shift at 4 kHz, in the 10 mM group, the shift reached 11 dB,
and in the 20 and 40 mM groups, the shift reached 20 dB. At 8 and
16 kHz, the shift in the control and 5 mM groups was 12–15 dB,
however, there was a shift of 20–30 dB in the 10, 20 and 40 mM
groups. At 24 and 32 kHz, the shift was <20 dB in the control, 5
and 10 mM groups, but was >20 dB in the other groups. The ABR
shifts increased with the frequency of the toneburst (Fig. 1).
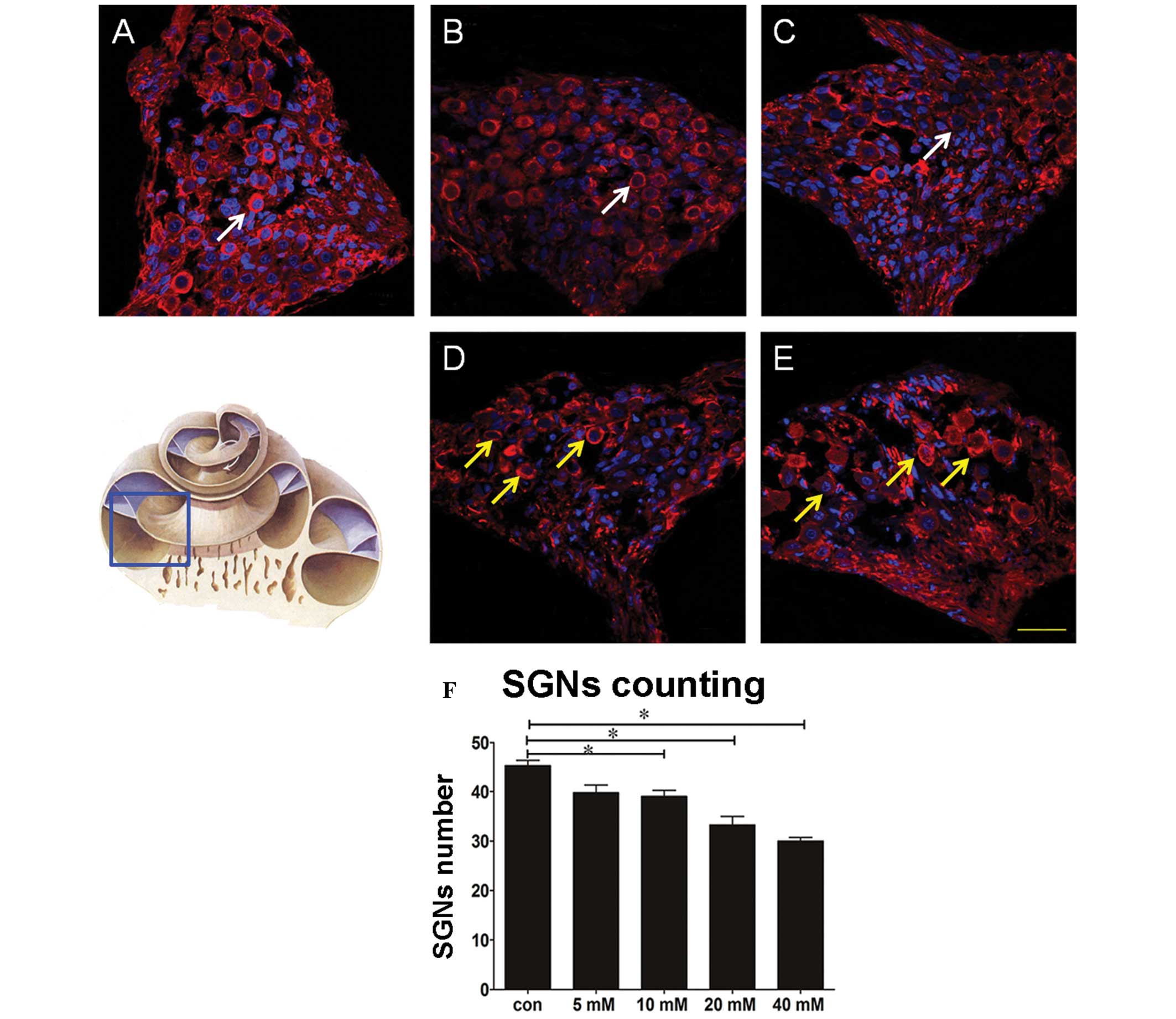
Glutamate toxicity reduces the number of
cochlear SGNs and alters their morphology
A total of three successive sections of the cochlea
middle axis were selected for tubulin staining. Images were
captured using a laser scanning confocal microscope and the mean
number of basal SGNs present in each group were counted by two or
more individuals. The Glu perfusion groups were compared with the
control, providing the rates of SGN loss. The results demonstrated
that the mean number of SGNs was reduced by a magnitude of between
two and three in the 5 and 10 mM Glu groups, compared with the
control group, and by 8–9-fold in the 20 and 40 mM Glu groups (data
not shown). The statistical analyses revealed that the numbers of
SGNs in the latter groups were significantly lower compared with
those in the that of the 5 and 10 mM Glu groups (Fig. 2). TEM also revealed that treatment
with Glu at ≥10 mM generated a high number of cytoplasmic vacuoles
and a significant quantity of heterochromatin around the nuclei in
the SGNs, in addition to swelling of the mitochondria and
endoplasmic reticulum (Fig. 3). It
was, therefore, concluded that excessive Glu damaged the SGNs in
the cochlea, in a dose-dependent manner.
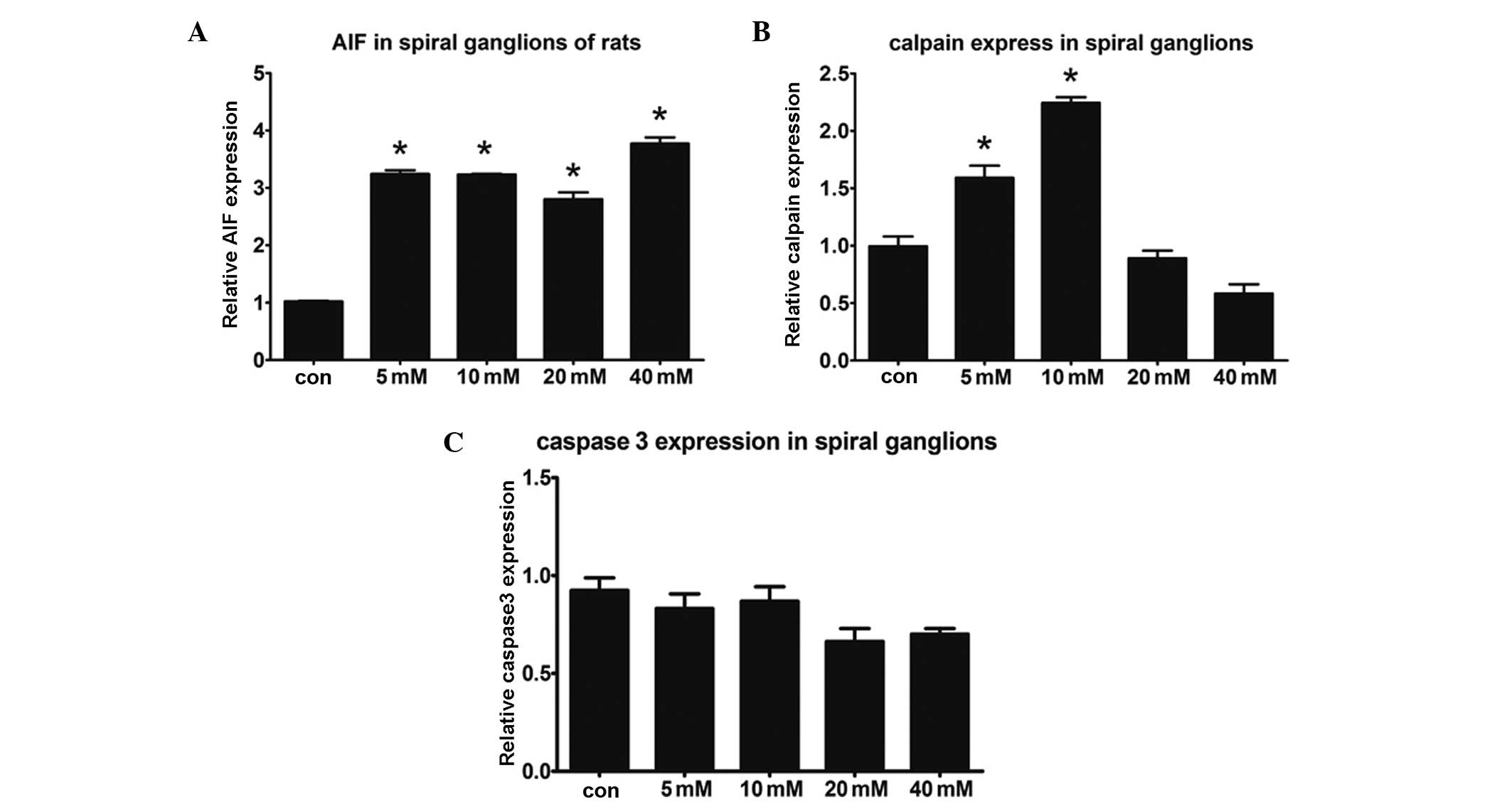
Expression levels of caspase 3 are
unchanged, whereas those of AIF and calpain are altered in Glu
toxicity-induced SGNs
The perfused rats were decapitated on a clean bench,
Rosenthal’s canal and the middle axis were separated and the
basilar membrane and spiral ligament were removed. RT-qPCR was
performed to detect the expression levels of AIF, calpain and
caspase 3. Increased expression levels of AIF were observed in the
Glu intervention groups, whereas the expression levels of caspase 3
were not significantly different. Thus, SGN damage did not
upregulate the expression of caspase 3. Changes in the expression
levels of calpain were also observed in all the treatment groups.
In the 5 and 10 mM Glu groups, the expression levels of calpain
were significantly higher compared with those of the 20, 40 mM and
control groups (P<0.05; Fig.
4). In conclusion, AIF, rather than caspase 3, initiated the
Glu toxicity-induced SGN apoptosis. Calpain was also involved in
the damage of the SGNs.
AIF is translocated into the nuclei
following exposure to Glu at concentrations ≥20 mM
To assess the localisation and expression levels of
AIF, the subcellular location of AIF was examined in SGNs following
Glu perfusion. The results demonstrated that, in the groups treated
with ≥20 mM Glu, the red fluorescence of AIF overlapped with the
blue nuclear staining in the laser scanning confocal microscopy;
however, concentric circles of red labelling surrounded the blue
labelling were observed in the control group. The staining patterns
of the perfused groups were irregular and included incomplete
nuclear staining and polygonal cytoplasmic labelling. These altered
patterns of expression suggested that the AIF was initally
translocated into the nuclei of the SGNs (Fig. 5).
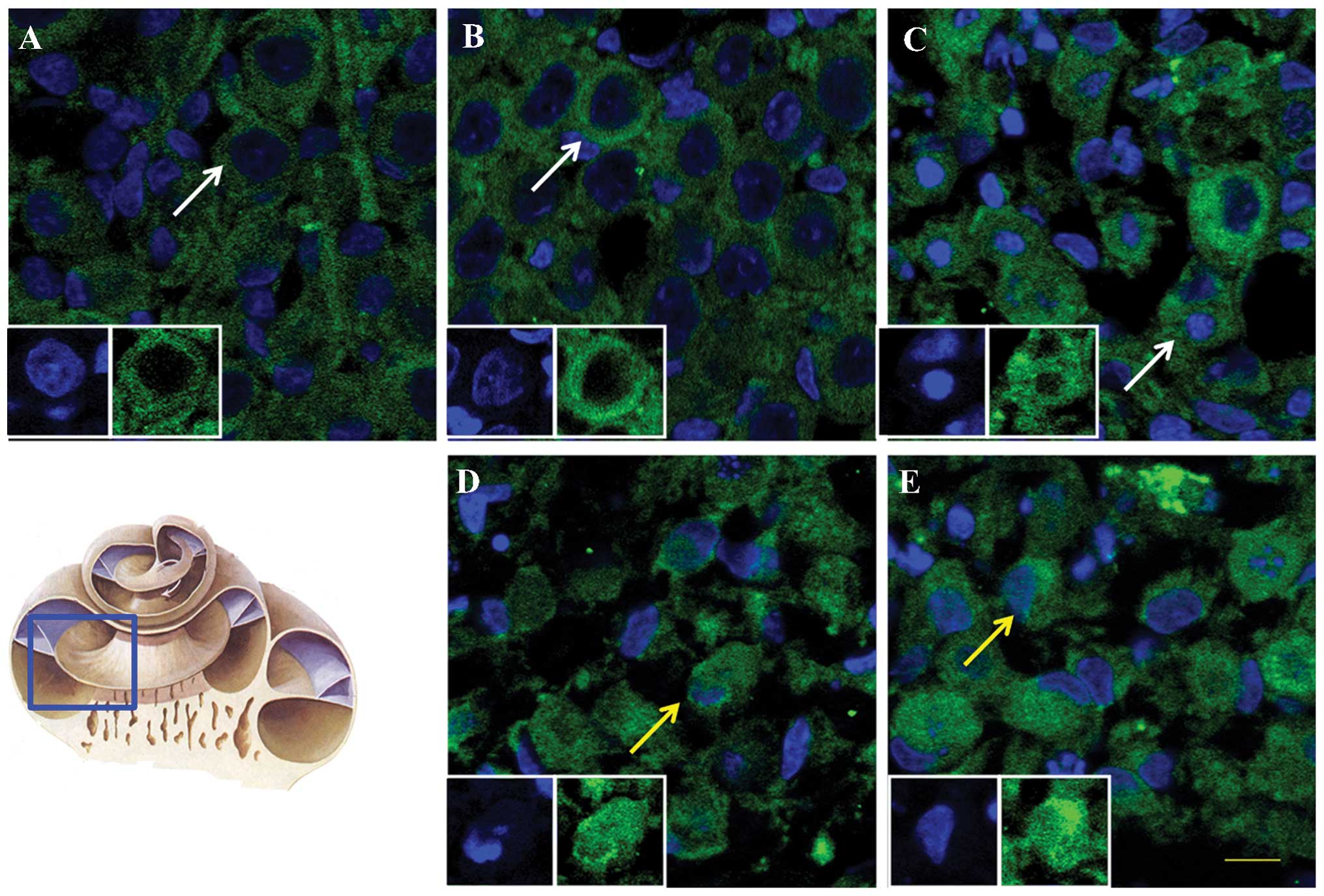 | Figure 5Immunofluorescence results of the
distribution of AIF in SGNs perfused with Glu. Immunofluorescence
images at in the (A) control, and at Glu perfusion concentrations
of (B) 5, (C) 10, (D) 20 and (E) 40 mM. Scale bar, 50 µm.
AIF (green) is located in the cytoplasm in the normal group,
whereas the nuclei (blue) are visible in the central regions of the
SGNs. In the 10 mM, 20 mM and 40 mM groups, the green and blue
signals overlap. White arrows indicate cells, in which AIF was
distributed in the cytoplasm, and yellow arrows indicate cells, in
which AIF overlapped with the nuclei. AIF, apoptosis-inducing
factor; SGNs, spiral ganglion neurons; Glu, glutamate. |
AIF is translocated into the nucleus and
the expression levels of AIF and calpain, but not caspase 3 are
upregulated in Glu-treated SGNs in vitro
To confirm the mRNA expression levels of AIF and
calpain, the SGNs were incubated in medium, containing different
concentrations of Glu. The mRNA expression levels of AIF and
calpain increased following the addition of Glu for 48 h, and the
expression levels of AIF were significantly higher, compared with
the control, in the 10, 20 and 40 mM groups (P<0.05). The
expression levels of calpain were also higher in the 20 and 40 mM
groups compared with the control (P<0.05). However, the
expression levels of caspase 3 were not significantly different in
the four groups (P>0.05). These results demonstrated that AIF
and calpain were upregulated in Glu-treated SGNs in vitro,
however, the levels of caspase 3 were unchanged (Fig. 6).
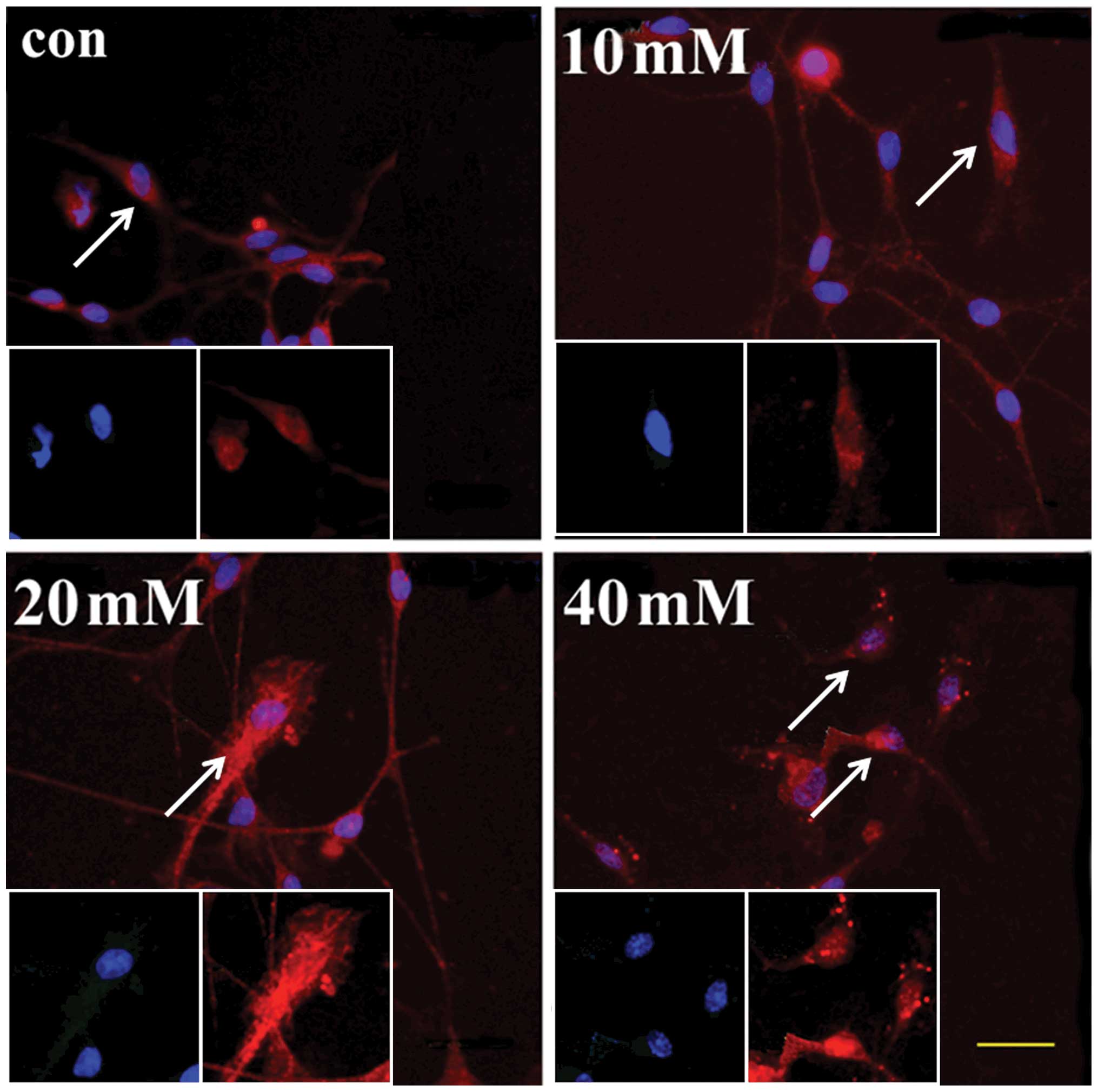
To observe the distribution of AIF, an
immunostaining assay was performed. In the control group, the
red-stained AIF was distributed in the cytoplasm only. However, in
the 20 mM group, AIF had translocated into the nuclei of certain
bipolar neurons, which was observed in greater number of cells in
the 40 mM group (Fig. 7). In
conclusion, the addition of a high concentration of Glu induced the
translocation of AIF into the nuclei, and the upregulation of AIF
and calpain in the cultured SGNs.
Discussion
Spiral ganglia, as the first afferent nerves,
transmit acoustic information to the auditory centre, by which they
are regulated (8). Excessive Glu
has been reported to damage SGNs, inducing sensorineural hearing
loss and auditory neuropathy (13). Auditory neuropathy is a disease, in
which the inner hair cells fail to transmit acoustic information to
the brain. This disorder clinically presents with elevated hearing
thresholds without a parallel loss of otoacoustic emissions, for
which damage to the SGNs may be the cause (3). Increased Glu binds with Glu
receptors, including NMDAR2 and mGluRIs, and initiates calcium
regulation and oxidative damage, injuring the SGNs (14), which leads to subsequent hearing
loss. In the present study, ABR threshold shifts increased with
increasing concentrations of perfused Glu. The 20 and 40 mM Glu
groups had a greater shift, between 4 kHz and 32 kHz, indicating
damage mediated by Glu. The number of SGNs was significantly
reduced with increasing concentrations of Glu, and TEM identified
heterochromatin surrounding the nuclei, which confirmed the damage
to the SGNs by excessive Glu and demonstrated a successful model of
SGN-damage. These results provide support in further examining the
mechanisms of auditory neuropathy. Investigating higher
concentrations of Glu may complete experiment data and provide
clearer presentation of Glu-damaged SGNs.
Caspase has been a focus of early investigations of
cochlea sensory cells. Noise and ototoxic drugs can induce caspase
upregulation in outer hair cells, however, Steinbach and Lutz
observed that addition of the Z-VAD-FMK caspase inhibitor to SGN
cultures prevents the apoptosis of SGNs (14). Abaamrane et al injected
Z-VAD-FMK into the cochlea, which resulted in incomplete recovery
in deafness, following detonation (15). In the present study, qPCR revealed
that the levels of caspase 3 were not upregulated in response to
Glu perfusion or SGN culture, which supported the hypothesis that
the caspase pathway has a limited effect on Glu toxicity in SGNs
(14). However, the RT-qPCR
results demonstrated that the mRNA expression levels of AIF
increased following Glu treatment, compared with the control group,
and the immunostaining assay indicated that AIF was translocated
into the nuclei at Glu concentrations of ≥20 mM perfusion, in the
perfusion and cultured SGNs. These results demonstrated that AIF
may be induced in SGN apoptosis and contribute to Glu-mediated SGN
injury in sensory hearing loss and auditory neuropathy.
Calpain has been identified as being important in
the association between Glu toxicity and AIF in apoptosis (16). Vosler et al demonstrated
that calpain can be activated by apoptotic signals, releasing AIF
from the inner mitochondrial membrane into the cytoplasm (17), while other studies have observed
that AIFis then transferred between the cytoplasm and the nucleus
to induce nuclear DNA fragmentation (3,18),
which results in an influx of Ca2+ into the cytoplasm
through the ion channels of the NMDA Glu receptors and the
activation of calpain. In the RT-qPCR results of the present study,
the expression of calpain was upregulated subsequent to Glu acting
on the SGNs via Glu perfusion or following the addition of Glu to
cultured SGNs, highlighting the important role of calpain. TEM
demonstrated morphological alterations of the endoplasmic reticulum
in the SGNs, and suggested that endogenous Ca2+ was
involved in calpain activation in the cytoplasm (19). The results suggested that the
calpain-AIF upregulation pathway was important in Glu-mediated
damage of SGNs.
In conclusion, the present study demonstrated an
explanation for the mechanism underlying Glu-mediated SGN damage in
tympanic canal perfusion and in vitro SGNs intervention.
This may assist in understanding the relevant mechanisms of
auditory neuropathy.
Acknowledgments
This study was supported by grants from the Major
State Basic Research Development Program of China (973 Project;
grant no. 2011CB504505) and the National Natural Science Foundation
of China (grant nos. 81120108008, 30930098, 81300832 and
81271069).
References
|
1
|
Mizukoshi S, Nakazawa M, Sato K, Ozaki T,
Metoki T and Ishiguro S: Activation of mitochondrial calpain and
release of apoptosis-inducing factor from mitochondria in RCS rat
retinal degeneration. Exp Eye Res. 91:353–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Q, Paillard M, Gomez L, et al:
Activation of mitochondrial µ-calpain increases AIF cleavage in
cardiac mitochondria during ischemia-reperfusion. Biochem Biophys
Res Commun. 415:533–538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu C, Wang X, Deinum J, et al:
Cyclophilin A participates in the nuclear translocation of
apoptosis-inducing factor in neurons after cerebral
hypoxia-ischemia. J Exp Med. 204:1741–1748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joseph B, Marchetti P, Formstecher P,
Kroemer G, Lewensohn R and Zhivotovsky B: Mitochondrial dysfunction
is an essential step for killing of non-small cell lung carcinomas
resistant to conventional treatment. Oncogene. 21:65–77. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Norberg E, Gogvadze V, Ott M, et al: An
increase in intracellular Ca2+ is required for the
activation of mitochondrial calpain to release AIF during cell
death. Cell Death Differ. 15:1857–1864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan Y, Shi F, Yin Y, et al:
Ouabain-induced cochlear nerve degeneration: synaptic loss and
plasticity in a mouse model of auditory neuropathy. J Assoc Res
Otolaryngol. 15:31–43. 2014. View Article : Google Scholar :
|
|
7
|
Zhang YM, Ma B, Gao WY, Wen W and Liu HY:
Role of glutamate receptors in the spiral ganglion neuron damage
induced by acoustic noise. Acta Physiologica Sinica. 59:103–110.
2007.In Chinese.
|
|
8
|
Starr A: Auditory neuropathy and inner
hair cell and synapses. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za
Zhi. 43:323–326. 2008.In Chinese. PubMed/NCBI
|
|
9
|
Fu Y, Ding D, Wei L, Jiang H and Salvi R:
Ouabain-induced apoptosis in cochlear hair cells and spiral
ganglion neurons in vitro. BioMed Res Int. 2013:6280642013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmutzhard J, Glueckert R, Pritz C, et
al: Sepsis otopathy: experimental sepsis leads to significant
hearing impairment due to apoptosis and glutamate excitotoxicity in
murine cochlea. Dis Model Mech. 6:745–754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rebillard G and Bryant GM: Effects of in
vivo perfusion of glutamate dehydrogenase in the guinea pig cochlea
on the VIIIth nerve compound action potential. Brain Res.
494:379–382. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu W, Fan Z, Han Y, Zhang D, Li J and
Wang H: Intranuclear localization of apoptosis-inducing factor and
endonuclease G involves in peroxynitrite-induced apoptosis of
spiral ganglion neurons. Neurol Res. 34:915–922. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang M, Sun H and Zhang YQ: Excitotoxic
effect of glutamate on the afferent neurons in guinea pigs. Zhong
Nan Da Xue Xue Bao Yi Xue Ban. 29:170–173. 2004.In Chinese.
|
|
14
|
Steinbach S and Lutz J: Glutamate induces
apoptosis in cultured spiral ganglion explants. Biochem Biophys Res
Commun. 357:14–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abaamrane L, Raffin F, Schmerber S and
Sendowski I: Intracochlear perfusion of leupeptin and z-VAD-FMK:
influence of antiapoptotic agents on gunshot-induced hearing loss.
Eur Arch Otorhinolaryngol. 268:987–993. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozaki T, Yamashita T and Ishiguro S:
Mitochondrial m-calpain plays a role in the release of truncated
apoptosis-inducing factor from the mitochondria. Biochim Biophys
Acta. 1793:1848–1859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vosler PS, Sun D, Wang S, et al: Calcium
dysregulation induces apoptosis-inducing factor release: cross-talk
between PARP-1- and calpain-signaling pathways. Exp Neurol.
218:213–220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sanges D, Comitato A, Tammaro R and Marigo
V: Apoptosis in retinal degeneration involves cross-talk between
apoptosis-inducing factor (AIF) and caspase-12 and is blocked by
calpain inhibitors. Proc Natl Acad Sci USA. 103:17366–17371. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Norberg E, Gogvadze V, Ott M, et al: An
increase in intracellular Ca2+ is required for the activation of
mitochondrial calpain to release AIF during cell death. Cell Death
Differ. 15:1857–1864. 2008. View Article : Google Scholar : PubMed/NCBI
|