Introduction
Osteoporosis is an age-associated systemic disease
that is characterized by a progressive loss of bone mass, decreased
bone strength and increased fracture risk (1). Onset of the disease often has no
warning sign or symptoms, and therefore may remain undiagnosed
until the pain induced by an osteoporotic fracture attracts
attention. Normal maintenance of bone tissue strength and integrity
is a dynamic process that requires constant remodeling of the
mineralized bone matrix by two opposing processes. New bone tissue
is formed by specialized osteoblast cells (bone formation), and
existing bone matrix is broken down by osteoclast cells to be
re-absorbed by the body (bone resorption). If these processes are
in balance, the bone mass is maintained. However, if osteoblast
activity is increased compared with osteoclast activity, then a net
gain in bone mass will occur. In osteoporosis, the loss of bone
mass, commonly detected as a decrease in bone mineral density
(BMD), is the result of an imbalance between the rates of bone
formation and resorption.
Postmenopausal women are particularly susceptible to
this disorder of bone metabolism. During child-bearing years,
estrogen aids the maintenance of bone mass in women by stimulating
osteoblast activity. The reduced estrogen levels in postmenopausal
women lead to reduced rates of bone formation by osteoblasts,
resulting in a net increase in bone resorption (1). In addition, decreased proliferation
of osteoblasts in postmenopausal women results in enhanced
differentiation and hyperactivity of osteoclasts (OCs), resulting
in bone loss. Considerable evidence indicates that the
osteoprotegerin (OPG)/receptor activator of nuclear factor κB
ligand (RANKL)/RANK system is necessary for osteoclast development
(2,3). RANKL, also known as tumor necrosis
factor superfamily member 11 (TNFSF11), is secreted by T
lymphocytes, B lymphocytes, stromal cells and osteoblasts (4,5).
RANKL binds to a membrane-bound receptor (RANK or TNFRSF11A) on the
surface of osteoclast precursor cells, promoting the
differentiation, maturation and activity of OCs. The soluble
receptor OPG (TNFRSF11B), secreted by stromal and osteogenic cells
(including osteoblasts), competes with RANKL to bind to RANK,
thereby preventing the differentiation and activation of OCs
(3,6).
Numerous drugs have been investigated for use in the
treatment of osteoporosis for postmenopausal women with elevated
bone resorption rates. Hormone replacement therapy may inhibit bone
loss; however, long-term use of hormone treatment may cause venous
thromboembolism, coronary heart disease and stroke (7–9).
Bisphosphonates (BPs) have been reported to have high affinity for
hydroxyapatite, a structural component of the bone matrix, and are
therefore absorbed by bone tissues. BPs are then able to suppress
bone resorption by OCs by inhibiting intracellular mevalonic acid
metabolism and inducing apoptosis (10). However, BPs are strong inhibitors
of osteoclast function, which can lead to the inhibition of normal
bone turnover. In certain cases, this inhibition results in a
severe complication associated with the administration of BPs,
known as bisphosphonate-associated osteonecrosis of the jaw
(11). Salmon calcitonin (sCT) is
a classic anti-osteoporosis drug, and is an active peptide,
comprised of 32 amino acids with a molecular weight of 3,500 Da
(12). Experimental administration
of sCT in rats increases cancellous (spongy) bone volume and
trabecular number and may reduce the number of osteoclasts
(13,14). sCT has been used for the long-term
treatment of metabolic bone diseases associated with bone turnover,
including Paget’s disease of bone and hypercalcemia associated with
bone cancer. sCT rarely causes hypocalcemia and has been used as a
routine drug for the clinical treatment of postmenopausal
osteoporosis (15).
A daily low dose of aspirin may prevent
cardiovascular disease and has few adverse reactions. Solheim et
al (16) previously revealed
that aspirin (ASA), also known as acetylsalicylic acid, may have a
role in the inhibition of bone resorption, and subsequent
epidemiological reports also found that regular use of low-dose ASA
or other NSAIDs is frequently associated with increased bone
density (17,18). Through acting on the Fas/FasL
signaling pathway, ASA intervenes in the development of
osteoporosis by inhibiting the apoptotic effect of T lymphocytes on
bone marrow-derived mesenchymal cells, promoting osteogenic
differentiation and inhibiting osteoclast differentiation (19,20).
Additionally, cyclooxygenase-2 (COX-2) may promote bone resorption
by altering the expression of matrix metalloproteinase-1 (MMP-1)
and interleukin (IL)-6 (21). ASA
acts as an effective inhibitor of COX-2, and may also reduce COX-2,
inhibiting the development of bone resorption in osteoporosis
(17). Therefore, ASA has
significant potential as an effective, cheaper alternative to the
drugs currently used to treat osteoporosis.
In the present study, experiments were conducted to
assess the effectiveness of combined therapy with sCT and ASA
(sCT+ASA) in rats that were ovariectomized (OVX) via bilateral
oophorectomy, an animal model that has been accepted as an
effective simulation of osteoporosis in postmenopausal women with
characteristic bone resorption (22). ASA or sCT was administered to
ovariectomized rats as a monotherapy (OVX+sCT or OVX+ASA) or in
combination (OVX+sCT+ASA) to inhibit bone resorption. Dual energy
X-ray absorptiometry, bone biomechanics and serum markers of bone
turnover were evaluated to assess the therapeutic effects of the
drug combination, and to study the impact of the drug combination
on osteoclast activity and the OPG/RANKL/RANK system in
vivo.
Materials and methods
Experimental design
A total of 40 healthy, female Sprague-Dawley (SD)
rats (3-months old, weighing 245–300 g each) were purchased from
Guangdong Province Medical Experimental Animal Center (license no.
SCXK 2008-0002; Guangdong, China). The rats were reared in an
environment with an ambient temperature of 24–26°C, relative
humidity of 45–65% and 12 h of daylight with ad libitum
access to food and water. The care of the experimental animals met
the requirements of Guidance Suggestions for the Care and Use of
Laboratory Animals, issued by The Ministry of Science and
Technology of the People’s Republic of China (http://www.calas.org.cn/html/jypx/zcfg/20111129/1309.html).
All surgical and treatment procedures were approved by the Animal
Ethics Committee of Guangdong Medical College (Zhanjiang,
China).
Following adaptive feeding, according to weight, the
rats were divided randomly into five groups (n=8 rats/group): The
sham group (Sham), the aspirin group (OVX+ASA or ASA), the
calcitonin group (OVX+sCT or sCT), the combined treatment group
(OVX+sCT+ASA or sCT+ASA) and the placebo group (OVX). Following an
intraperitoneal injection of 7% chloral hydrate (0.5 ml/100 g;
Guangzhou Chemical Reagent Factory, Guangzhou, China), rats in the
sCT, ASA, sCT+ASA and OVX placebo groups underwent bilateral
oophorectomies, while those in the Sham group underwent abdominal
incision and suture without concomitant oophorectomy (23). Postoperatively, all rats were
provided with free access to food and water. No treatment was
administered during the first two weeks following the operation, to
allow for the development of osteoporosis. Beginning two weeks
post-operation, the groups were administered treatment intervention
as follows: i) Rats in the aspirin group (OVX+ASA) were given a
gavage of low-dose aspirin (Hunan Xinhui Pharmaceutical Industry
Ltd. Co., Hunan, China). The daily dosage of aspirin for rats (per
square meter of body surface) was calculated to be similar to that
of a low-dose aspirin regimen in humans (34.4 mg/kg/day); ii) rats
in the calcitonin group (OVX+sCT) were injected subcutaneously in
the neck with 2 U/kg/day sCT (Miacalcic; Beijing Novartis
Pharmaceutical Co. Ltd., Beijing, China); iii) rats in the
OVX+sCT+ASA group were injected subcutaneously with sCT (2 U/kg/d)
and administered a gavage of aspirin (34.4 mg/kg/d) and iv) rats in
the Sham and OVX groups were administered a subcutaneous injection
of saline (0.5 ml/kg/day) and a gavage of saline (5 ml/kg/day;
Guangzhou Jinhuada Chemical Reagent Co., Ltd.).
Sample collection
All rats were sacrificed 12 weeks following the
commencement of treatment intervention. Blood was collected from
the right ventricle under anesthesia with intraperitoneal injection
of chloral hydrate (0.5 ml/10 g, 7%). The blood samples were placed
into a 4°C refrigerator for 3 h and then centrifuged (989 x g, 4°C,
20 min). The resulting serum supernatants were recovered at 10,000
x g, placed in Eppendorf tubes (Thermo Fisher Scientific, Waltham,
MA, USA) and stored at −80°C until required for further analysis.
Following sacrificing the rats, the right femurs were rapidly
removed and the muscles, ligaments and other tissues on the surface
were cleaned off. The femurs were then washed with alcohol and
rinsed three times with sterile saline. Each end of the femur was
cut with sterile surgical instruments under sterile conditions to
expose the marrow cavity and 1 ml low-sugar Dulbecco’s modified
Eagle’s medium (DMEM; Thermo Fisher Scientific) was drawn with a
2.5-ml disposable sterile syringe (Guangzhou Jinhuada Chemical
Reagent Co., Ltd.) to flush the marrow cavity repeatedly. The
bone-marrow cell suspension was placed into a 2-ml freezing tube
and stored at −80°C until prior to analysis by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). The
left femurs and the fourth lumbar vertebrae were removed and,
following separation of the surrounding soft tissues, these bones
were wrapped with saline-soaked gauze and aluminum foil and stored
at −20°C until required for measurements of BMD and bone
biomechanics. The third lumbar vertebrae and right tibias were cut
from the adhering connective tissues, fixed with (5 ml, 2.5%)
paraformaldehyde for 24 h and then soaked in 5 ml of 10% EDTA
(Guangzhou Jinhuada Chemical Reagent Co., Ltd.) for decalcification
at room temperature. The EDTA solution was replaced every three
days, and following three weeks in EDTA, when there was no
resistance when using a pin to puncture the bones, the metaphyseal
bones were paraffin-embedded and sectioned for hematoxylin and
eosin (H&E) staining. The right tibias were sectioned and
stored at 4°C for immunohistochemical staining (IHC).
Serological marker detection
Serum calcium (Ca), phosphorus (P) and alkaline
phosphatase (ALP) concentrations were determined using an automated
biochemical analyzer (Olympus AU2700; Olympus, Tokyo, Japan) at the
Affiliated Hospital of Guangdong Medical College (Zhanjiang,
China). Serum concentrations of proteins were determined using
ELISA kits (BGP ELISA kit; PICP ELISA kit and ICTP ELISA kit;
RapidBio, West Hills, CA, USA): Osteocalcin/bone
γ-carboxyglutamic-acid (GLA)-containing proteins (OC), procollagen
I C-terminal peptide (PICP) and type I collagen cross-linked
telopeptide (ICTP) expression was evaluated.
Measurement of BMD and bone
mechanics
The left femurs and fourth lumbar vertebrae were
sent to the Department of Nuclear Medicine, Guangzhou Overseas
Chinese Hospital (Guangzhou, China), affiliated to Southern Medical
University (Guangzhou, China), for examination by Lunar Prodigy
dual-energy X-ray absorptiometry (DXA; GE Healthcare Life Sciences,
Little Chalfont, UK). The placement positions of each femur and
lumbar were consistent. The scanning results were analyzed using
the small animal software supplied (combined with the Lunar Prodigy
dual energy X-ray; GE Healthcare, Madison, WI, USA). Subsequently,
the specimens were soaked in 50% alcohol, the femurs were assessed
using a three-point stress test and the bone biomechanics of the
fourth lumbar vertebrae were assessed with a lumbar compression
test.
Isolation of RNA and RT-qPCR
analysis
Total RNA was extracted from the cryopreserved bone
marrow cells, which were ground in liquid nitrogen into fine
powders using TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). β-actin was used as an internal
control. PCR primer sets (Thermo Fisher Scientific) were designed
and synthesized for the three proteins as follows: OPG forward,
5′-GCCCAGACGAGATTGAGAGA-3′ and reverse, 5′-ACGGTTTTGGGA
AAGTGGTA-3′; RANKL forward, 5′-CCGTGCAAAGGG AATTACAA-3′ and
reverse, 5′-GGATGTCGGCAGCAT TGAT-3′; β-actin forward,
5′-AGGGAAATCGTGCGT GACAT-3′ and reverse, 5′-AGGGAAATCGTGCGTGA
CAT-3′. The RT conditions were as follows: 42°C for 1 h and 0°C for
2 min (HiScript® 1st Strand cDNA Synthesis kit; Vazyme
Biotech Co., Ltd., Nanjing, China). Using these primer sets, the
predicted amplicons for OPG, RANKL and β-actin were 160, 150 and
150 bp, respectively. SYBR® Green-labeled products were
used (Bio-Rad Laboratories (Shanghai) Co., Ltd., Guangzhou, China).
The incorporation of SYBR Green into the PCR products was monitored
in real-time following each PCR cycle, resulting in the calculation
of the threshold cycle (Ct value), which defines the PCR cycle
number at which the exponential growth of PCR products begins.
The PCR cycling conditions were as follows: Initial
denaturation at 95°C for 5 min, followed by 40 cycles of
denaturation at 95°C for 15 sec, annealing at 60°C for 15 sec and
extension at 72°C for 32 sec (72°C for 32 sec also facilitated
detection of the fluorescence signal following each round of
extension). A melting curve analysis from 60 to 95°C was also
performed. Gene expression of OPG and RANKL was quantified relative
to β-actin as the internal control.
H&E staining and
immunohistochemistry
The third lumbar vertebrae and right tibias
underwent gradient ethanol dehydration, and were then embedded in
paraffin and cut into 5-µm sections for H&E staining (Guangzhou
Jinhuada Chemical Reagent Co., Ltd.). Following H&E staining,
the sections were observed using a Nikon E400 low-magnification
microscope (x40; Nikon Corp., Tokyo, Japan), and images were
captured with the DS-U1 digital camera built into the
microscope.
Right tibia slices were immunohistochemically
stained according to a previously described protocol (24), and images were recorded with a x250
magnification microscope camera (Olympus DP71; Olympus). The
staining intensity was assessed using Image-Pro Plus version 6.0
(Media Cybernetics, Rockvilled, MD, USA). The antibodies used were
as follows: OPG primary antibody (1:80; BA1475; Wuhan Boster
Biological Technology Co. Ltd., Wuhan, China; rabbit anti-rat OPG),
RANKL primary antibody (1:80; BA1323; Wuhan Boster Biological
Technology Co. Ltd.; rabbit anti-rat OPG ligand) and rabbit
anti-rat biotinylated secondary antibody (SP-0023; Beijing
Biosynthesis Biotechnology Co., Ltd., Beijing, China). The
antibodies used were as follows: OPG primary antibody (rabbit
anti-rat OPG polyclonal antibody at a dilution of 1:80, incubated
overnight at 4°C; cat. no. BA1475; Wuhan Boster Biological
Technology Co., Ltd., Wuhan, China); RANKL primary antibody (rabbit
anti-rat OPG ligand polyclonal antibody at a dilution of 1:80,
incubated overnight at 4°C; cat. no. BA1323; Wuhan Boster
Biological Technology Co. Ltd.) and rabbit anti-rat biotinylated
secondary antibody (at a dilution of 1:1,000 and incubated for 2 h
at 37°C; cat. no. SP-0023; Beijing Biosynthesis Biotechnology Co.,
Ltd., Beijing, China).
Statistical analysis
All experimental results are expressed as the mean ±
standard deviation, and were processed using SPSS software version
17.0 for Windows (SPSS, Inc., Chicago, IL, USA). All data were
initially tested for normality and homogeneity of variance. Data
among the groups were compared using one-way analysis of variance
and Student-Newman-Keuls analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
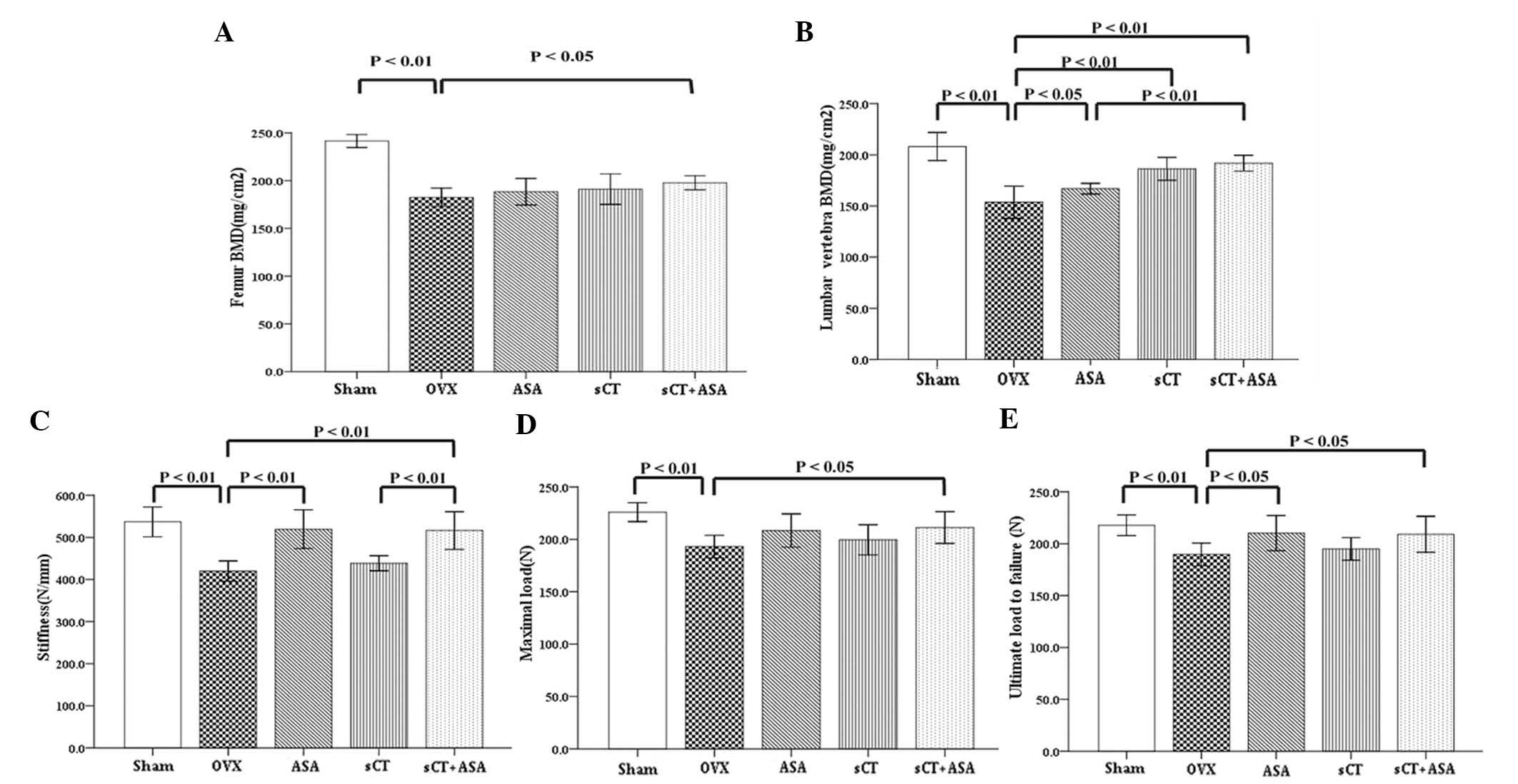
Combined treatment significantly enhances
BMD in OVX rats
At 14 weeks post-surgery, BMD in the femur (Fig. 1A) and fourth lumbar spine (Fig. 1B) of the OVX group was decreased,
by 24.48% and 26.18%, respectively, compared with that of the Sham
group (P<0.01). Compared with the OVX group at 12 weeks
following the commencement of treatment, rats that received ASA or
sCT monotherapy demonstrated no significant differences in the BMD
of the femur, while the combined sCT+ASA therapy resulted in an
8.42% increase in BMD (P<0.05). Compared with the OVX group, the
BMD of the fourth lumbar spine was increased by administration of
ASA alone (P<0.05) and significantly increased by a single
injection of sCT; however, there was no significant difference
detected between the OVX+ASA and OVX+sCT groups. With the
combination treatment of sCT+ASA, the BMD of the fourth lumbar
spine was significantly increased, by up to 24.90%, compared with
that of the OVX group, and greater than that of the OVX+ASA group
(P<0.05).
Combined treatment enhances femur bone
biomechanics in OVX rats
At 14 weeks post-surgery, all measurements of femur
bone biomechanics, including stiffness (Fig. 1C), maximal load (Fig. 1D) and ultimate failure load
(Fig. 1E), were decreased in the
OVX group compared with those of the Sham group (all P<0.01),
demonstrating the validity of the OVX rat model for osteoporosis.
Compared with the OVX group, at 12 weeks following commencement of
therapy, rats in the OVX+sCT group indicated no significant
improvement in femur bone biomechanics (P>0.05), while rats in
the OVX+ASA group benefited from an increase the femur ultimate
failure load (P<0.05) and a significant increase in stiffness
(P<0.01). Compared with the OVX group, combination therapy in
the OVX+sCT+ASA group increased the ultimate failure load and
maximal load of the femur (P<0.05) and significantly improved
femur stiffness in comparison to that of the sCT group (P<0.05).
As with measurements of femur bone biomechanics, all OVX rats
demonstrated significant losses in bone strength of the fourth
lumbar vertebrae, measured by stiffness, maximal load and ultimate
load compared with those of the Sham group; however, none of the
treatment groups exhibited any significant improvements in
vertebral bone strength compared with that of untreated OVX rats
(data not shown).
Combined treatment rescues bone
morphology of OVX rats
The third lumbar vertebrae and the right proximal
tibia of rats in all groups underwent H&E staining to
facilitate the observation of histological changes (Fig. 2). The cancellous bones of the rats
in the Sham group demonstrated neatly arranged and normal bone
trabeculae, which were bulky, plump and connected to a network
(Fig. 2A and F). The bone
trabeculae of rats in the OVX group were arranged sparsely, were
disorderly and indicated significant resorption, were thinner or
had disappeared altogether and the remaining connections were
incomplete (black arrow; Fig. 2B and
G). Distortions and fractures were common, the gaps became
larger and the bone layer under the osteoepiphysis was markedly
thinner (red arrow; Fig. 2B and
G). The bone trabecula arrangement in rats in the OVX+ASA group
was improved compared with that of the OVX group; the number of
trabecula was increased and trabecular connections were enhanced.
However, the trabecular rods in the OVX+ASA group were slightly
smaller, trabecular continuity was not significantly improved and
there were still apparent signs of resorption, fractures and
incomplete connections (black arrow). The bone layer under the
osteoepiphysis was recovered to a certain extent, but had not
reached normal levels (red arrow; Fig.
2C and H).
The bone trabeculae of rats in the OVX+sCT group
were more orderly arranged than those in the OVX group. The
trabecular number in the OVX+sCT group was not markedly increased,
but the trabecular bones were significantly thicker, although still
prone to fracture and resorption (black arrow). The bone layer
under the osteoepiphysis in rats treated with sCT was recovered to
a certain extent (red arrow; Fig. 2D
and I).
Compared with monotherapy and combined groups, the
trabecular bones were thickened, the number was increased and the
connections were compact. Fractures and absorption were reduced and
the bone layer under the osteoepiphysis was also improved to a
certain extent (red arrow; Fig. 2E and
J). The bone trabeculae of rats in the OVX+sCT+ASA group were
more neatly and uniformly arranged, compared with those in the OVX
group, the structure was clear and the network structure and
trabecular morphology was almost fully recovered. Furthermore, the
trabecular bones were thickened, the number was increased, and the
connections were compact. Fractures and absorption were reduced,
and the bone layer under the osteoepiphysis was also improved to a
certain extent (red arrow; Fig. 2E and
J).
Combined treatment rescues expression of
bone metabolic biomarkers of OVX rats
The serological analysis results of the various
groups are shown in Fig. 3. There
was no significant difference in serum Ca and P levels amongst any
group (P>0.05; Fig. 3A and B).
As shown in Fig. 3C–E and Table I, levels of serum bone metabolism
indicators, including ALP, OC, PICP and ICTP, were significantly
upregulated in the OVX group compared with those of the Sham group
(P<0.05 for OC; P<0.01 for PICP and ICTP). A significant
decline in ALP levels was detected in all the treated groups,
compared with those of the OVX group (P<0.05); whereas compared
with the Sham group, ALP was elevated in all treatment groups
(P<0.05) and increased most markedly in the OVX+sCT+ASA group.
Compared with the OVX group, serum levels of OC, PICP and ICTP in
each treatment group were decreased (P<0.05 for OC; P<0.01
for PICP and ICTP), and the largest decrease was observed in the
combined treatment group. There were no statistically significant
differences detected in the serum levels of OC, PICP or ICTP
between any treatment group and the Sham group.
 | Table IEffects of combined ASA and sCT
treatment on serum parameters. |
Table I
Effects of combined ASA and sCT
treatment on serum parameters.
| Serum
component | Sham | OVX | OVX+ASA | OVX+sCT | OVX+sCT+ASA |
|---|
| Ca (mmol/l) | 2.67±0.27 | 2.47±0.14 | 2.55±0.22 | 2.52±0.20 | 2.45±0.25 |
| Pi (mmol/l) | 1.95±0.15 | 2.09±0.37 | 2.04±0.24 | 2.07±0.19 | 2.12±0.13 |
| ALP (U/l) | 87.65±32.99 |
202.96±55.16b |
126.67±32.17ad |
136.93±18.60ad |
148.34±29.07bd |
| PICP (µg/l) | 7.59±1.24 | 9.62±1.04a | 7.92±1.81c | 7.76±1.29c | 7.71±0.65c |
| ICTP (µg/l) | 6.20±0.48 | 7.19±0.70b | 6.39±0.78d | 5.67±0.40d | 5.62±0.41d |
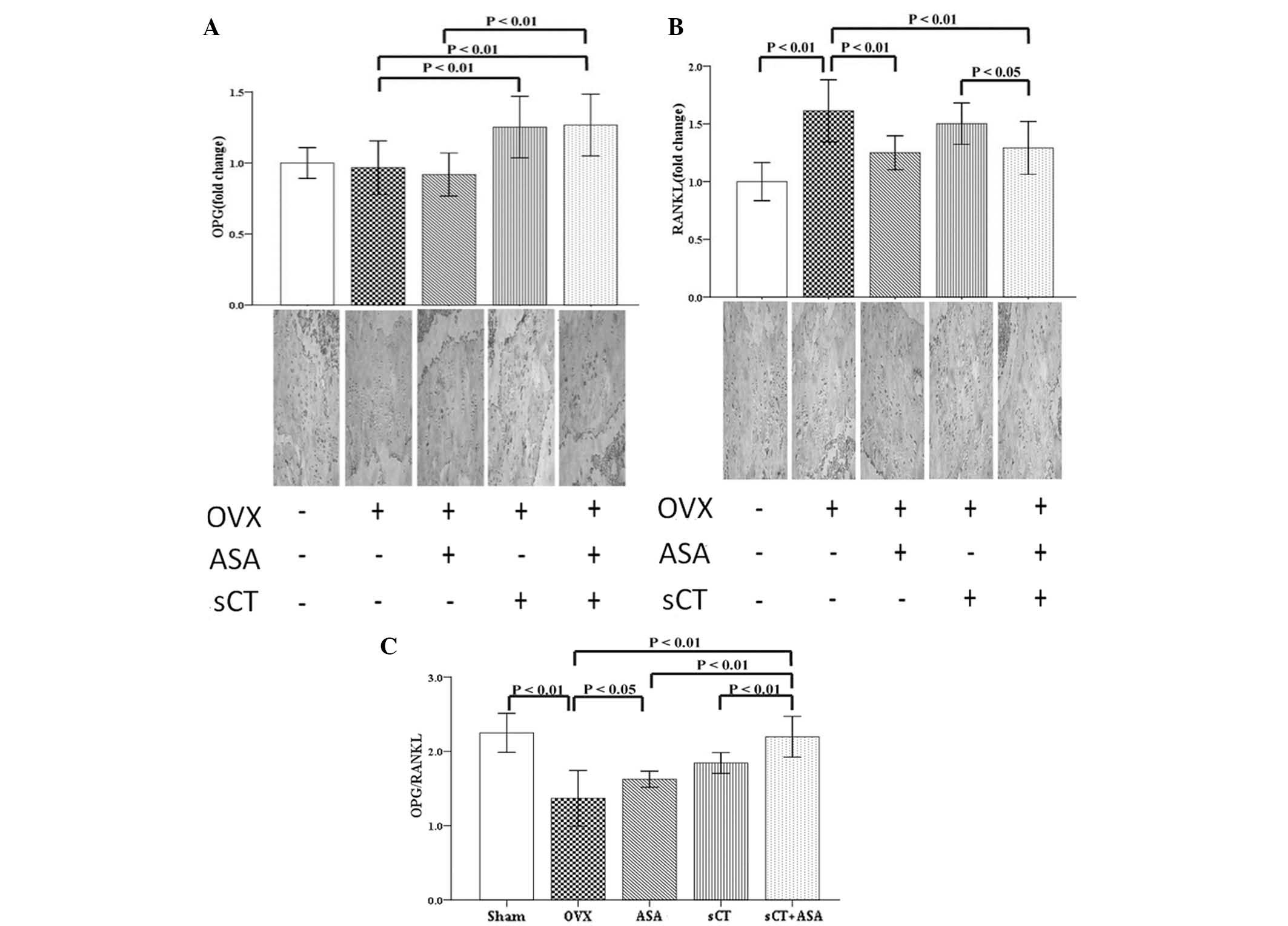
Combined treatment attenuates the
decrease in OPG/RANKL ratio induced in OVX rats
In order to evaluate the changes in gene expression
of OPG and RANKL following ovariectomy and drug treatments of OVX
rats, RT-qPCR was used to measure the relative quantities of their
mRNA expression levels in bone marrow cells obtained from rat
femurs (Fig. 4). As shown in
Fig. 4B, the mRNA expression of
RANKL was increased significantly in rats of the OVX group
(P<0.05 vs. the Sham group). However, no significant differences
were observed in OPG gene expression between the Sham and OVX
groups (P>0.05; Fig. 4A). The
OPG/RANKL ratio, an index of osteoclastogenic inhibition, was
significantly decreased following ovariectomy (P<0.01 vs. Sham
group; Fig. 4C). The mRNA
expression of RANKL was significantly decreased in the OVX+ASA and
OVX+sCT+ASA groups, compared with that of the Sham group
(P<0.05; Fig. 4B). However, no
significant difference was observed in OVX+sCT rats (P>0.05;
Fig. 4B). The mRNA expression of
OPG was increased significantly following sCT and sCT+ASA
treatments (P<0.01; Fig. 4A),
and compared with that of the OVX group, mRNA expression of OPG was
increased with ASA treatment, but the difference was not
significant (P>0.05; Fig. 4A).
The OPG/RANKL ratio increased with all drug treatments (P<0.01;
Fig. 4C), and the ratios were
similar between the ASA and sCT groups. The combination treatment
(sCT+ASA) induced the highest OPG/RANKL ratio, but there was no
statistically significant difference between this ratio and that of
the other treatment groups.
Combined treatment rescues RANKL and OPG
protein expression in the proximal tibiae
Cells positive for OPG and RANKL exhibited brown
staining by IHC in the proximal tibiae of all groups. Significant
RANKL and OPG protein staining was observed in all chondrocytes of
the epiphyseal growth plate, including the articular cartilage.
Control sections that were stained with the secondary antibody only
exhibited no staining, confirming the specificity of the response
(Fig. 5). IHC staining revealed
that the levels of RANKL in the right proximal tibia of the OVX
group were increased significantly, compared with those of the Sham
group (P<0.01; Fig. 5B).
However, no difference in OPG protein expression was detected
between the two groups (P>0.05; Fig. 5A). These results indicated that the
OPG/RANKL ratio was significantly decreased following ovariectomy
(P<0.01; Fig. 5C). None of the
parameters tested differed significantly between the sCT-treated
group and the OVX group (P>0.05; Fig. 5B). In the OVX+ASA and OVX+sCT+ASA
groups, the levels of RANKL in proximal tibia were decreased
significantly, when compared with those of the OVX group
(P<0.01; Fig. 5B). In the
OVX+sCT and OVX+sCT+ASA groups, the levels of OPG in the proximal
tibia were significantly increased, compared with those of the OVX
group (P<0.01; Fig. 5A). No
significant differences in OPG protein expression were observed in
the ASA group (P>0.05; Fig.
5A). The lower OPG/RANKL ratio detected in the OVX group was
reversed in all of the treatment groups, but the OPG/RANKL ratio
was highest in the OVX+sCT+ASA (Fig.
5C).
Discussion
Following the increase in the numbers and proportion
of the elderly population in more economically developed countries,
osteoporosis has become a significant global public health concern.
Once osteoporotic fractures occur, they can result in substantial
economic losses and emotional burden to the affected individuals
and their families. Furthermore, since much of the cost of
healthcare for elderly patients is borne by government agencies,
there is a significant societal burden associated with
osteoporosis. Nasal sprays containing sCT are relatively
inexpensive and have previously been approved for use in the
treatment of osteoporosis in multiple countries; however, recently
two experts at the US Food and Drug Association have recommended
that the marketing of sCT for osteoporosis be stopped due to a lack
of demonstrated efficacy (25).
The most effective bone-sparing drugs for use in the treatment of
osteoporosis tend to be relatively expensive and may only be
approved by insurers in patients with more severe symptoms of OA
(26). Therefore, the development
of cost-effective treatments for osteoporosis is a high priority
for biomedical research.
It has previously been reported that once female
rats are ovariectomized, the major manifestations of osteoporosis
observed are an increase in the number of osteoclasts, enhanced
osteolysis and high-turnover bone metabolism, with bone resorption
surpassing bone formation. Thus, there are numerous similarities
between the alterations to bone metabolism observed in human
postmenopausal osteoporosis and those of ovariectomized (OVX) rats,
and OVX rats are therefore considered an effective animal model of
osteoporosis in menopausal women (22,27).
The current study was designed to explore the use of combined
therapy of sCT and ASA to prevent bone loss in estrogen-deficient
rats. OVX rats were treated with a combination of sCT and ASA for
12 weeks. The results revealed that the strength of the femoral
shaft bone was increased (but not the load-bearing capacity of the
lumbar spine) and trabecular bone structure had been improved. The
combination treatment (sCT+ASA) was also able to reduce the
expression of bone turnover markers OC, PICP and ICTP. It was also
found that sCT+ASA increased OPG gene expression and reduced RANKL
gene expression in bone marrow cells.
BMD measurement is considered to be the standard
test for the diagnosis of osteoporosis and DXA has been used in
previous studies to measure BMD in rats (28,29).
In the present study, at 14 weeks following ovariectomy, the lumbar
spine and femoral BMD in OVX rats was significantly decreased,
compared with that of the Sham group (P<0.01), indicating that
the bone mass was reduced. Combined treatment of OVX rats with
sCT+ASA increased the bone mass in the lumbar spine (P<0.01) and
femur (P<0.05), compared with that of the OVX group. However,
when sCT or ASA treatment was used alone, femoral BMD was not
significantly improved (P>0.05). This may have been due to the
major part of the femur being composed of compact bone, and the
change or loss of compact bone in the femur following ovariectomy
was slow. Therefore, when dual-energy X-ray was used to measure the
total femur BMD, following treatment with sCT or ASA alone, the
change in femoral BMD was smaller. Similarly to the results of a
study by Kavuncu et al (30), sCT treatment alone increased rat
lumbar spine BMD (P<0.01), while ASA treatment alone did not
(P<0.05).
BMD can only indicate the degree of bone
mineralization, which represents one component of bone strength
(31). Bone strength is also
reflected by bone quality. Bone quality parameters include damage
accumulation, bone microarchitecture and bone mineralization
(32). The combined sCT+ASA
treatment enhanced femur stiffness (P<0.01), maximal load
(P<0.05) and ultimate failure loads (P<0.05). ASA treatment
increased femoral stiffness (P<0.01) and ultimate failure load
(P<0.05), perhaps as ASA was able to improve the bone trabeculae
and increase the quantity of cortical bone in OVX rats (20), while sCT treatment alone failed to
enhance the various parameters of the femur. In humans, calcitonin
(CT) treatment alone is not effective in the long-term reduction of
hip fracture risk; this may be due to the fact that the metabolism
of cancellous bone in humans is more vulnerable to the impact of
bone metabolism changes than cortical bone (33,34).
However, in OVX rats, sCT appears to be effective in the prevention
of cancellous bone loss (35).
Whether sCT and ASA were used alone or in
combination, there was no significant effect on the biomechanical
parameters of the rat lumbar bone. There are several reasons that
may explain this result. Rat lumbar motion differs from that of
human upright walking, therefore the rat lumbar spine is not the
main axial load-bearing bone. In addition, the rat lumbar
compression test indicates that there may be certain defects in the
drug treatments. The ability of bone to withstand pressure also
depends on the quality and arrangement of the trabecular bone
(36,37). Histological methods indicated that
the trabeculae of cancellous bones in OVX rats were sparse,
fractured and disordered in arrangement. At 12 weeks post-treatment
with sCT and ASA, the trabecular bone structure in the tibia and
lumbar spine was significantly improved compared with that of
treatment with either drug alone.
DXA measurement is considered to be the ‘gold
standard’ for the diagnosis of osteoporosis, recognized by the
international academic and medical communities. DXA may directly
reflect the BMD, but not all individuals with low BMD have
fractures, and relying solely on BMD changes underestimates the
degree of osteoporotic fracture risk (38). Bone turnover markers, which do not
depend upon BMD, have been widely used to assess bone
reconstruction and to assess the effects of drugs on bone
metabolism in vivo. The biochemical indicators primarily
used for evaluating bone formation are OC and PICP, and a marker of
bone resorption is ICTP. The results of the present study indicated
that the levels of OC (P<0.01), PICP (P<0.05) and ICTP
(P<0.01) were all increased in the OVX group. This may be due to
the lack of estrogen resulting in hyperactivity in osteoclastic
bone resorption and leading to a subsequent increase in bone
resorption markers (39).
Regardless of whether sCT and ASA were used alone or in
combination, they were able to inhibit the high bone turnover
observed in OVX rats; however, there was no significant difference
between the effectiveness of the treatments, alone or in
combination.
During the processes of macrophage/monocyte
differentiation into osteoclasts and osteoclast activation, the
differentiation and maturation of osteoclasts are inseparable from
activation of the OPG/RANKL/RANK system (40). When osteoporosis occurs, there are
differing levels of disorder detected in the expression of OPG and
RANKL (41). Previous studies have
found that a calcitonin receptor-like receptor (CLR) is expressed
on the osteoblast surface (42)
and that CLR has a high affinity for calcitonin gene-related
peptide (CGRP), which can exhibit pharmacological effects similar
to those of CT (43). CT promotes
cartilage formation through the ERK1/2 signaling pathway (44), and CGRP is able to promote the
expression of the osteoblast OPG gene (45). In aged rats administered an agonist
of prostaglandin E, RANKL gene expression in the rat vertebrae was
increased, while OPG was unaffected. In cultured bone marrow cells
in vitro, prostaglandin E agonists increased RANKL gene
expression in bone marrow cells and reduced OPG gene expression
(46). Prostaglandin production is
primarily mediated by COX-2 in osteoblasts (47). ASA as a non-selective COX inhibitor
and is therefore able to inhibit prostaglandin production.
Long-term ASA treatment may systemically alter multiple serum
markers, reducing RANKL and increasing OPG in OVX mice.
In the current study, it was demonstrated that once
OVX rats were treated with a combination of sCT and ASA, OPG gene
expression in bone marrow cells and OPG protein expression in the
tibia metaphysis were increased (P<0.01), while RANKL gene
expression in bone marrow cells (P<0.05) and RANKL protein
expression in the tibial metaphysis (P<0.01) were reduced. In
addition, the OPG/RANKL ratio, which reflects osteoblast activity,
was increased (P<0.01). However, following treatment with ASA
alone, it was found that only RANKL expression was decreased
(P<0.05), while OPG expression was unaffected (P>0.05).
Following sCT treatment alone, it was found that only OPG
expression was increased (P<0.01) and RANKL expression was
unaffected (P>0.05).
The results of the present study demonstrated that
sCT (2 U/kg/d) and ASA (34.4 mg/kg/d) in combination prevented
trabecular bone loss in OVX rats (as evidenced by a higher BMD in
the femur and vertebrae), improved the structure of the trabecular
bone in vertebrae, increased femoral shaft strength and reduced
serum markers of bone turnover. The combined therapy increased OPG
gene expression in bone marrow cells and OPG protein expression in
the tibial metaphysis in OVX rats, while reducing RANKL gene
expression in bone marrow cells and RANKL protein expression in the
tibial metaphysis, resulting in a significant elevation of the
OPG/RANKL ratio (P<0.01). At the protein level, it was apparent
that sCT and ASA in vivo impacted distinct factors in the
OPG/RANKL/RANK pathway to inhibit bone resorption: ASA monotherapy
only lowered RANKL protein expression in bone marrow cells, while
CT monotherapy only increased OPG protein expression. However,
combination therapy (sCT+ASA) lowered RANKL protein expression and
increased OPG protein expression. These results suggested a
synergistic effect of the two drugs that may explain the superior
performance of combination therapy in increasing femur bone
strength, normalizing morphology of the trabecular networks in
vertebrae and increasing BMD in the femurs and vertebrae.
It was therefore hypothesized that sCT and ASA
combination therapy may regulate osteoclast activity and inhibit
bone resorption through the OPG/RANKL/RANK pathway, and these
experiments may provide the basis for future clinical trials. ASA
is cheap, sCT is relatively inexpensive and ASA+sCT combined
therapy is suggested to have fewer adverse reactions compared with
bisphosphonates or hormone-replacement therapies. However, the
safety and efficacy of ASA+sCT combination therapy requires further
assessment in clinical trials.
Acknowledgments
The present study was supported by a grant from the
Project of Science and Technology of Guangdong Province (no.
2010B031600288).
References
|
1
|
Raisz LG: Pathogenesis of osteoporosis:
concepts, conflicts, and prospects. J Clin Invest. 115:3318–3325.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu XH, Kirschenbaum A, Yao S and Levine
AC: Interactive effect of interleukin-6 and prostaglandin E2 on
osteoclastogenesis via the OPG/RANKL/RANK system. Ann NY Acad Sci.
1068:225–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Udagawa N, Takahashi N, Akatsu T, et al:
Origin of osteoclasts: mature monocytes and macrophages are capable
of differentiating into osteoclasts under a suitable
microenvironment prepared by bone marrow-derived stromal cells.
Proc Natl Acad Sci USA. 87:7260–7264. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kung YY, Felge U, Sarosi I, et al:
Activated T cells regulate bone loss and join destruction in
adjuvant arthritis through osteoprotegerin ligand. Nature.
402:304–309. 1999. View
Article : Google Scholar
|
|
6
|
Simonet WS, Lacey DL, Dunstan CR, et al:
Osteoprotegerin: a novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jacobs HS: Postmenopausal hormone
replacement therapy and breast cancer. Medscape Women’s Health.
5:E22000.
|
|
8
|
Grady D, Wenger NK, Herrington D, et al:
Postmenopausal hormone therapy increases risk for venous
thromboembolic disease. The heart and estrogen/progestin
replacement study. Ann Intern Med. 132:689–696. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rossouw JE, Anderson GL, Prentice RL, et
al Writing Group for the Women’ Health Initiative Investigators:
Risks and benefits of estrogen plus progestin in healthy
postmenopausal women: principal results From the Women’s Health
Initiative randomized controlled trial. JAMA. 288:321–333. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luckman SP, Hughes DE, Coxon FP, et al:
Nitrogen-containing bisphosphonates inhibit the mevalonate pathway
and prevent post-translational prenylation of GTP-binding proteins,
including Ras. J Bone Miner Res. 13:581–589. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pechalova P, Bakardjiev A, Zaprianov Z, et
al: Bisphosphonate-associated osteonecrosis of the jaws – report of
three cases in Bulgaria and review of the literature. Acta Clin
Croat. 50:273–279. 2011.
|
|
12
|
Andreotti G, Méndez BL, Amodeo P, et al:
Structural determinants of salmon calcitonin bioactivity: The role
of the Leu-based amphipathic alpha-helix. J Biol Chem.
281:24193–24203. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen Y, Li M and Wronski TJ: Calcitonin
provides complete protection against cancellous bone loss in the
femoral neck of ovariectomized rats. Calcif Tissue Int. 60:457–461.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ogawa K, Hori M, Takao R and Sakurada T:
Effects of combined elcatonin and alendronate treatment on the
architecture and strength of bone in ovariectomized rats. J Bone
Miner Metab. 23:351–358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reginster JY: Management of high turnover
osteoporosis with calcitonin. Bone. 13(Suppl 2): S37–S40. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solheim LF, Rönningen H and Langeland N:
Effects of acetylsalicylic acid on heterotopic bone resorption and
formation in rats. Arch Orthop Trauma Surg. 105:142–145. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carbone LD, Tylavsky FA, Cauley JA, et al:
Association between bone mineral density and the use of
nonsteroidal anti-inflammatory drugs and aspirin: impact of
cyclooxygenase selectivity. J Bone Miner Res. 18:1795–1802. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vestergaard P, Hermann P, Jensen JE, et
al: Effects of paracetamol, nonsteroidal anti-inflammatory drugs,
acetylsalicylic acid and opioids on bone mineral density and risk
of fracture: results of the Danish Osteoporosis Prevention Study
(DOPS). Osteoporos Int. 23:1255–1265. 2012. View Article : Google Scholar
|
|
19
|
Kataoka T, Shinohara N, Takayama H, et al:
Concanamycin A, a powerful tool for characterization and estimation
of contribution of perforin- and Fas-based lytic pathways in
cell-mediated cytotoxicity. J Immunol. 156:3678–3686.
1996.PubMed/NCBI
|
|
20
|
Yamaza T, Miura Y, Bi Y, et al:
Pharmacologic stem cell based intervention as a new approach to
osteoporosis treatment in rodents. PLoS One. 3:e26152008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin SK, Kok SH, Kuo MY, et al: Sequential
expressions of MMP-1, TIMP-1, IL-6, and COX-2 genes in induced
periapical lesions in rats. Eur J Oral Sci. 110:246–253. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Frost HM and Jee WS: On the rat model of
human osteopenias and osteoporoses. Bone Miner. 18:227–236. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui L, Wu T, Liu YY, et al: Tanshinone
prevents cancellous bone loss induced by ovariectomy in rats. Acta
Pharmacol Sin. 25:678–684. 2004.PubMed/NCBI
|
|
24
|
Van Poznak C, Cross SS, Saggese M, et al:
Expression of osteoprotegerin (OPG), TNF related apoptosis inducing
ligand (TRAIL) and receptor activator of nuclear factor κB ligand
(RANKL) in human breast tumours. J Clin Pathol. 59:56–63. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lowry F: FDA panel says to stop marketing
salmon calcitonin for osteoporosis. Medscape Medical News. March
6–2013, http://www.medscape.com/viewarticle/780323.
Accessed August 10, 2013.
|
|
26
|
Byrne P: Quality and outcomes framework
indicators for osteoporosis. Prescriber. 23:14–22. 2012. View Article : Google Scholar
|
|
27
|
de Freitas PH, Hasegawa T, Takeda S, et
al: Eldecalcitol, a second-generation vitamin D analog, drives bone
minimodeling and reduces osteoclastic number in trabecular bone of
ovariectomized rats. Bone. 49:335–342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanis JA, Melton LJ III, Christiansen C,
et al: The diagnosis of osteoporosis. J Bone Miner Res.
9:1137–1141. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rico H, Valencia MJ, Villa LF, et al:
Calcitonin versus clodronate in the prevention of
ovariectomy-induced osteopenia in rats. Clin Rheumatol. 19:47–50.
2000.PubMed/NCBI
|
|
30
|
Kavuncu V, Sahin S, Baydas G, et al: A
comparison of estrogen and two different doses of calcitonin in
ovariectomized rats. Yonsei Med J. 44:508–516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Friedman AW: Important determinants of
bone strength: beyond bone mineral density. J Clin Rheumatol.
12:70–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
NIH Consensus Development Panel on
Osteoporosis Prevention, Diagnosis, and Therapy: Osteoporosis
prevention, diagnosis, and therapy. JAMA. 285:785–795. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li XJ, Jee WS and Li YL: Flurbiprofen
enhances growth and cancellous and cortical bone accumulation in
rapidly growing long bones. Bone. 10:35–44. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dempster DW, Birchman R, Xu R, et al:
Temporal changes in cancellous bone structure of rats immediately
after ovariectomy. Bone. 16:157–161. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li M, Shen Y, Burton KW, et al: A
comparison of the skeletal effects of intermittent and continuous
administration of calcitonin in ovariectomized rats. Bone.
18:375–380. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van der Linden JC, Homminga J, Verhaar JA
and Weinans H: Mechanical consequences of bone loss in cancellous
bone. J Bone Miner Res. 16:457–465. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zioupos P, Cook RB and Hutchinson JR: Some
basic relationships between density values in cancellous and
cortical bone. J Biomech. 41:1961–1968. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee J and Vasikaran S: Current
recommendations for laboratory testing and use of bone turnover
markers in management of osteoporosis. Ann Lab Med. 32:105–112.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yasumizu T, Okuno T, Fukada Y and Hoshi K:
Age-related changes in bone mineral density and serum bone-related
proteins in premenopausal and postmenopausal Japanese women. Endocr
J. 47:103–109. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Trouvin AP and Goëb V: Receptor activator
of nuclear factor-κB ligand and osteoprotegerin: maintaining the
balance to prevent bone loss. Clin Interv Aging. 5:345–354.
2010.
|
|
41
|
Liu HY, Wu AT, Tsai CY, et al: The balance
between adipogenesis and osteogenesis in bone regeneration by
platelet-rich plasma for age-related osteoporosis. Biomaterials.
32:6773–6780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Villa I, Mrak E, Rubinacci A, et al: CGRP
inhibits osteoprotegerin production in human osteoblast-like cells
via cAMP/PKA-dependent pathway. Am J Physiol Cell Physiol.
291:C529–C537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huebner AK, Schinke T, Priemel M, et al:
Calcitonin deficiency in mice progressively results in high bone
turnover. J Bone Miner Res. 21:1924–1934. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen WH, Zeng R, Lo WC, et al: The role of
the ERK1/2 pathway as an alternative to the aging-diminished cyclic
AMP pathway in calcitonin-mediated chondrogenesis in human nucleus
pulposus. Biomaterials. 33:8256–8264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu J, Kauther MD, Hartl J, et al: Effects
of alpha-calcitonin gene-related peptide on osteoprotegerin and
receptor activator of nuclear factor-κB ligand expression in MG-63
osteoblast-like cells exposed to polyethylene particles. J Orthop
Surg Res. 5:832010. View Article : Google Scholar
|
|
46
|
Downey ME, Holliday LS, Aguirre JI and
Wronski TJ: In vitro and in vivo evidence for stimulation of bone
resorption by an EP4 receptor agonist and basic fibroblast growth
factor: Implications for their efficacy as bone anabolic agents.
Bone. 44:266–274. 2009. View Article : Google Scholar
|
|
47
|
Raisz LG: Potential impact of selective
cyclooxygenase-2 inhibitors on bone metabolism in health and
disease. Am J Med. 110(Suppl 3A): 43S–45S. 2001. View Article : Google Scholar : PubMed/NCBI
|