Introduction
Epilepsy is a common neurological disease,
characterized by unpredictable recurrent seizures. The disease
affects all age groups and, in the majority of cases, the cause is
unknown. Susceptibility to epilepsy may be genetic, however,
environmental factors, including stroke, brain cancer, brain
trauma, and drug and alcohol misuse are also risk factors (1). Seizures are the direct result of the
excessive and abnormal discharge of neurons (2). The majority of antiepileptic drugs
work by interfering with ion channel function, to reduce or inhibit
excitatory neurophysiological activity. However, they rarely affect
epileptogenesis (3).
Previous studies have indicated that the mammalian
target of rapamycin (mTOR) signaling pathway may be important in
the course of epilepsy and epileptogenesis, and may offer a
promising molecular target in epilepsy therapy. mTOR, a
serine/threonine kinase, is a critical regulator of neuronal
functions, including cellular metabolism, survival, growth,
proliferation and plasticity (4).
It can be activated through the phosphoinositide 3-kinase
(PI3K)/Akt and Ras/mitogen-activated protein kinase kinase
(MEK)/extracellular signal-regulated kinase (ERK) pathways, and
inhibited by the energy sensor, 5′adenosine
monophosphate(AMP)-activated protein kinase (5). mTOR exists as two complexes, mTORC1
and mTORC2. mTORC1 activates the p70 ribosomal protein S6 kinase
and inactivates eIF4E binding protein 1, to promote cell growth,
however, the role of mTORC2 remains to be fully elucidated
(6,7).
The peroxisome proliferator-activated receptors
(PPARs) constitute a class of nuclear transcription factors
belonging to the nuclear receptor superfamily. There are three
subtypes, α, β (or δ) and γ, among which PPAR-γ has been
investigated extensively. PPAR-γ is involved in immune regulation,
differentiation, glucose metabolism and the synthesis of
triglycerides. It is expressed in the central nervous system and
has a neuroprotective role (8).
Natural ligands of PPAR-γ include the fatty acid metabolites,
9-hydroxyoctadecadienoic acid and 13-hydroxyoctadecadienoic acid,
12-hydroxy-5,8,10,14 eicosatetraenoic acid, 15-hydroxy-5,8,11,14
eicosatetraenoic acid and prostaglandins (9). Synthetic ligands of PPAR-γ include
predominantly thiazolidinedione ketones. Among these, pioglitazone
and rosiglitazone are used clinically, and ciglitazone and
troglitazone are used experimentally. The thiazolidinedione ketones
also comprise non-steroidal anti-inflammatory drugs, including
diclofenac and anti-nfan (10).
The association between PPAR-γ agonists and the mTOR
pathway is complex and remains to be fully elucidated. Indirect
evidence suggests that PPAR-γ agonists may suppress the mTOR
pathway (11), whereas other
studies have suggested they may function as activators (12,13).
The present study aimed to investigate the effect of the
pioglitazone PPAR-γ agonist on the mTOR pathway in
pentylenetetrazol (PTZ)-induced status epilepticus (SE), and
examine the expression levels of the IL-1β and IL-6 inflammatory
factors.
Materials and methods
All the experimental protocols and procedures were
approved by the Ethics Committee of Harbin Medical University
(Harbin, China).
SE animal model and seizure
monitoring
Adult male Sprague-Dawley rats (n=126; Laboratory
Animal Center of the Second Affiliated Hospital of Harbin Medical
University) weighing 200–300 g were used in the present study. The
rats were acclimatized in a dedicated animal room under a 12 h
light/dark cycle at 22±2°C, and allowed free access to food and
water.
SE was induced by repetitive intraperitoneal
injections of subconvulsive doses of PTZ (10 mg/ml in saline;
Sigma-Aldrich, St. Louis, MO, USA), as previously described
(14–17). Briefly, a dose of 40 mg/kg was
administered initially, followed by 20 mg/kg 10 min later.
Subsequent doses of 10 mg/kg were administered to the rats at 10
min intervals until SE occurred (18). This procedure led to an SE lasting
at least 30 min and consisting of prolonged episodes of seizures,
interrupted by postictal depression phases with no return to a
quadruped posture or consciousness. When SE lasted for ≥1 h, or if
death appeared imminent, intraperitoneal injection of SE diazepam
(4 mg/kg; Sigma-Aldrich), was administered. If a rat died during
the establishment of SE, another rat was randomly selected for
replacement.
Experimental procedure and drug
administration
The present study determined the time course of
activation of the mTOR signaling pathway in SE. The rats were
randomly distributed into either an SE group or a control group
(n=21 each). In each group, three rats were sacrificed by
decapitation under anesthesia with intraperitoneal sodium
pentobarbital (20 mg/ml in saline; 40 mg/kg; Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) at each of
seven specific time points following modeling, at 1, 8, and 16 h,
and on days 2, 3, 5 and 7. Western blot analyses were performed to
detect the protein levels of mTOR, phosphorylated (p-)mTOR, S6 and
p-S6 subsequent to acquisition of hippocampal tissues. The peak
time-period of mTOR signaling pathway activation was then used in
the subsequent experiments.
The effect of the pioglitazone PPAR-γ agonist on the
activation of the mTOR signaling pathway in SE was then assessed.
The rats were randomly assigned to four groups (11 rats/group), and
received daily intragastric treatments for 5 days, as follows:
Normal control group, 10 mg/kg saline (vehicle);
pioglitazone-treated group, 10 mg/kg pioglitazone (Sigma-Aldrich);
vehicle+SE goup, 10 mg/kg saline, with SE triggered 30 min
following the final intraperitoneal injection of PTZ; and the
pioglitazone+SE group, 10 mg/kg pioglitazone, with SE similarly
triggered 30 min after the final intraperitoneal injection of
PTZ.
All the rats were sacrificed at the time-point when
mTOR signaling changes reached their peak, as determined in the
first experiment. The entire hippocampal tissues were obtained,
Nissl staining was performed to detect neuronal loss in the CA3
area. An enzyme-linked immunosorbent assay (ELISA) was performed to
quantify the levels of IL-1β and IL-6 and immu-nohistochemical
detection was performed to assess the levels of p-mTOR in the CA3
area. Western blot analysis was performed to quantify the protein
levels of mTOR, p-mTOR, S6 and p-S6.
ELISA for the detection of IL-1β and IL-6
in hippocampal tissues
The levels of IL-1β and IL-6 in the hippocampal
tissues 24 h after SE were analyzed using an ELISA. The procedures
were performed, according to the manufacturer’s instructions
(Neobioscience Technology, Beijing, China).
The entire hippocampus was homogenized, ground and
centrifuged for 30 min (4°C; 10,000 × g). The supernatant was then
collected. The protein concentrations were determined using the
Bradford method (19). Equal
quantities of the lysates were used for the analyses of IL-1β and
IL-6, with values expressed as pg/ml protein.
Immunohistochemistry
Staining of p-mTOR (S2448) was performed, as
follows: Following the embedding of the hippocampal tissues in
paraffin (Shanghai Hualing Recovery Appliance Factory, Shanghai,
China), sections (5 µm) from the CA3 area were transferred
to slides (Leica RM 2135, Leica Microsystems GmbH, Wetzlar,
Germany) and deparaffinized. The slides were then incubated with
citrate buffer (pH 6; Beijing Solarbio Science & Technology
Co., Ltd.) for 5 min in a microwave oven twice (with a 10 min
interval between) and cooled to room temperature. The slides were
then incubated with rabbit p-mTOR polyclonal antibody (Ser2448;
1:65; YP0176; ImmunoWay Biotechnology Company, Newark, DE, USA) at
4°C overnight, and then in the anti-rabbit secondary immunoglobulin
(Ig)G antibody conjugated to horseradish peroxidase (PV6001;
OriGene Technologies, Inc., Beijing, China) for 30 min. A
3,3′-diaminobenzidine kit (ZLI-9017; OriGene Technologies, Inc.)
was used for visualization, and slides were counterstained with
hematoxylin (Beijing Solarbio Science & Technology Co., Ltd.).
The average integrated optical density value was obtained by
analyzing five fields per slide using Image-Pro Plus software (v.
6.0; Media Cybernetics, Carlsbad, CA, USA).
Nissl staining
Nissl staining was performed, as described
previously (20). Briefly,
following deparaffinization in xylene (Tianjin Fuyu Fine Chemical
Co., Ltd., Tianjin, China) and hydration in 100% alcohol twice for
5 min, 95% alcohol for 3 min and 70% alcohol for 3 min, the
sections were stained in 0.1% cresyl violet solution
(Sigma-Aldrich) for 10 min at room temperature. The slides were
then rinsed rapidly in distilled water, differentiated in 95% ethyl
alcohol for 2–30 min, in order to determine the optimal time of 10
min, and checked under a microscope. Slides were then dehydrated
and mounted. The average integrated optical density was obtained by
analyzing five fields per slide using Image-Pro Plus software (v.
6.0). The nissl bodies appeared blue-purple and cell nuclei
appeared blue.
Western blot analysis
Western blot analysis was performed in accordance
with previously desctribed methods (21). The hippocampal tissues were
homogenized and lysed with radioimmunoprecipitation lysis buffer
(150 µl/20 mg tissue; Beyotime Institute of Biotechnology,
Haimen, China) and phenylmethanesulfonylfluoride (Beyotime
Institute of Biotechnology). Following centrifugation at 10,000 × g
for 30 min at 4°C, the supernatant was collected. Equivalent
quan-tites of protein (10 µl/lane) were resolved via 10%
SDS-PAGE (Beyotime Institute of Biotechnology) and transferred onto
a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA).
Western blot analyses were performed using the
following rabbit polyclonal antibodies from ImmunoWay Biotechnology
Company: mTOR (1:500; YT2915), p-mTOR (Ser2448; 1:500; YP0176), S6
(1:500; YT4139), p-S6 (1:500; YP0893) and β-actin antibodies
(1:1,000; YT0099) for 2 h at 37°C or overnight at 4°C. The membrane
was then incubated with the secondary antibody, alkaline
phosphatase-conjugated anti-rabbit IgG (1:500; ZB2308; OriGene
Technologies, Inc.) and detected using Western Blue Stabilized
Substrate for Alkaline Phosphatase (Promega Corporation, Madison,
WI, USA). The protein levels were normalized against β-actin, and
the phosphorylation of the proteins were compared with the total
protein.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). Unless
otherwise stated, all data were analyzed using Student’s t-test or
one way analysis of variance. The data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Behavioral changes and activation of mTOR
signaling in SE
The present study first determined whether mTOR
signaling was activated in PTZ-induced SE. SE was successfully
elicited in the experimental rats (n=40). At the beginning of SE,
the rat typically engaged in washing of the face, nodding and
rhythmic chewing for <3 min in each episode. Following this,
recurrent, generalized tonic-clonic seizures, with standing,
falling and tumbling became apparent. The mortality rate of the
PTZ-treated rats was 47.5% (19/40 rats).
The levels of mTOR, p-mTOR, S6, and p-S6 were then
determined using western blot analysis, and the p-mTOR/mTOR and
p-S6/S6 ratios were calculated to determine the activation of mTOR
signaling between 1 h and 7 days. The results demonstrated no
significant changes in the expression of mTOR or S6 in the SE group
(P>0.05), while the expression levels of p-mTOR and p-S6 were
signifi-cantly increased on the 2nd day and peaked on the 3rd day
(P<0.05; Fig. 1). No
significant changes in mTOR, p-mTOR, S6, or p-S6 were observed in
the control group. Similarly, the p-mTOR/mTOR and p-S6/S6 ratios in
the SE group were significantly increased on the 2nd day, and
peaked on the 3rd day, compared with those in the control group
(P<0.05).
The results of the present study indicated that mTOR
signaling was activated in PTZ-induced SE, and the most marked
changes in the p-mTOR/mTOR and p-S6/S6 ratios occurred at the day
3. Therefore, in subsequent investigations, the 3rd day was
selected as the point of examination.
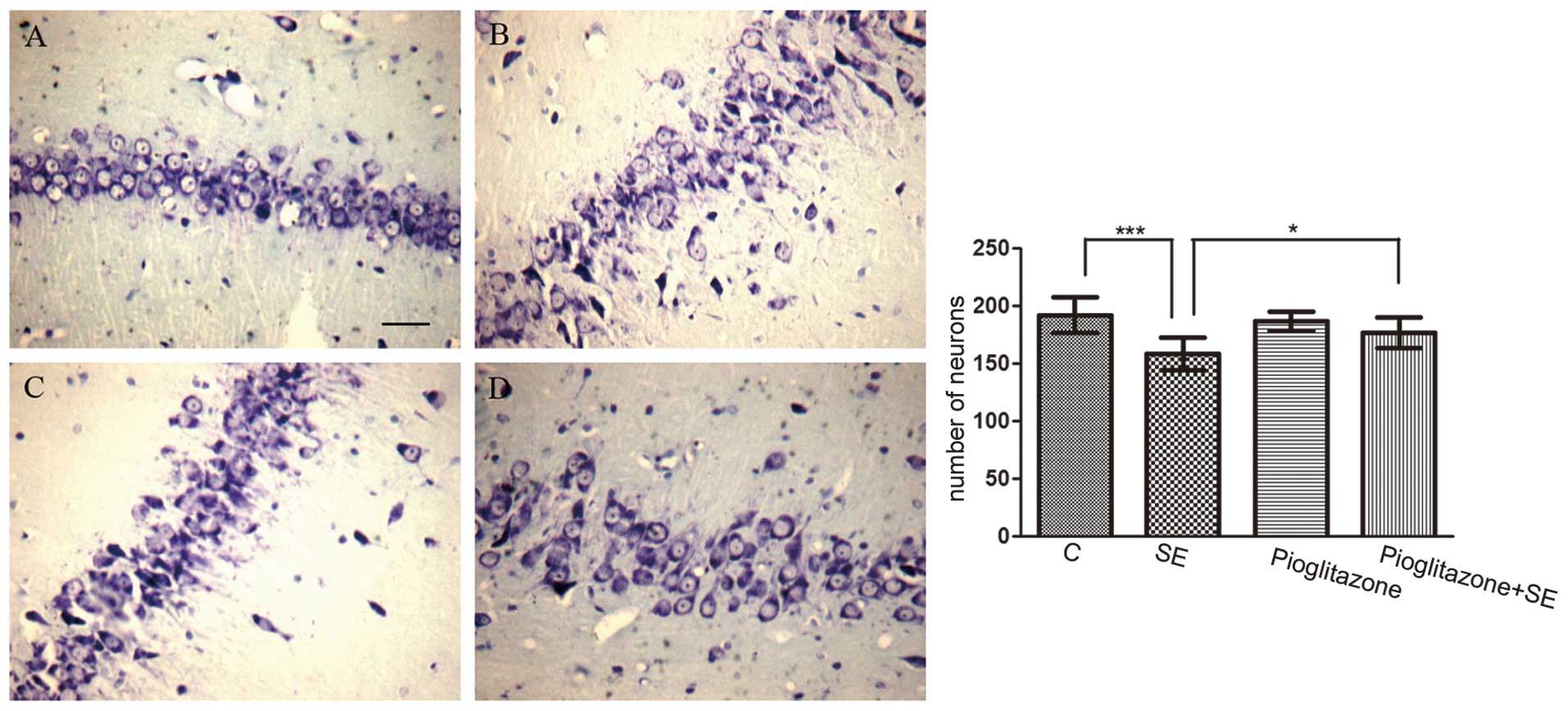
In addition, neuronal loss was detected subsequent
to SE. The number of surviving neurons in the CA3 area in the
vehicle treated-SE group was significantly reduced compared with
that in the control group (P<0.001; Fig. 2A and B).
Effects of pioglitazone on
neuroprotection, activation of the mTOR pathway and the levels of
IL-1β/IL-6 in SE
The effect of the pioglitazone PPAR-γ agonist on the
SE-induced changes was also examined. The mean duration required to
trigger SE was 31.30±6.22 min in the vehicle-treated SE group,
compared with 44.70±14.27 min in the pioglitazone-treated SE group
(P<0.05). In addition, the number of surviving neurons decreased
in the SE group, while the number of surviving neurons in the
pioglitazone-treated SE group was higher, compared with the number
in the vehicle treated-SE group (P<0.05; Fig. 2). These data indicated that
pioglitazone had a neuroprotective role in SE.
To assess whether pioglitazone was associated with
lower levels of proinflammatory cytokines, the levels of IL-1β and
IL-6 in the hippocampal tissues were examined using ELISA. The
results revealed that the levels of IL-1β and IL-6 in the
vehicle-treated SE group were significantly increased compared with
the control group (P<0.001). The levels of IL-1β and IL-6 in the
pioglitazone-treated SE group were increased compared with the
pioglitazone group (P<0.001), however, the levels of IL-1β
(P<0.5) and IL-6 (P<0.001) in the pioglitazone-treated SE
group were significantly decreased compared with the
vehicle-treated SE group (Fig. 3).
These results suggested that proinflammatory cytokines were
involved in SE, and that the PPAR-γ agonist inhibited
inflammation.
The activation of the mTOR pathway was also detected
by immunohistological analysis (Fig.
4). The cells positive for p-mTOR in the vehicle treated-SE
group were significantly increased compared with those in the
control group (P<0.001).
The present study also examined whether pioglitazone
had an effect on the mTOR pathway (Fig. 4). Although the number of cells
positive for p-mTOR in the pioglitazone-treated SE group were
higher compared with those in the pioglitazone group (P<0.01),
those in the pioglitazone-treated SE group were lower compared with
those in the vehicle-treated SE group (P<0.05). Similar results
were detected using western blot analysis (Fig. 5). The p-mTOR/mTOR and p-S6/S6
ratios in the vehicle-treated SE group were significantly higher
compared with the ratios in the control group (P<0.01), while
the ratios in the pioglitazone-treated SE group were significantly
lower compared with the vehicle-treated SE group (P<0.05). These
data suggest that the pioglitazone PPAR-γ agonist inhibited the
mTOR pathway.
Discussion
Epileptogenesis occurs in the period between the
start of brain damage, due to cerebral trauma, infection or genetic
deficiency, and the first spontaneous seizure (22–24).
SE is a more severe form of epilepsy compared with other forms, and
is usually accompanied by damage to the brain. There is evidence
suggesting that spontaneous recurrent seizures may occur in the
days or weeks following SE recovery (25). In the present study, the
time-points at which measurements were obtained fell within this
interval of epileptogenesis.
The hippocampus has a more important role in
epilepsy compared with the amygdala or cerebral cortex due to its
plasticity and adaptability (26).
The general structure of the hippocampus can be divided into the
hippocampal cortex and the dentate gyrus. The hippocampal cortex
can be further divided into the CA1, CA2, CA3 and CA4 areas
(27). The CA1 and CA3 areas are
particularly vulnerable to injury, as these areas lack blood
vessels and are more susceptible to hypoxic-ischemic damage. In
addition, N-methyl-D-aspartate (NMDA) receptors are more abundant
in the CA3 area, resulting in increased vulnerability to excitatory
amino acids. Therefore, the CA3 area was selected for examination
in the present study.
Several drugs have been used to induce epilepsy in
rats, including pilocarpine, kainate and PTZ (28). PTZ can convert inactivated
K+ channels to open channels, decrease the action
potential threshold and increase neuronal excitation, making PTZ an
effective epileptic agent, which is frequently used to establish
epilepsy models (29–35). In addition, intraperitoneal
injection of PTZ does not disturb the structure of brain tissue,
and SE can be successfully induced (36), which is the reason for the use of
PTZ to induce SE in the present study.
Several studies have indicated that mTOR signaling
is associated with epileptogenesis (37–39).
Zeng et al (40) observed
biphasic activation of the mTOR pathway in a kainate-induced SE
model, with one peak within hours and another peak within days. It
was concluded that the first peak, found in the hippocampus and
cortex, may have been caused by activation of glutamate receptors,
while the second peak may have occurred due to epileptogenesis,
observed only in the hippocampus. Zhang et al (41) found that acute seizures, triggered
by a single large dose of PTZ (75 mg/kg), induced the transient
activation of mTOR in the hippocampus and cortex, which lasted 6 h
and returned to baseline after 16 h.
In the present study, the mTOR signaling pathway was
activated in the hippocampus of PTZ-induced SE rat models. However,
unlike the results of Zeng et al (40), only a single peak of mTOR
activation and downstream S6 activation was observed, on the third
day. This may have been the result of the epileptogenic mechanisms
of PTZ and kainatediffer. PTZ-induced seizures involve blocking of
the Cl-related type A γ-aminobutyric acid receptor and NMDA
receptor-mediated transmission (42), while kainate is an analogue of
glutamate, an excitatory amino acid neurotransmitter in the brain
(43). In addition, a second peak
may have occur, beyond the observation period of the present study.
PPAR-γ is an important transcription factor in adipogenesis. The
results in the present study demonstrated that the pioglitazone
PPAR-γ agonist inhibited the mTOR signaling pathway, which was
detected by western blot and immunochemical analyses. A previous
study (9) demonstrated that PPAR-γ
agonists increase the expression of PTEN, a phosphatase and tensin
homolog, in adipocytes and skeletal muscle cells. PTEN is a
negative regulator of mTOR, therefore, a PPAR-γ agonist may repress
the mTOR pathway. Another study suggested that mTOR inhibitors can
inhibit the positive feedback loop of transcription between
CCAAT-enhancer-binding protein-α and PPAR-γ to suppress their
expressio, thereby suppressing adipogenesis and adipocyte
differentiation (44), and
indicating that the inhibition of the mTOR pathway inhibits the
expression of PPAR-γ. This appears to contradict the results of the
present study, and a negative feedback loop may have been
present.
There are multiple feedback loops in the mTOR
pathway. For example, the mTORC1-Akt feedback loop has been
demonstrated in a variety of cancer cells and xenograft tissues;
rapamycin may elevate levels of p-Akt which is upstream of mTOR,
but it also inhibits mTORC1 (45,46).
In addition, an mTORC1-MAPK/ERK feedback loop has been reported
(47,48). MAPK/ERK can positively regulate the
activity of mTORC1, and elevated levels of ERK phosphorylation,
induced by rapamycin, have been observed in in vitro cell
culture, in a murine tumor model and in patients with cancer
(47,48). The presence of these feedback loops
may act to offset the inhibitory effect of drugs on the mTOR
pathway and cause resistance. The existence of such loops requires
further investigation.
Inflammation is important in epilepsy. A previous
study (49) demonstrated that
levels of IL-1β, IL-6 and tumor necrosis factor-α were
significantly elevated in the seizure focus. It also reported that
the mTOR pathway is involved in the activation of microglia, and
that phosphatidic acids can activate the mTOR pathway (50). The activation of mTOR can also
promote the release of the IL-10 inflammatory factor (51), whereas inhibition of mTOR can
reduce the activity of macrophages and microglial cells, thereby
reducing nerve inflammation (52).
The neuroprotective role of PPAR-γ agonists has been
previously observed in models of central nervous system diseases,
including acute cerebral ischemia, Parkinson’s disease and
Alzheimer’s disease (53–55). In the present study, the
pre-administration of pioglitazone reduced neuronal loss following
SE. The results also demonstrated that, in SE, the pioglitazone
PPAR-γ agonist inhibited the mTOR pathway, and decreased the levels
of IL-1β and IL-6 in the hippocampus. These results suggested that
a PPAR-γ agonist may assist in relieving SE by inhibiting the mTOR
pathway and by decreasing inflammation factors. Further experiments
are required to investigate the associations among the PPAR-γ
agonists, mTOR pathway and anti-inflammatory effects.
Notably, the present study demonstrated that
neuronal loss in the pioglitazone-treated group was greater
compared with that in the control group, although the difference
was not statistically significant. It was suggested that this was
due to hypoglycemia following pioglitazone treatment; however, the
rats were found to be normoglycemic following pioglitazone
administration. Therefore, there may be additional mechanisms or
pioglitazone may act as a double-edged sword, which requires
further investigation.
In conclusion, the results of the presents study
suggested that the mTOR pathway was activated in PTZ-induced SE
rats, and its activation peaked on the third day. In addition, the
PPAR-γ agonist was associated with anti-inflammatory effects and
inhibition of the mTOR pathway. Further investigations are required
to examine the associations among PPAR-γ agonists, the mTOR
pathway, and inflammatory factors, and to delineate a PPAR-γ-mTOR
feedback loop.
Abbreviations:
|
4E-BP1
|
eukaryotic translation initiation
factor 4E-binding protein 1
|
|
9-13-HODE
|
9-hydroxyoctadecadienoic acid and
13-hydroxyoctadecadienoic acid
|
|
12-HETE
|
12-hydroxy- 5,8,10,14 eicosatetraenoic
acid
|
|
15-HETE
|
15-hydroxy-5,8,11,14 eicosatetraenoic
acid
|
|
AMPK
|
5′adenosine monophosphate-activated
protein kinase
|
|
mTOR
|
mammalian target of rapamycin
|
|
PPAR
|
peroxisome proliferator-activated
receptor
|
|
PPAR-γ
|
peroxisome proliferator-activated
receptor γ
|
|
PTZ
|
pentylenetetrazol
|
|
S6K1
|
p70 ribosomal protein S6 kinase
|
|
SE
|
status epilepticus
|
References
|
1
|
Pierzchała K and Machowska-Majchrzak A:
Late onset of epilepsy. Wiad Lek. 56:577–581. 2003.In Polish.
|
|
2
|
Sliwa A, Plucinska G, Bednarczyk J and
Lukasiuk K: Post-treatment with rapamycin does not prevent
epileptogenesis in the amygdala stimulation model of temporal lobe
epilepsy. Neurosci Lett. 509:105–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong M: Rapamycin for treatment of
epilepsy: antiseizure, antiepileptogenic, both, or neither?
Epilepsy Curr. 11:66–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho CH: Frontier of epilepsy research-mTOR
signaling pathway. Exp Mol Med. 43:231–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inoki K, Zhu T and Guan KL: TSC2 mediates
cellular energy response to control cell growth and survival. Cell.
115:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park IH, Bachmann R, Shirazi H and Chen J:
Regulation of ribosomal S6 kinase 2 by mammalian target of
rapamycin. J Biol Chem. 277:31423–31429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reinhard C, Thomas G and Kozma SC: A
single gene encodes two isoforms of the p70 S6 kinase: activation
upon mitogenic stimulation. Proc Natl Acad Sci USA. 89:4052–4056.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loane DJ, Deighan BF, Clarke RM, Griffin
RJ, Lynch AM and Lynch MA: Interleukin-4 mediates the
neuroprotective effects of rosiglitazone in the aged brain.
Neurobiol Aging. 30:920–931. 2009. View Article : Google Scholar
|
|
9
|
Limor R, Sharon O, Knoll E, Many A,
Weisinger G and Stern N: Lipoxygenase-derived metabolites are
regulators of peroxisome proliferator-activated receptor gamma-2
expression in human vascular smooth muscle cells. Am J Hypertens.
21:219–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bernardo A and Minghetti L: Regulation of
glial cell functions by PPAR-gamma natural and synthetic agonists.
PPAR Res. 2008:8641402008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zang L, Mu YM, Lu ZH, et al: LRP16 gene
causes insulin resistance in C2-C12 cells by inhibiting the IRS-1
signaling and the transcriptional activity of peroxisome
proliferator actived receptor gamma. Zhonghua Yi Xue Za Zhi.
91:1408–1412. 2011.In Chinese. PubMed/NCBI
|
|
12
|
Kim KY, Cho HS, Jung WH, Kim SS and Cheon
HG: Phosphatase and tensin homolog deleted on chromosome 10
suppression is an important process in peroxisome
proliferator-activated receptor-gamma signaling in adipocytes and
myotubes. Mol Pharmacol. 71:1554–1562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sozio MS, Lu C, Zeng Y, Liangpunsakul S
and Crabb DW: Activated AMPK inhibits PPAR-{alpha} and PPAR-{gamma}
transcriptional activity in hepatoma cells. Am J Physiol
Gastrointest Liver Physiol. 301:G739–747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motte JE, da Silva Fernandes MJ, Marescaux
C and Nehlig A: Effects of pentylenetetrazol-induced status
epilepticus on c-Fos and HSP72 immunoreactivity in the immature rat
brain. Brain Res Mol Brain Res. 50:79–84. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nehlig A and Pereira de Vasconcelos A: The
model of pentylenetetrazol-induced status epilepticus in the
immature rat: short- and long-term effects. Epilepsy Res.
26:93–103. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pineau N, Charriaut-Marlangue C, Motte J
and Nehlig A: Pentylenetetrazol seizures induce cell suffering but
not death in the immature rat brain. Brain Res Dev Brain Res.
112:139–144. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Erdogan F, Golgeli A, Arman F and Ersoy
AO: The effects of pentylenetetrazole-induced status epilepticus on
behavior, emotional memory and learning in rats. Epilepsy Behav.
5:388–393. 2004. View Article : Google Scholar
|
|
18
|
el Hamdi G, de Vasconcelos AP, Vert P and
Nehlig A: An experimental model of generalized seizures for the
measurement of local cerebral glucose utilization in the immature
rat. I Behavioral characterization and determination of lumped
constant. Brain Res Dev Brain Res. 69:233–242. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Augustinack JC, van der Kouwe AJ,
Blackwell M1, et al: Detection of entorhinal layer II using 7Tesla
[corrected] magnetic resonance imaging. Ann Neurol. 57:489–494.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Peng X, Yu W, et al:
Alpha-tocopheryl succinate enhances doxorubicin-induced apoptosis
in human gastric cancer cells via promotion of doxorubicin influx
and suppression of doxorubicin efflux. Cancer Lett. 307:174–181.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bovolenta R, Zucchini S, Paradiso B, et
al: Hippocampal FGF-2 and BDNF overexpression attenuates
epileptogenesis-associated neuroinflammation and reduces
spontaneous recurrent seizures. J Neuroinflammation. 7:812010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Turrin NP and Rivest S: Innate immune
reaction in response to seizures: implications for the
neuropathology associated with epilepsy. Neurobiol Dis. 16:321–334.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soller M, Tautenhahn A, Brune B, et al:
Peroxisome proliferator-activated receptor gamma contributes to T
lymphocyte apoptosis during sepsis. J Leukoc Biol. 79:235–243.
2006. View Article : Google Scholar
|
|
25
|
Leite JP, Garcia-Cairasco N and Cavalheiro
EA: New insights from the use of pilocarpine and kainate models.
Epilepsy Res. 50:93–103. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Covolan L, Ribeiro LT, Longo BM and Mello
LE: Cell damage and neurogenesis in the dentate granule cell layer
of adult rats after pilocarpine- or kainate-induced status
epilepticus. Hippocampus. 10:169–180. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sendrowski K and Sobaniec W: Hippocampus,
hippocampal sclerosis and epilepsy. Pharmacol Rep. 65:555–565.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lian XY, Zhang Z and Stringer JL:
Anticonvulsant and neuro-protective effects of ginsenosides in
rats. Epilepsy Res. 70:244–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dhir A: Pentylenetetrazol (PTZ) kindling
model of epilepsy. Curr Protoc Neurosci. Chapter 9: Unit 9.
37:2012. View Article : Google Scholar
|
|
30
|
Visweswari G, Siva Prasad K, Lokanatha V
and Rajendra W: The antiepileptic effect of Centella asiatica on
the activities of Na+/K+, Mg2+ and
Ca2+-ATPases in rat brain during
pentylenetetrazol-induced epilepsy. Indian J Pharmacol. 42:82–86.
2010.
|
|
31
|
Bao YY and Ding MP: C-Fos expression in
the hippocampus of rats with pentylenetetrazol-induced epilepsy.
Zhejiang Da Xue Xue Bao Yi Xue Ban. 31:111–114. 2002.In
Chinese.
|
|
32
|
Ekonomou A and Angelatou F: Upregulation
of NMDA receptors in hippocampus and cortex in the
pentylenetetrazol-induced ‘kindling’ model of epilepsy. Neurochem
Res. 24:1515–1522. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eells JB, Clough RW, Browning RA and Jobe
PC: Fos in locus coeruleus neurons following audiogenic seizure in
the genetically epilepsy-prone rat: comparison to electroshock and
pentylenetetrazol seizure models. Neurosci Lett. 233:21–24. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Madeja M, Stocker M, Musshoff U, et al:
Potassium currents in epilepsy: effects of the epileptogenic agent
pentylenetetrazol on a cloned potassium channel. Brain Res.
656:287–294. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lathers CM and Schraeder PL: Autonomic
dysfunction in epilepsy: characterization of autonomic cardiac
neural discharge associated with pentylenetetrazol-induced
epileptogenic activity. Epilepsia. 23:633–647. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Planas AM, Soriano MA, Ferrer I and
Rodriguez Farré E: Regional expression of inducible heat shock
protein-70 mRNA in the rat brain following administration of
convulsant drugs. Brain Res Mol Brain Res. 27:127–137. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Berdichevsky Y, Dryer AM, Saponjian Y, et
al: PI3K-Akt signaling activates mTOR-mediated epileptogenesis in
organotypic hippocampal culture model of post-traumatic epilepsy. J
Neurosci. 33:9056–9067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McDaniel SS and Wong M: Therapeutic role
of mammalian target of rapamycin (mTOR) inhibition in preventing
epileptogenesis. Neurosci Lett. 497:231–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Russo E, Citraro R, Donato G, et al: mTOR
inhibition modulates epileptogenesis, seizures and depressive
behavior in a genetic rat model of absence epilepsy.
Neuropharmacology. 69:25–36. 2013. View Article : Google Scholar
|
|
40
|
Zeng LH, Rensing NR and Wong M: The
mammalian target of rapamycin signaling pathway mediates
epileptogenesis in a model of temporal lobe epilepsy. J Neurosci.
29:6964–6972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang B and Wong M:
Pentylenetetrazole-induced seizures cause acute, but not chronic,
mTOR pathway activation in rat. Epilepsia. 53:506–511. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang LT, Yang SN, Liou CW, et al:
Pentylenetetrazol-induced recurrent seizures in rat pups: time
course on spatial learning and long-term effects. Epilepsia.
43:567–573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Z, Duan RS, Quezada HC, et al:
Increased microglial activation and astrogliosis after intranasal
administration of kainic acid in C57BL/6 mice. J Neurobiol.
62:207–218. 2005. View Article : Google Scholar
|
|
44
|
Kim DJ, Akiyama TE, Harman FS, et al:
Peroxisome proliferator-activated receptor beta (delta)-dependent
regulation of ubiquitin C expression contributes to attenuation of
skin carcinogenesis. J Biol Chem. 279:23719–23727. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Svejda B, Kidd M, Kazberouk A, Lawrence B,
Pfragner R and Modlin IM: Limitations in small intestinal
neuroendocrine tumor therapy by mTor kinase inhibition reflect
growth factor-mediated PI3K feedback loop activation via ERK1/2 and
AKT. Cancer. 117:4141–4154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shi Y, Yan H, Frost P, Gera J and
Lichtenstein A: Mammalian target of rapamycin inhibitors activate
the AKT kinase in multiple myeloma cells by up-regulating the
insulin-like growth factor receptor/insulin receptor
substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther.
4:1533–1540. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Carracedo A, Ma L, Teruya-Feldstein J, et
al: Inhibition of mTORC1 leads to MAPK pathway activation through a
PI3K-dependent feedback loop in human cancer. J Clin Invest.
118:3065–3074. 2008.PubMed/NCBI
|
|
48
|
Wang X, Hawk N, Yue P, et al: Overcoming
mTOR inhibition-induced paradoxical activation of survival
signaling pathways enhances mTOR inhibitors’ anticancer efficacy.
Cancer Biol Ther. 7:1952–1958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Binder DK and Steinhauser C: Functional
changes in astroglial cells in epilepsy. Glia. 54:358–368. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Foster DA: Phosphatidic acid signaling to
mTOR: signals for the survival of human cancer cells. Biochim
Biophys Acta. 1791:949–955. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Weichhart T and Saemann MD: The multiple
facets of mTOR in immunity. Trends in immunology. 30:218–226. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dello Russo C, Lisi L, Tringali G and
Navarra P: Involvement of mTOR kinase in cytokine-dependent
microglial activation and cell proliferation. Biochem Pharmacol.
78:1242–1251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zuhayra M, Zhao Y, von Forstner C, et al:
Activation of cerebral peroxisome proliferator-activated receptors
gamma (PPARgamma) reduces neuronal damage in the substantia nigra
after transient focal cerebral ischaemia in the rat. Neuropathol
Appl Neurobiol. 37:738–752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hunter RL, Choi DY, Ross SA and Bing G:
Protective properties afforded by pioglitazone against
intrastriatal LPS in Sprague-Dawley rats. Neurosci Lett.
432:198–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zolezzi JM, Silva-Alvarez C, Ordenes D, et
al: Peroxisome proliferator-activated receptor (PPAR) γ and PPARα
agonists modulate mitochondrial fusion-fission dynamics: Relevance
to reactive oxygen species (ROS)-related neurodegenerative
disorders? PloS One. 8:e640192013. View Article : Google Scholar
|