Introduction
Multiple myeloma (MM) is a malignant tumor derived
from B cells. It is characterized by the uncontrolled proliferation
of monoclonal plasma cells in the bone marrow and the presence of
monoclonal immunoglobulin in the serum or urine. It is often
accompanied by multiple osteolytic lesions, hypercalcemia, anemia,
kidney damage and suppression of the immune system, and carries a
poor prognosis. MM has a high incidence in middle-aged to elderly
individuals and remains an incurable disease (1). Since the molecular etiology and
mechanisms of drug resistance are not clear, there are currently no
treatments that result in a complete cure. Therefore, further
studies into the pathogenesis of MM are required, in order to
develop clinical diagnostic and therapeutic approaches to this
disease.
In humans, cytokine-induced apoptosis inhibitor 1
(CIAPIN1) is located on the long arm of chromosome 16. Shibayama
et al (2,3) first identified CIAPIN1 as a
regulatory molecule in the rat sarcoma signal transduction pathway,
which is independent of apoptotic B cell lymphoma 2 and the
cysteine-dependent aspartate-directed protease family. CIAPIN1 is
widely distributed in healthy fetal and adult tissues, and the gene
is expressed in differentiated tissue and metabolically active
tissue (4). However, its
expression has been shown to be inhibited in certain types of
cancerous tissues, including gastric cancer and clear cell renal
cell carcinoma (5,6). These findings suggest that the
expression of the CIAPIN1 gene may be associated with the
suppression of tumor development.
In the present study, the expression and effects of
CIAPIN1 were investigated in multiple myeloma.
Materials and methods
Immunohistochemistry
Paraffin-embedded tissue samples from diseased and
healthy bone marrow, were obtained from 32 patients with MM in whom
the disease had been pathologically confirmed and healthy controls,
respectively. The healthy controls ranged from 32–60 years old (23
males and 9 females) and the bone marrow examination did not reveal
malignant blood disorders. Samples were obtained by Dr Xiaobo Wang
from patients who underwent orthopedic surgery at The First
Affiliated hospital of Sun Yan-sen University (Guangzhou, China)
between July 2009–March 2013. The patients ranged from 37–69 years
old (20 males and 12 females). The patients had not previously
received radiotherapy, chemotherapy or biological response modifier
therapy. A mouse anti-CIAPIN1 monoclonal antibody was produced by
Abcam (ab154904; Cambridge, UK). All specimens were obtained from
patients who had provided informed consent for research purposes.
The protocols used in the present study were approved by the
Hospital’s Protection of Human Subjects Committee of Sun Yat-sen
University (Guangzhou, China). The use of human tissue in this
study was approved by the institutional review board of The First
Affiliated hospital, Sun Yat-sen University and was conducted in
accordance with international guidelines for the use of human
tissue. All slides were treated with polylysine in preparation for
immunohistochemistry. In order to recover antigens, slides were
treated with boiling 0.01 mol/l citrate buffer (Jingyan Chemicals
Co., Ltd, Shanghai, China) followed by incubation at 95°C for 5
min. Following natural cooling of the buffer to room temperature,
slides were washed with phosphate-buffered saline (PBS; Jingyan
Chemicals Co., Ltd) three times, every 2 min. The mouse monoclonal
anti-CIAPIN1 primary antibody was diluted to 1:1,000 and the
secondary antibody, biotinylated goat anti-mouse Immunoglobulin G
(IgG; 33207ES60, Yeasen Biotechnology Co., Ltd., Shanghai, China),
was diluted to 1:500. The positive control consisted of
CIAPIN1-stained human SkHep1 xenograft tissue. For the negative
control, 0.01 M PBS was used instead of the primary antibody.
Criteria for positively stained tumor cells: Presence of
brown-yellow staining of the tumor cells nuclei and/or irregular
thickness and shades of granules in the cytoplasm (only tumor cells
were assessed). Two types of CIAPIN1 cells were identified: CIAPIN1
(−), ≤25% tumor nuclei containing brown staining or granules; and
CIAPIN1 (+), >25% tumor nuclei containing brown staining or
granules.
Cell culture
Exponentially growing RPMI-8226 cells and WIL2-S B
lymphocytes (American Type Culture Collection, Manassas, VA, USA)
were cultured in RPMI-1640 complete media (Cyagen Biosciences,
Guangzhou, China) containing 15% fetal bovine serum (FBS;
Invitrogen Life Technologies, Carslbad, CA, USA), 100 U/ml
penicillin and 100 µg/ml streptomycin (Minsheng Chemical
Co., Ltd., Beijing, China). 293T cells were cultured in
high-glucose Dulbecco’s modified Eagle’s Medium (DMEM; Cyagen
Biosciences) containing 10% FBS, 100 U/ml penicillin and 100
µg/ml streptomycin. Cells were cultured in a 37°C, 5%
CO2 incubator with saturated humidity. When cells had
reached 80–90% confluence, they were passaged, and adherent 293T
cells were removed using 0.25% trypsin (Cyagen Biosciences)
digestion.
Extraction of bone marrow mononuclear
cells
Bone marrow (3–5 ml) was extracted from the
posterior iliac spines of patients and controls, and transpired
into 0.5 ml anticoagulant heparin saline solution (1,200 U/ml;
Minsheng Chemical Co., Ltd.). Subsequently, 2-4X volume of PBS was
added to the solution. The diluted bone marrow solution was slowly
added to a lymphocyte separation medium (bone marrow : lymphocyte
separation medium = 1:1) for gradient centrifugation at 100 × g for
20 min at 20°C. Following centrifugation, the solution was
separated into four layers. The top layer consisted of the plasma
containing platelets. The second layer consisted of a white film,
which contained the mononuclear cells; the third layer consisted of
the separation medium; and the fourth layer contained the
granulocytes and erythrocytes. Mononuclear cells were aspirated
into a new tube, mixed with 5 ml PBS and centrifuged at 60 × g for
10 min at 20°C. Cells were washed twice with PBS and resuspended in
PBS for counting.
Construction of CIAPN1-expressing
lentiviral vector
The primers designed for CIAPIN1 gene amplification
were based on the GenBank CIAPIN1 sequence. In order to facilitate
the expression of the vector in eukaryotic cells, a CACC sequence
was added to the primer’s 5′ end and the terminator sequence was
removed from the primer’s 3′ end. The following primers were
synthesized by GENEWIZ, Inc. (Suzhou, China): 5′CCG CTC GAG ATG GCA
GAT TTT GGG ATC TCT GC3′ for ciapin1XhoIF and 5′ATA AGA ATG CGG CCG
CCT AGG CAT CAT GAA GAT TGC TATC 3′ for ciapin1NotIR. The CIAPIN1
gene was amplified using the high fidelity enzyme KOD-Plus-Neo DNA
polymerase (Toyobo, Osaka, Japan) using the full CAIPIN1 cDNA as a
template, with the ciapin1XhoIF and ciapin1NotIR primers [2 mM
deoxyribonucleotide mixture; 2.5 µl 10X KOD buffer; 1.5
µl (25 mM) MgSO4; 0.3 µl ciapin1XhoIF; 0.3
µl ciapin1NotIR; 0.3 µl KOD-Plus-Neo; and 17.1
µl double distilled (dd)H2O]. The cycling
conditions were as follows: 94°C for 5 min and 30 cycles of 98°C
for 30 sec; followed by 58°C for 30 sec; and then 68°C for 1 min
and 68°C for 5 min. The amplified products were purified and mixed
with pLVX-IRES-ZsGreen1 vector. Subsequently, they were digested
with the restriction enzymes XhoI and NotI at 37 °C for 3 h and
recovered using a DNA gel extraction kit (Dongsheng Biotech Ltd.,
Guangzhou, China). In brief, polymerase chain reaction (PCR)
products were electrophoresed, sectioned and added to BD solution
(Novoprotein Scientific, Inc., Shanghai, China) at 60°C for 10 min.
PCR products were then transferred to a DNA purification column
(held for 2 mins, centrifuged at 12,000 × g for 1 min; Novoprotein
Scientific, Inc.) and the supernatant was removed, then centrifuged
following resuspension in 500 µL PE at 1,000 × g for 1 min
and the supernatant was removed. Subsequently, the DNA was eluted
at 60°C in 30 µl ddH2O and centrifuged at 400 × g
for 1 min. PCR products were inserted into the vector using T4 DNA
ligase (D2011A; Takara Biotechnology Co., Ltd., Dalian, China) at
16°C for 2 h. Competent DH5α cells (Invitrogen Life Technologies)
were transformed, plated on lysogeny broth (LB; Jingyan Chemicals
Co., Ltd) medium containing ampicillin (Minsheng Chemical Co.,
Ltd.) and incubated at 37°C for 18 h. Colonies were selected and
cultured in LB mediu containing ampicillin at 37°C. Plasmids were
then purified for sequence analysis in order to obtain the
CIAPIN1-expressing lentiviral vector pLVX-IRES-ZsGreen1. In brief,
1.5 ml gel was centrifuged at 4°C for 30 sec at 12,000 × g and the
supernatant was removed. The pellet was then resuspended in 120 ml
lysis buffer STET (Rong Bio-Science Technology Co., Ltd., Shanghai,
China) for 50 sec, 10 ml lysozyme (10 mg/ml) and boiling water for
50 sec, prior to further centrifugation at 4°C for 10 min at 12,000
× g to remove bacterial debris. BLAST sequence analysis was then
performed (NCBI/BLAST/blastnsuite-2sequences/).
Construction of CIAPIN1-small interfering
RNA (si-RNA) lentiviral vector
The specific CIAPIN1 siRNA target was designed by
Sigma-Aldrich (St. Louis, MO, USA). To facilitate cloning into the
vector, the sequences CACC and AAAA were annealed to the 5′ ends of
the following complementary single-stranded DNA hairpins
respectively: 5′GAT
CCGTGTTCAGCCCTGACTCTTCTCGAGAAGAGTCAGGGCTGAACACTTTTTG3′ for
ciapin1siRNABamHIF and 5′AATTCAAAAAGTGTTCAGC
CCTGACTCTTCTCGAGAAGAGTCAGGGCTGAA CACG’ for ciapin1siRNAEcoRIR.
These single-stranded DNA hairpins were synthesized by GENEWIZ Inc.
The pLVX-shRNA2 vector was mixed with the ciapin1siRNABamHIF and
ciapin1siRNAEcoRIR annealed products and digested using the
restriction enzymes BamHI and EcoRI (Sigma-Aldrich)
for 4 h at 37°C. The digestion products were recovered using a DNA
gel extraction kit (Dongsheng Biotech Ltd.), and either the
ciapin1siRNA (Sigma-Aldrich) or the unrelated control siRNA was
inserted into the vector using T4 DNA ligase (D2011A; Takara
Biotechnology Co., Ltd.) at 16°C for 2 h. Competent DH5α cells
(Invitrogen Life Technologies) were transformed, plated on an LB
medium containing ampicillin and incubated at 37°C for 18 h.
Colonies were selected and cultured, and plasmids were purified for
sequence analysis in order to obtain the CIAPIN1-siRNA lentiviral
vector and the unrelated control vector, pLVX-NC.
Lentiviral packaging
Recombinant lentiviral plasmids and the two
packaging plasmids were extracted using a Plasmid Extraction kit
(PD1212-01; Biomiga, Inc., San Diego, CA, USA) according to the
manufacturer’s instructions. These plasmids were endotoxin-free.
After 8 h, the 293T cells were transfected and cultured in a
complete media using Lipofectamine 2000® according to
the manufacturer’s instructions (Invitrogen Life Technologies). The
cells were harvested at 24 h and 48 h, centrifuged at 200 × g for 5
min and filtered separately through a 0.45 µm needle filter
(Zhejiang Aijiren Technology Co., Ltd., Shanghai, China). The virus
pellet was collected by centrifuging at 50,000 × g for 2 h at 4°C,
re-suspended in 1 ml PBS, further filtered by a 0.22 µm
needle filter (Zhejiang Aijiren Technology Co., Ltd.) and stored at
−80°C.
Virus infection and screening
RPMI-8226 cells were seeded at 2×105
cells/well in 24-well plates and cultured at 37°C for 18 h. The
CIAPIN1-expressing lentivirus, CIAPIN1-siRNA lentivirus and the
unrelated control siRNA lentivirus were transfected into RPMI-8226
cells separately and polybrene (Jingyan Chemicals Co., Ltd) was
added to obtain a final concentration of 6 µg/ml. The
multiplicity of infection (ratio of virus to cell number) was 10:1.
Following culture for 6 h, the cells were replenished with fresh
media and blasticidin (SelleckChem, Houston, TX, USA) was added to
produce a final concentration of 5 µg/ml for screening.
Stably transduced cells were obtained after 2–3 weeks.
Reverse transcription-quantitative PCR
(RT-qPCR)
Using ABI’s Primer Express software 3.3 and the
glyceraldehydes 3-phosphate dehydrogenase (GAPDH) gene as an
internal control, primers and probes were synthesized by Biosune
Biotech Ltd Inc. (Nanjing, Jiangsu, China). Using a small RNA
extraction kit (Dongsheng Biotech Ltd.), total RNA was extracted
from CIAPIN1 overexpressing RPMI-8826 cells,
CIAPIN1-siRNA-RPMI-8826 cells, unrelated siRNA-RPMI-8826 cells and
control RPMI-8826 cells, and amplified using the ABI 7500 Fast
& 7500 RT-PCR PCR system, according to the manufacturer’s
instructions (Applied Biosystems Life Technologies, Beijing,
China). The gene copy number was calculated according to the
corresponding cycle threshold value. The ratio of CIAPIN1 gene copy
number to internal GAPDH gene copy number in the samples was
interpreted as the relative expression level of the CIAPIN1
gene.
Western blotting
Cells (5×105) were collected and
sonicated. Protein content was measured using coomassie blue
staining protocol (7) and the
samples were run on a SDS-PAGE. The gels were electronically
transferred to polyvinylidene difluoride (PVDF) membranes (Hangzhou
Kaijie Membrane Separation Technology Co., Ltd., Hangzhou, China),
followed by incubation in 5% non-fat milk (Nestle, York, PA, USA)
at 4°C for 6 h. The membranes were incubated with mouse anti-human
CIAPIN1 monoclonal IgG (cat. no. sc-271298) overnight at 4°C and
further incubated with mouse anti-human (E030170; Earthox,
Millbrae, CA, USA) or goat anti-human (PL03b-0297M; PL
Laboratories, Port Moody, BC, Canada) horseradish
peroxidase-conjugated secondary antibodies for 2 h. A
chemiluminescence kit (cat. no. RPN2106; Amersham Pharmacia
Biotech, Uppsala, Sweden) was used to detect levels of CIAPIN1,
insulin-like growth factor-1U (IGF-1, dilution 1:500; cat. no.
sc-1422; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
mouse monoclonal cyclin-dependent kinase 2 (CDK2; cat. no. 610145),
mouse monoclonal CDK4, p27 (dilution 1:500; cat. no. 559677; BD
Biosciences, San Jose, CA, USA) and rabbit monoclonal
retinoblastoma protein (Rb, dilution 1:500; cat. no. 8516; Cell
Signaling Technology, Inc. Beverly, MA, USA) in the PVDF
membranes.
MTT assay
Exponentially growing cells in each group were
diluted into 4×104/ml single-cell suspension and seeded
in 96-well cell culture plates at 200 µl/well. The cells
were cultured at 37°C in 5% CO2, for 24, 48, 72 and 96
h. Subsequently, the cells were cultured in 20 µl of 5 mg/ml
MTT for 4 h. The supernatants were discarded and 150 µl
dimethylsulfoxide (Jingyan Chemicals Co., Ltd) was added to each of
the cell cultures, followed by vortexing for 10 min in order to
completely dissolve the crystals. The optical density (OD) of 490
nm was measured in a microplate reader (iMARK; Bio-Rad, Hercules,
CA, USA). A cell growth curve was created in Excel.
Cell cycle analysis
Synchronized cells in each group were collected at
1×106 cells per sample. The cells were washed twice with
PBS, and ice-cold ethanol (75%; Jingyan Chemicals Co., Ltd) was
added for overnight fixation at 4°C. Fixed cells were washed once
with PBS and 100 µg/ml RNase was then added, followed by
propidium iodine staining (Jingyan Chemicals Co., Ltd) in darkness
for 30 min. Flow cytometric analysis (BD FACSCalibur; BD
Biosciences) was used to detect changes in cell cycle progression
and ModFit 4.0 software (Verity Software House, Topsham, ME, USA)
was used to analyze data in the FC files.
Soft agar colony formation assay
Each cell line was diluted in RPMI-1640 culture
medium containing 20% FBS (500 cells/ml). Each cell suspension
sample (9 ml) was mixed with 1 ml of 3% low-melting point agarose
solution (Jingyan Chemicals Co., Ltd) in order to produce the
agarose suspension. The agarose suspension was seeded in 6-well
cell culture plates at 3 ml/well (three copies for each cell line).
Subsequently, the cells were solidified at 4°C for approximately 10
min prior to incubation. Following 18 days of culturing at 37°C and
5% CO2, cells were stained with 0.05% crystal violet dye
(Jingyan Chemicals Co., Ltd) and observed under a microscope (BX51;
Olympus Corp., Tokyo, Japan).
Cellular tumorigenicity in nude mice
In total, 24 male BALB/c nude mice (age 4–6 weeks;
weight, 18–20 g), were randomly divided into four groups with six
mice per group. Cells (0.2 ml; 1×107 cells/ml) in the
exponential growth phase from each group were inoculated
subcutaneously into the forelimb of nude mice to form a diameter of
~3 mm around the skin rash caused by the tumor cells, using a 1 ml
sterile disposable syringe (Minsheng Chemical Co., Ltd.). At 20
days post-inoculation, the nude mice were sacrificed by cervical
dislocation and tumors were removed and measured using a vernier
caliper. Tumor volume was measured according to the following
equation: V = a (long diameter) × b2 (short diameter)/2.
A portion of tumor tissue from each group was fixed in 10% neutral
formalin (Jingyan Chemicals Co., Ltd), and stained with hematoxylin
and eosin (Jingyan Chemicals Co., Ltd) for pathological
confirmation. The remaining tissue was stored at −80 °C.
Statistical analysis
Data were analyzed using SPSS 20.0 (IBM SPSS,
Armonk, NY, USA) and the results are presented as the mean ±
standard deviation. One-way analysis of variance and Student’s
t-tests were conducted. P<0.05 was considered to indicate a
statistically significant difference.
Results
Reduced expression of CIAPIN1 in MM
cells
The measurement of CIAPIN1 expression in 32 MM and
adjacent healthy bone marrow tissue samples using
immunohistochemistry, suggested that CIAPIN1 is primarily located
in the cytoplasm and cell membranes of MM cells (Fig. 1). MM tissue samples consisted of
28.2% (9/32) CIAPIN1 (+) cells, which was significantly lower than
the proportion in the healthy bone marrow tissue of 53.1% (17/32;
P<0.05; Table I). Compared with
adjacent healthy bone marrow tissue, there was a reduction in the
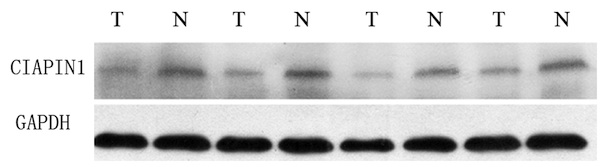
expression of CIAPIN1 in the MM tissue (Fig. 2). CIAPIN1 expression was also lower
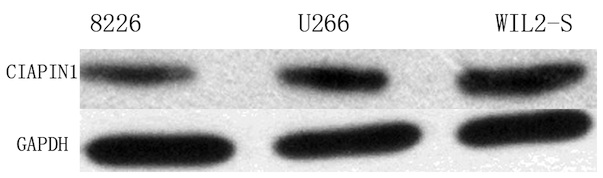
in human MM cell lines than in human WIL2-S B lymphocyte cells
(Fig. 3).
 | Figure 1CIAPIN1 protein expression in MM
tissues and adjacent paired normal bone marrow tissues. (A) Healthy
bone marrow tissues and (B) MM bone marrow tissues.
Immunohistochemical images were captured from MM tissues (32 cases)
and adjacent tissues (32 cases) at different magnifications A1,
x100; A2, x200; A3, x400; A4, negative control, x100; B1, x100; B2,
x200; B3, x400; B4, negative control, x100. The percentage of
CIAPIN1 (+) samples in MM was 28.2% (9/32). This was significantly
lower than 53.1% (17/32) in healthy bone marrow tissues
(P<0.05). CIAPIN1, cyto-kine induced apoptosis inhibitor 1; MM,
multiple myeloma. |
 | Table ICIAPIN1 expression in multiple myeloma
tissues and adjacent healthy tissues. |
Table I
CIAPIN1 expression in multiple myeloma
tissues and adjacent healthy tissues.
| Group | Total cases | CIAPIN1 gene (n)
| Positive % |
|---|
| Positive cases | Negative cases |
|---|
| Multiple myeloma | 32 | 9 | 23 | 28.2a |
| Healthy bone
marrow | 32 | 17 | 15 | 53.1 |
Overexpression of the CIAPIN1 protein
inhibits growth and tumorigenicity of MM cells in vitro and in
vivo
Transduction of the CIAPIN1 gene, Ad-CIAPIN1, in the
RPMI-8226 cells, caused the CIAPIN1 protein levels to increase
(Fig. 4), which significantly
inhibited RPMI-8226 cell growth (Fig.
5, P<0.05). However, the siRNA group exhibited the opposite
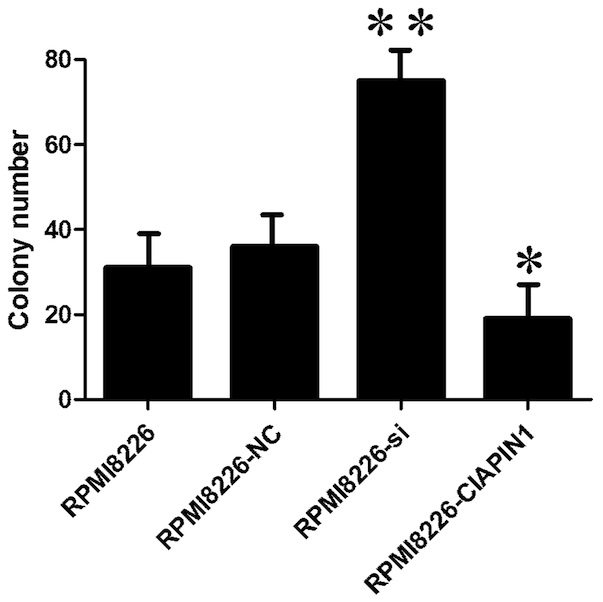
effect (Fig. 4). In the colony
formation assay, Ad-CIAPIN1-transfected tumor cells formed
significantly fewer colonies compared with the control cells
(Fig. 6, P<0.05). Furthermore,
in the in vivo subcutaneous tumor formation experiments,
inoculation with RPMI-8226-CIAPIN1 cells produced tumors of
markedly reduced sizes, compared with the inoculation of parental
cells with empty vector-transfected cells (Fig. 7, P<0.05). However, the siRNA
cells exhibited the opposite effect. Thus, the results of in
vitro and in vivo tests suggest that CIAPIN1 inhibits
tumor cell proliferation.
CIAPIN1 proteins target cell
cycle-regulatory genes, induce cell cycle arrest in the G1/S phase
and inhibit IGF-1 secretion
Flow cytometric analyses showed that the level of
expression of CIAPIN1 proteins had a significant effect on the cell
cycle in RPMI-8226 cells (Table
II). At 48 h following transduction, 74.04% of
RPMI-8226-CIAPIN1 cells were in the G1 phase, whereas only 59.41%
of RPMI-8226-NC cells were in the G1 phase (Table II, P<0.05). No significant
differences were observed in the percentage of cells in the G2
phase between groups. However, the RPMI-8226-siRNA group exhibited
a substantial reduction in the proportion of cells in the G1 phase
(Table II, P<0.05). These
results indicate that CIAPIN1 inhibits cell entry into the S phase.
Therefore, CIAPIN1 directly acts on cells in order to inhibit cell
cycle progression, which may in part explain the inhibitory effect
of CIAPIN1 on the growth of MM cells. In order to investigate the
molecular mechanisms underlying CIAPIN1-induced cell cycle arrest,
the levels of cell cycle regulatory genes (CDK2, CDK4 and IGF-1)
were analyzed. The results indicated that upregulation of CIAPIN1
protein reduces the expression of CDK2, CDK4 and IGF-1, but
enhances that of p27 and Rb proteins. By contrast, the
downregulation of CIAPIN1 by specific siRNA led to the opposite
effect (Fig. 8). Therefore,
CIAPIN1 appears to inhibit the growth of MM cells through the
regulation of various proteins involved in the G1/S progression.
Furthermore, CIAPIN1 may disrupt IGF-1 expression and reduce the
growth, survival, adhesion, migration and drug resistance of MM
cells.
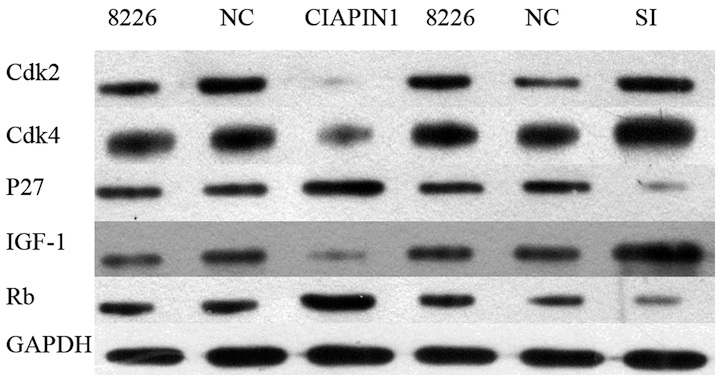 | Figure 8Expression of cell cycle-regulatory
proteins and ICF-1. All gene expression levels were quantitatively
analyzed and are expressed as the ratio of the levels of GAPDH. The
expression of Cdk2, Cdk4, Rb, p27 and IGF-1 proteins were evaluated
before and after infection using western blot. Cdk,
cyclin-dependent kinase; CIAPIN1, cytokine induced apoptosis
inhibitor 1; IGF-1, insulin-like growth factor-1; Rb,
retinoblastoma protein; 8226, RPMI8226; NC, negative control; SI,
small interfering RNA. |
 | Table IICell cycle test results of RPMI-8226
cells in each experimental group. |
Table II
Cell cycle test results of RPMI-8226
cells in each experimental group.
| Clones expressing
CIAPIN1 protein | RPMI-8266
|
|---|
| G1 | S | G2 |
|---|
| RPMI-8226 | 60.85 | 31.60 | 7.55 |
| RPMI-8226-NC | 59.41 | 32.82 | 7.77 |
| RPMI-8226-siRNA | 41.12a | 51.80 | 7.09 |
|
RPMI-8226-CIAPIN1 | 74.04a | 20.01 | 5.95a |
Discussion
MM remains a clinical challenge due to its complex
pathogenesis. Certain genes have been identified that are involved
in the progression of MM. However, further research is required in
order to identify diagnostic markers and therapeutic target
proteins in MM. The present study showed that CIAPIN1 gene
expression was significantly lower in MM tissues, suggesting that
CIAPIN1 expression may be correlated with the development of MM and
thus act as a potential novel diagnostic marker.
CIAPIN1 expression was modified in MM cell lines by
lentiviral transduction, and in vitro and in vivo
cell proliferation was subsequently investigated. The results
suggested that CIAPIN1 arrested the cell cycle at the G1/S phase
and inhibited the growth of MM cells. In order to further explore
how CIAPIN1 inhibits the growth of MM cells, the expression of
molecules involved in cell cycle regulation (CDK and CDK inhibitor
molecules) was investigated in primary MM and transfected cells.
CDK2 and CDK4 are involved in G1/S transition (8,9). The
expression of cyclin D and CDK4 promotes the phosphorylation of Rb
during the early to mid-G1 phase of the cell cycle (10). At the mid-G1 point of the cell
cycle and towards the end of the G1/S phase, the expression of CDK2
also enhances the phosphorylation of Rb (11). The p27 protein negatively regulates
cell cycle progression and inhibits the activity of a variety of
cyclins and CDKs. However, p27 primarily inhibits kinases that are
involved in the G1 phase, such as the cyclin E-CDK2 and cyclin
D-CDK4 kinase complexes, p27 therefore prevents cells from
progressing to the S phase (12,13).
The present study indicates that CIAPIN1 is involved in cell cycle
progression, in part by inhibiting the expression of CDK4 and CDK2.
In addition, it enhances the expression of p27, thereby inhibiting
Rb phosphorylation. Furthermore, the proliferative effects of IGF-1
on the interleukin-6-dependent and non-dependent MM cell lines have
previously been investigated (14). Following chemotherapy, patients
with low levels of serum IGF-1 exhibited reduced utilization of
IGF-1 in the bone marrow, a reduction in growth stimulated by
paracrine signaling and a remission of malignant clone (15). The results of the present study
suggest that the upregulation of CIAPIN1 expression suppresses the
expression of IGF-1 and may inhibit IGF-1-induced proliferation,
apoptosis, adhesion, migration and drug resistance in MM cells.
In conclusion, the present study suggests that
CIAPIN1 may function as a tumor suppressor due to its reduced
expression in MM cells. The results indicate that CIAPIN1 may
suppress tumor growth through inhibition of CDKs and IGF-1.
However, a more precise mechanism remains to be further
elucidated.
Acknowledgments
This study was supported by grants from the National
clinical key subject construction project funds in China.
References
|
1
|
Sirohi B and Powles R: Multiple myeloma.
Lancet. 363:875–887. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shibayama H, Takai E, Matsumura I, et al:
Identification of a cytokine-induced antiapoptotic molecule
anamorsin essential for definitive hematopoiesis. J Exp Med.
199:581–592. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanakura Y: Regulation and dysregulation
of hematopoiesis by a cytokine-induced antiapoptotic molecule
anamorsin. Hematology. 10(Suppl 1): 73–75. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hao Z, Li X, Qiao T, Zhang J, Shao X and
Fan D: Distribution of CIAPIN1 in normal fetal and adult human
tissues. J Histochem Cytochem. 54:417–426. 2006. View Article : Google Scholar
|
|
5
|
Li X, Hao Z, Fan R, et al: CIAPIN1
inhibits gastric cancer cell proliferation and cell cycle
progression by downregu-lating CyclinD1 and upregulating P27.
Cancer Biol Ther. 6:1539–1545. 2007. View Article : Google Scholar
|
|
6
|
He L, Wang H, Jin H, et al: CIAPIN1
inhibits the growth and proliferation of clear cell renal cell
carcinoma. Cancer Lett. 276:88–94. 2009. View Article : Google Scholar
|
|
7
|
Pan Y, Zhao L, Liang J, Liu J, Shi Y, Liu
N, Zhang G, Jin H, Gao J, Xie H, Wang J, Liu Z and Fan D: Cellular
prion protein promotes invasion and metastasis of gastric cancer.
FASEB J. 20:1886–1888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pagano M, Pepperkok R, Lukas J, Baldin V,
Ansorge W, Bartek J and Draetta G: Regulation of the cell cycle by
the Cdk2 protein kinase in cultured human fibroblasts. J Cell Bio.
121:101–111. 1993. View Article : Google Scholar
|
|
9
|
Harbour JW, Luo RX, Dei Santi A, Postigo
AA and Dean DC: Cdk phosphorylation triggers sequential
intramolecular interactions that progressively block Rb functions
as cells move through G1. Cell. 98:859–869. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Satyanarayana A and Rudolph KL: p16 and
ARF: activation of teenage proteins in old age. J Clin Invest.
114:1237–1240. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nevins JR: The Rb/E2F pathway and cancer.
Hum Mol Genet. 10:699–703. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheaff RJ, Groudine M, Gordon M, Roberts
JM and Clurman BE: Cyclin E-cdk2 is a regulator of p27Kip1. Genes
Dev. 11:1464–1478. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Polyak K, Kato JY, Solomon MJ, Sherr CJ,
Massague J, Roberts JM and Koff A: p27Kip1, a cyclin-Cdk inhibitor,
links transforming growth factor-beta and contact inhibition to
cell cycle arrest. Genes Dev. 8:9–22. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Georgii-Hemming P, Wiklund HJ, Ljunggren O
and Nilsson K: Insulin-like growth factor 1 is a growth and
survival factor in human multiple myeloma cell lines. Blood.
88:2250–2258. 1996.PubMed/NCBI
|
|
15
|
Standal T, Borset M, Lenhoff S, et al:
Serum insulin-like giowth factor is not elevated in patients with
multiple myeloma but is still a prognostic factor. Blood.
100:3925–3929. 2002. View Article : Google Scholar : PubMed/NCBI
|