Introduction
Pituitary adenomas are common intracranial tumors,
with an incidence of 15% in intracranial neoplasms (1–4).
Clinically, aggressive pituitary adenomas are observed in up to 33%
of pituitary adenomas (5,6). Despite surgery combined with
radiation- and chemotherapy, patients with aggressive pituitary
adenomas still have a poor prognosis due to aggressive tumor
characteristics and the lack of effective therapies, which remains
an enormous therapeutic challenge (7–10).
Temozolomide (TMZ) is currently considered the most
promising chemotherapeutic drug in the treatment of aggressive
pituitary adenomas resistant to conventional standard therapies
(11–15). However, the efficacy of TMZ
treatment in aggressive pituitary adenoma patients in clinical
trials varies largely (12,16–18).
Numerous patients who were treated at our clinic were classified as
TMZ nonresponders. Furthermore, patients displaying an initial
response to TMZ treatment may present with tumor relapse after a
certain number of treatment cycles due to increasing TMZ resistance
(16,17). These treatment shortfalls justify
an urgent requirement for novel therapeutic approaches to improve
the TMZ efficacy in pituitary adenomas.
In a previous study by our group using primary
cultured human pituitary adenoma cells, experimental evidence
suggested that O6-methylguanine-DNA methyltransferase (MGMT) is the
key factor responsible for chemoresistance to TMZ, and
2-methoxyestradiol (2ME) may be applied as a mediator of TMZ
resistance via the downregulation of MGMT expression (19). Besides 2ME, disulfiram (DSF),
having the ability to cross the blood-brain barrier, has recently
been reported to be a potential inhibitor of MGMT in glioblastoma
cells (20). Whether DSF can
inhibit MGMT expression and improve the efficacy of TMZ in human
pituitary adenomas has remained elusive. In the present study, the
effect of DSF on MGMT protein expression levels and on the
anti-tumor effect of TMZ on primary cultured human pituitary
adenoma cells and isolated CD133+ nestin+ phenotype stem like cells
was investigated in vitro. Furthermore, the sensitizing
effect was also assessed in differentiated human pituitary adenoma
cells in vivo.
Materials and methods
Patients and samples
The present study was approved by the Research
Ethics Committee of the Henan University of Science and Technology
(Luoyang, China). Prior informed consent was obtained from the
patients harboring huge aggressive pituitary adenomas. The
aggressive pituitary adenoma characteristic of the patients was
classified based on magnetic resonance imaging (MRI) results and
history of illness prior to surgery, which showed pituitary
adenomas of large size, massive invasion of surrounding anatomical
structures and rapid growth indicated by comparison with their
previous MRI scans. Between June 2012 and January 2014, after
surgery for patients harboring huge aggressive pituitary adenomas,
the pituitary adenoma fragments were divided into three parts; one
part was sent to the Pathology Department, one part was
investigated for MGMT and Ki67 using western blot analysis, and
another part was primarily cultured for further possible analysis.
Two MGMT-negative human pituitary-null cell adenoma tissues were
identified and used as controls. Whenever western blot analysis of
tumor tissues showed that the pituitary adenoma was strongly
MGMT-positive, further primary culture or tumor implantation in
nude mice was performed. Twelve strongly MGMT-positive pituitary
adenoma samples and two MGMT-negative pituitary adenoma samples
were successfully primarily cultured or transplanted into nude mice
in the present study.
Primary culture of pituitary adenoma
tissue
The human pituitary adenoma tissues was processed
according to the standard protocols as described in a previous
study by our group (19) directly
following surgery. The tissue was enzymatically digested using the
Human Tumor Dissociation Kit (#130-095-929; Miltenyi Biotec,
Bergisch Gladbach, Germany) with the gentle MACS Dissociator
(#130-093-235; Miltenyi Biotec). After dissociation, the sample was
filtered using anti-human Fibroblast MicroBeads (#130-050-601;
Miltenyi Biotec). The cells were then counted and cultured in
16-(106 cells/well) or 96-well plates (104
cells/well) in complete DMEM/F12 medium (Gibco Life Technologies,
Carlsbad, CA, USA) with 2 mM glutamine (Gibco Life Technologies),
15% horse serum (Gibco Life Technologies) and 2.5% fetal bovine
serum (Gibco Life Technologies) for primary culture, with reference
to the study by Xu et al (21).
Isolation for pituitary adenoma stem-like
cells
The isolation of stem-like cells was performed as
described in a previous study by our group (22). In brief, the human pituitary
adenoma cells in primary culture were dispersed, counted and
suspended (108 cells) in magnetic micro beads buffer
(#130-091-376; Miltenyi Biotec) in a final volume of 600 µl.
The suspension was added in FcR blocking reagent (#130-059-901; 200
µl) and 200 µl CD133 MACS micro beads (#130-050-801;
Miltenyi Biotec). The suspension was then mixed and incubated for
15 min at 4°C. Using a MACS Separator (#130-042-602; Miltenyi
Biotec), the suspension was passed through a CD133 column, the
column was placed on a collection tube, and the CD133-positive
cells were obtained. Then, the resuspended cells were cultured with
mouse monoclonal anti-nestin antibody (#196908; R&D Systems,
Inc., Westerville, OH, USA) for 30 min at 4°C and were washed with
phosphate-buffered saline (PBS; Wuhan Boster Biological Technology,
Ltd., Wuhan, China). The cells were then mixed with anti-mouse IgG
MicroBeads (#130-048-401; Mitenyi Biotec) and proceeded to magnetic
separation as described above. In reference to the protocol of a
previous study by Xu et al (21) for primary cultural pituitary
adenoma cells prior to isolation, the cells were cultured in
DMEM/F12 (1:1; Gibco Life Technologies), B27 (1X; Gibco Life
Technologies), penicillin/streptomycin (200 U/ml; Invitrogen Life
Technologies, Carlsbad, CA, USA), fungizone (250 ng/ml; Peprotech,
Inc., Rocky Hill, NJ, USA), epidermal growth factor (20 ng/ml;
Peprotech, Inc.) and basic fibroblast growth factor (20 ng/ml;
Peprotech, Inc.) prior to and after isolation in order to avoid
cell differentiation.
Cell viability assay, western blot
analysis, mitotic catastrophe assessment, flow cytometry,
bromodeoxyuridine (BrdU) and terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
In the present study, the protocols and the reagents
of the cell counting kit (CCK)8 cell viability assay, western blot
analysis, mitotic catastrophe assessment and flow cytometry were
identical to those described in previous studies by our group
(23–27). For the CCK8 assay, the Cell
Counting Kit (CCK-8/WST-8) was used (AR1160-500; Wuhan Boster
Biological Technology, Ltd.). The BrdU assay was conducted using
the BrdU Cell Proliferation Assay kit (#551321; BD Biosciences, La
Jolla, CA, USA). Mice were injected intraperitoneally with 200
µl (3 mg/ml) BrdU solution 2 h prior to scarification.
Subsequently, cellular incorporation of BrdU was detected by
immunohistochemistry using anti-BrdU specific antibodies. The TUNEL
assay was performed using the ApopTag Peroxidase In situ Apoptosis
Detection kit (#S7100; Merck Millipore, Darmstadt, Germany). The
BrdU and TUNEL assays were performed following the manufacturer’s
instructions. In brief, for the CCK8 assay, DSF (T1132,
Sigma-Aldrich, St. Louis, MO, USA) and TMZ (T2577; Sigma-Aldrich)
were added at various concentrations to the primary culture of
human pituitary adenoma cells for 24 h, the CCK8 assay reagent was
added to each well of the 96-well plate, followed by 1 h of
incubation at 37°C. Absorbance was read at 450 nm using the Thermo
Multiskan Ascent plate reader (Thermo Fisher Scientific, Rockford,
IL, USA). For mitotic catastrophe assessment, the cells were fixed
with cold methanol (4°C; Maixin Biotech Co., Fuzhou, China) and
stained with DAPI for chromosome analysis under an Olympus BX41
fluorescence microscope (Olympus Corp., Tokyo, Japan). Mitotic
catastrophe is defined as the presence of ≥2 nuclear lobes within a
single cell. For western blot analysis, equal aliquots (30
µg) of protein were separated using 10–12% SDS-PAGE gel and
transferred to nitrocellulose membranes (EMD Millipore, Shanghai,
China). The membranes were incubated with relevant primary
antibodies overnight at 4°C, the following antibodies were used:
Rabbit polyclonal anti-MGMT antibody (FL-207) (1:1,000; sc-28241,
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), mouse monoclonal
anti-γH2AX antibody (1:500; JBW 301, Upstate Biotechnology,
Milford, MA, USA), rabbit polyclonal anti-Rad51 (H-92) antibody
(1:1,000; sc-8349, Santa Cruz Biotechnology, Inc.), mouse
monoclonal anti-GAPDH (A-3) antibody (1:3,000; sc-137179, Santa
Cruz Biotechnology, Inc.). Subsequently, the membranes were
incubated in horseradish peroxidase-conjugated secondary antibody
(goat anti-mouse IgG; 1:5,000; #31431; Thermo Fisher Scientific)
and goat anti-rabbit IgG (1:5,000; #31466; Thermo Fisher
Scientific) for 1.5 h at room temperature. The membranes were then
treated using Pierce ECL Western Blotting Substrate (Thermo Fisher
Scientific) and visualized using a BioRad Universal Hood II Imager
(Bio-Rad Laboratories, Inc., Kenilworth, NJ, USA). For surface
marker identification, the expression of the CD133 and nestin
markers in cells was determined by flow cytometry after surface
staining with anti-human mouse monoclonal CD133/1(AC133)-PE
(#130-080-801; Mitenyi Biotec) and mouse monoclonal anti-nestin
(Ab81216; Abcam, Cambridge, MA, USA) antibodies. The cells were
fixed with 4% paraformaldehyde (10 min; Solarbio, Beijing, China)
and then permeabilized with 0.1% phosphate-buffered saline
(PBS)-Tween (Wuhan Boster Biological Technology, Ltd.) for 20 min.
The cells were then incubated in 1 × PBS/10% normal goat serum
(Wuhan Boster Biological Technology, Ltd.)/0.3 M glycine (Solarbio)
to block non-specific protein-protein interactions followed by the
antibody (1:200 dilution) for 30 min at 22°C. The secondary
antibody used was DyLight-488 goat anti-mouse IgG (H+L) (ab96879;
Abcam) at 1:500 dilution for 30 min at 22°C. Flow cytometry was
performed on a FACSCanto II (BD Biosciences) and analyzed using BD
FCSDiva software, version 6 (BD Biosciences) and FCS Express 4
software (DeNovo Software, Glendale, CA, USA). For apoptosis
analysis, the cells were suspended in Annexin V-fluorescein
isothiocyanate (FITC) binding buffer (195 µl) and Annexin
V-FITC (5 µl) in the dark for 10 min. The cells were
centrifuged at 1,000 × g/min for 5 min and were suspended in
binding buffer (190 µl) and 10 µl propidium iodide
solution on ice in the dark for flow cytometric analysis.
Pituitary adenoma xenografts
Experiments on mice were conducted in line with
protocols from the Institutional Animal Care and Use Committee and
were approved by the Research Ethics Committee of the Henan
University of Science and Technology (Luoyang, China). In the
current study, 3–4 weeks old male BALB/c nude mice weighing 18–22 g
were purchased from Shanghai Animal Center (Shanghai, China) and
were housed five per cage in a specific pathogen-free (SPF)
environment. The mice were kept on a 12-hour reversed light/dark
cycle, the temperature was maintained at 25°C, they were fed with
SPF pellet diet (Beijing Huafukang Company, Beijing, China) and
water was supplied. As described in previous studies by our group
(23–25,27),
in order to increase the tumor formation rate, the tissues were
dissociated and then implanted subcutaneously into the three nude
mice. Once the tumors were formed after two weeks, the mice were
sacrificed by cervical dislocation and tumors from nude mice were
dissociated and equally implanted subcutaneously into the lower
rear flank of twelve six-week-old nude mice again. After the tumors
had formed (following two weeks), the mice were injected with
saline, TMZ (3 mg/kg), DSF (50 mg/kg) or TMZ (3 mg/kg) plus DSF (50
mg/kg) for five days (each group, n=3). Tumors were then harvested
for further protein and histology analysis.
Statistical analysis
Statistical analyses were conducted using SPSS 13.0
(SPSS, Inc., Chicago, IL, USA). Values are expressed as the mean ±
standard deviation. Results were assessed using the Student’s
t-test and analysis of variance. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
DSF decreases MGMT protein expression in
human pituitary adenoma cells in primary culture
DSF has recently been shown to induce loss of MGMT
protein in T98G and UW228 MGMT-proficient human glioblastoma cells
(20); however, whether DSF can
also downregulate the MGMT protein expression levels in human
primary cultural pituitary adenoma cells has not been studied. Due
to the lack of any well-established human pituitary adenoma cell
line, human pituitary adenoma cells were obtained via primary
culture of human pituitary adenoma tissue. DSF was added at various
concentrations (0, 10, 25 and 50 µmol/l) to the primary
culture of human pituitary adenoma cells for 24 h, followed by CCK8
assay and western blot analysis. As shown in Fig. 1A, the CCK8 assay showed that DSF
alone in serum-free medium had no significant effect on the
viability of the human pituitary adenoma cells, even at a
concentration of 50 µmol/l (P=0.835). However, DSF treatment
significantly inhibited the MGMT protein expression levels in human
primary cultural pituitary adenoma cells (Fig. 1B).
DSF inhibits protein expression of MGMT
in putative pituitary adenoma stem-like cells
Recently, putative pituitary adenoma stem-like cells
were isolated from the primary culture of human pituitary adenomas
(21). The CD133- and
nestin-expressing pituitary adenoma cells isolated from primary
culture in the present study were capable of self-renewal and
multipotent differentiation in vitro as well as initiation
of serially transplantable pituitary tumors in vivo
(21). The above evidence
supported, at least to a certain extent, the tumor stemness of the
isolated CD133+ nestin+ pituitary adenoma cells.
In previous studies by our group, cancer stem cells
were isolated using magnetic microbeads (26). Furthermore, human pituitary adenoma
cells from adenoma fragments were primarily cultured for studying
the role of MGMT in human pituitary adenomas (27). Based on these previous studies, the
present study also established primary cultures of human pituitary
adenoma samples and isolated seemingly pituitary adenoma stem-like
cells using CD133- and nestin-specific magnetic microbeads followed
by flow cytometric cell sorting. As shown in Fig. 2C and D, the percentages of the
CD133+ nestin+ cell sub-populations in the total pituitary adenoma
cells in primary culture and isolated CD133+ nestin+ stem-like
cells were 0.31±0.14% and 52.13±15.13%, respectively. Although the
pituitary adenoma stem-like cell phenotype has not been completely
defined, the CD133+ nestin+ pituitary adenoma cells in primary
culture indeed generated spheres for several passages in the
present study (Fig. 2A and B),
indicating the self-renewal ability of these cells. The cells from
spheres can also differentiate into cells with a morphology similar
to that of human pituitary adenoma cells (Fig. 2E). Moreover, a small number of
cells (10,000) that were injected intracranially and incubated for
6 weeks, formed xenograft tumors in the right hemisphere of nude
mice (Fig. 2F), suggesting the
differentiation capacity and high tumorigenic ability of the CD133+
nestin+ pituitary adenoma stem-like cells.
 | Figure 2Culture and characterization of the
CD133+ nestin+ cell population of the human pituitary adenoma
cells. (A) Culture of isolated CD133+ nestin+ cells growing as
non-adherent spheres (magnification, ×40). (B) Spheroids of CD133+
nestin+ cells (magnification, ×400). (C) Representative cytometric
dot-plots of CD133- and nestin-positive cells among human pituitary
adenoma cells, displayed by the flow cytometric cell sorting using
mouse monoclonal CD133 antibody and mouse monoclonal nestin
antibody. (D) Representative cytometric dot-plots of CD133- and
nestin-positive cells in isolated CD133+ nestin+ stem like cells,
displayed by the flow cytometric cell sorting using mouse
monoclonal CD133 antibody and mouse monoclonal nestin antibody. (E)
Differentiation capacity of the isolated CD133+ nestin+ cells
(magnification, ×100). (F) High tumorigenic ability of CD133+
nestin+ cells. Hematoxylin and eosin staining of tumors generated
from 10,000 CD133+ nestin+ cells in nude mice (magnification, ×10
and ×100). (G) As shown in the western blot, DSF (50
µmol/l)inhibited MGMT protein expression in isolated CD133+
nestin+ cells. GAPDH served as the loading control. Con, control;
MGMT, O6-methylguanine-DNA methyltransferase; DSF, disulfiram. |
Since DSF was shown to inhibit MGMT in
differentiated pituitary adenoma cells, the present study assessed
whether DSF can also downregulate the MGMT protein expression
levels in CD133+ nestin+ pituitary adenoma stem-like cells. As
shown in Fig. 2G, western blot
analysis showed that DSF (50 µmol/l) treatment inhibited the
MGMT protein expression levels in CD133+ nestin+ pituitary adenoma
stem-like cells.
DSF treatment sensitizes MGMT-proficient
human pituitary adenoma cells and the isolated CD133+ nestin+
stem-like cells to TMZ chemotherapy in vitro
A previous study by our group (27), it was demonstrated that
2-methoxyestradiol (2ME) increased the efficacy of TMZ in human
primary culture pituitary adenoma cells in vitro and in
vivo. In the present study, using the CCK8 assay on pituitary
adenoma in primary culture, the sensitizing effect of DSF of
pituitary adenoma cells from MGMT-negative and MGMT-positive
pituitary adenomas, respectively, to TMZ treatment was assessed.
Saline, TMZ (100 µmol/l), DSF (25 µmol/l) or TMZ plus
DSF was added to the primary cultural human pituitary adenoma cells
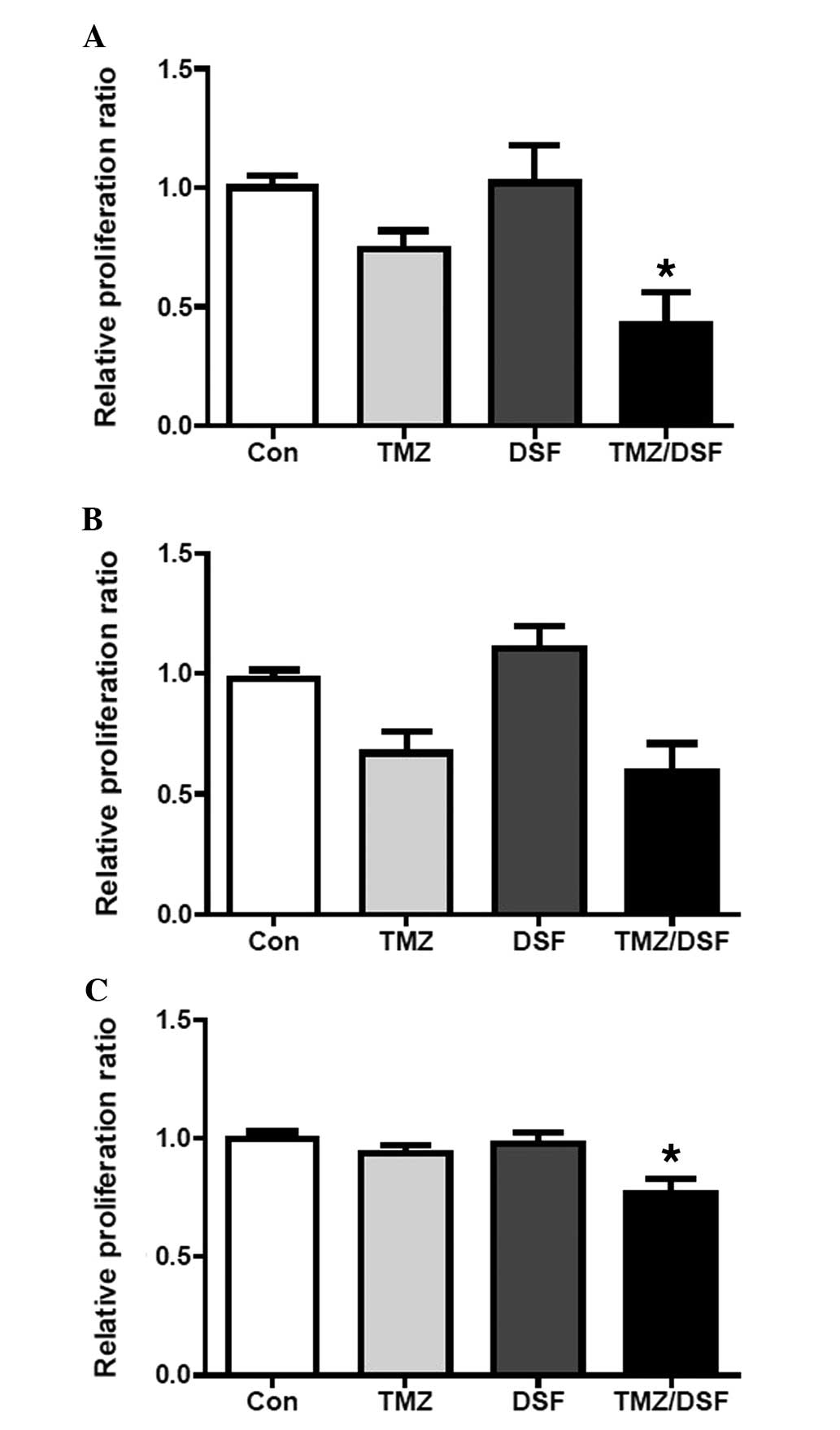
for 24 h. As shown in Fig. 3A, the
CCK8 assay showed that the relative proliferation ratio of strongly
MGMT-positive cells in the TMZ, DSF and TMZ plus DSF groups was
0.73±0.07, 1.02±0.16 and 0.44±0.16, respectively (TMZ vs. TMZ/DSF;
P=0.025), while the relative proliferation ratio of MGMT-negative
cells in the TMZ, DSF and TMZ plus DSF groups was 0.67±0.09,
1.10±0.11 and 0.59±0.12, respectively (TMZ vs. TMZ/DSF; P=0.405)
(Fig. 3B). These results indicated
that DSF significantly enhanced the sensitivity of MGMT-positive,
but not MGMT-negative cells, to TMZ.
The present study also determined this effect on
pituitary adenoma stem like cells. Saline, TMZ (100 µmol/l),
DSF (25 µmol/l) or TMZ plus DSF was added to the isolated
CD133+ nestin+ human pituitary adenoma stem-like cells in primary
culture for 24 h. As expected, the CCK8 assay showed that the
relative proliferation ratio of isolated CD133+ nestin+ human
pituitary adenoma stem-like cells in the TMZ, DSF and TMZ plus DSF
groups was 0.94±0.05, 0.98±0.08 and 0.75±0.13, respectively (TMZ
vs. TMZ/DSF; P=0.0425) (Fig. 3C),
indicating that DSF sensitized the isolated CD133+ nestin+ human
pituitary adenoma stem-like cells to TMZ.
DSF sensitizes MGMT-proficient human
pituitary adenoma xenografts to TMZ chemotherapy in vivo
To translate the in vitro findings of the
present study into murine models of pituitary adenoma, the TMZ
sensitization efficacy of DSF was evaluated in xenografts of
MGMT-positive human pituitary adenoma cells from primary culture in
athymic nude mice. As described above, once the subcutaneous
xenografts formed, the mice were treated with saline, TMZ (3
mg/kg), DSF (50 mg/kg) or TMZ plus DSF for five days. BrdU, TUNEL
and MGMT staining were then performed. Although no significant
difference in tumor size was identified between the different
groups, the ratio of apoptotic cells in the control, TMZ, DSF and
TMZ plus DSF groups was 0.31±0.11, 1.32±0.13, 0.35±0.14 and
3.04±0.93%, respectively (TMZ vs. TMZ/DSF; P=0.0380). The ratio of
BrdU-stained cells in the TMZ, DSF and TMZ plus DSF groups was
10.80±1.21, 5.33±1.27, 11.55±1.14 and 2.30±1.03%, respectively (TMZ
vs. TMZ/DSF; P=0.0739). The TMZ plus DSF group displayed the lowest
BrdU staining levels and highest TUNEL staining levels (Fig. 4). Compared with those in the other
groups, the MGMT expression levels were downregulated in the
DSF-treated groups (Fig. 4E).
These results suggested that DSF sensitized pituitary adenoma cells
to TMZ in vivo.
DSF treatment facilitates TMZ-induced
mitotic catastrophe and apoptosis
Apoptosis, mitotic catastrophe and cell cycle arrest
are known to be the main underlying mechanisms of the anti-tumor
effect of TMZ (28). To
characterize which of the above mechanisms of action of TMZ was
facilitated by DSF in pituitary adenoma cells, the apoptosis,
mitotic catastrophe and cell cycle arrest were determined in
pituitary adenoma cells treated with TMZ (100 µmol/l), DSF
(25 µmol/l), TMZ plus DSF or saline (control) for 24 h. As
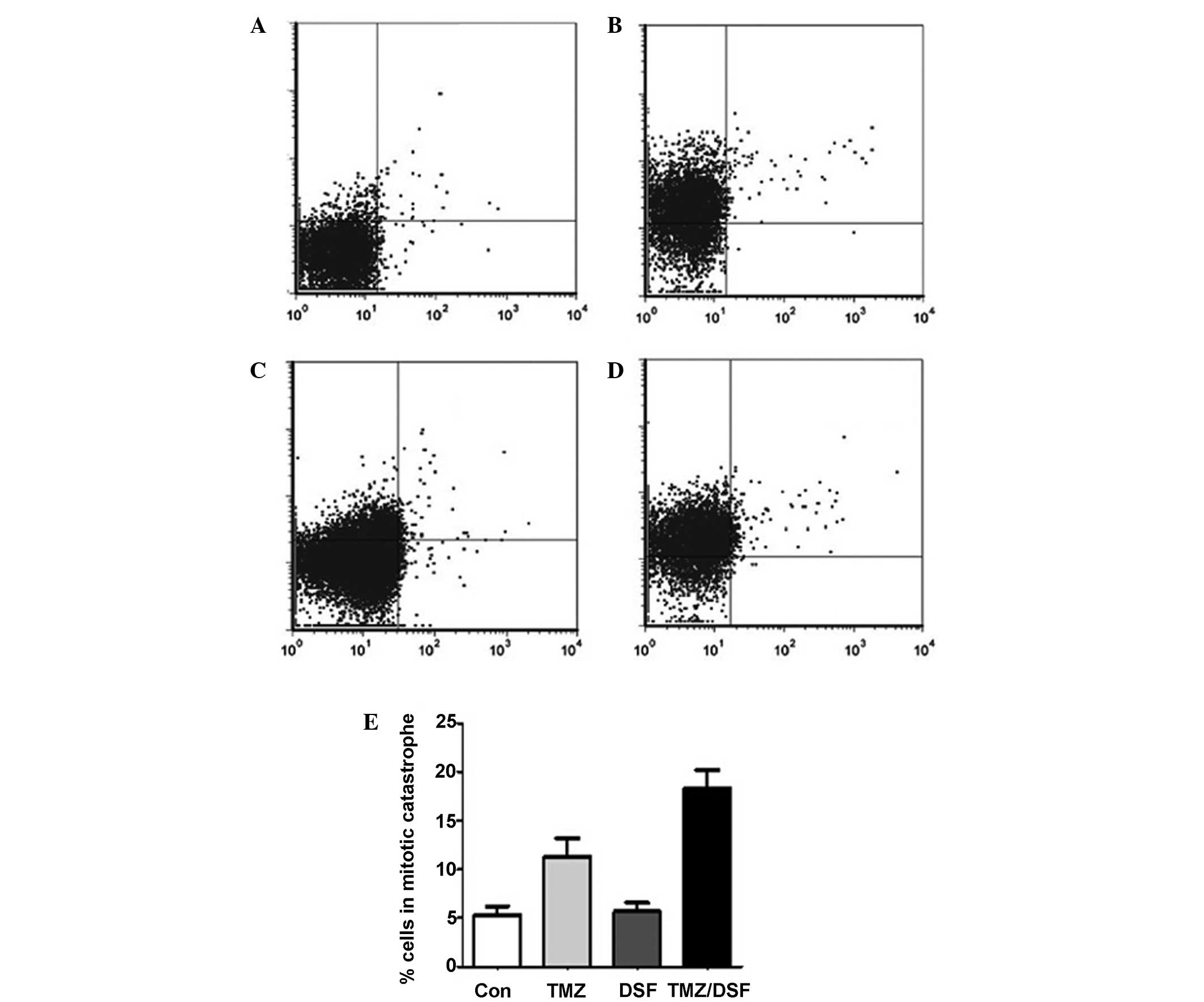
shown in Fig. 5, the ratio of
apoptotic cells in the control, TMZ, DSF and TMZ plus DSF groups
was 0.29±0.09, 0.81±0.23, 0.31±0.10 and 1.64±0.16%, respectively
(TMZ vs. TMZ/DSF; P=0.0427), which showed that the pituitary
adenoma cells treated with DSF plus TMZ displayed an increased
apoptotic cell population compared with cells exposed to TMZ alone.
As expected, the ratio of cells in mitotic catastrophe, defined as
the presence of ≥2 nuclear lobes within a single cell (29), in the control, TMZ, DSF and TMZ
plus DSF groups was 5.35±1.11, 12.91±2.21, 5.65±1.06 and
18.27±2.34%, respectively (TMZ vs. TMZ/DSF; P=0.0560; non
significant). Compared to cells treated with TMZ alone, increased
mitotic catastrophe was observed in the DSF plus TMZ-treated cells
(Fig. 5E). However, in contrast to
what was expected, no significant changes in the cell cycle were
detected in any of the groups (data not shown). As the saline group
displayed no changes in the cell cycle distribution compared with
that in the other groups in the present study, which was not in
line with the results of previous studies (28,30),
this may suggest that the dose of the drugs used in the present
study may not have been sufficient to affect the cell cycle;
furthermore, the diversity of the primarily cultured cells may also
have contributed to this inconsistency.
In spite of this inconsistency, the results still
indicate that DSF enhances the cytotoxicity of TMZ by increasing
the occurrence of mitotic catastrophe and induction of apoptosis.
The results of the present study neither confirm nor deny the
involvement of cell cycle arrest in DSF-facilitated TMZ
toxicity.
Pre-treatment with the proteasome
inhibitor PS-341 abrogates the inhibition of MGMT by DSF in
vitro
The effect of DSF on MGMT has been reported to be
eliminated through inhibition of the ubiquitin (ub)-proteasomal
route (20,31–34).
To determine whether the enhancement of MGMT by DSF observed in the
present study proceeded via the ubiquitin-proteasomal route, the
proteasome inhibitor PS-341 was used. In accordance with a previous
study by Paranjpe et al (20), the pituitary adenoma cells were
pre-treated with PS-341 (10 µmol/l) for 6 h, followed by 50
µmol/l DSF for 12 h in the present study. Subsequent western
blot analysis showed that DSF inhibited MGMT protein expression,
while PS-341 pre-treatment attenuated the DSF-induced loss of MGMT
protein expression (Fig. 6). These
results suggested that the interference of DSF with MGMT expression
proceeds via the ub-proteasomal route.
DSF treatment inhibits the repair of
TMZ-induced DNA double strand breaks (DSBs)
It is known that MGMT is a unique anti-mutagenic DNA
repair protein, which transfers alkyl groups to the active site of
the cysteine residue as part of the DNA damage response (18,35–37).
It was hypothesized that DSF inactivated the MGMT protein and
inhibited the repair function of MGMT, which in turn caused an
accumulation of DNA double strand breaks induced by TMZ, ultimately
resulting in cell death. To confirm this mechanism, pituitary
adenoma cells were treated with TMZ (100 µmol/l), DSF (25
µmol/l), TMZ plus DSF (25 µmol/l) or saline (control)
for 24 h. As phosphorylation of histone H2Ax, resulting in γH2AX,
is correlated with DSBs, and RAD51 is a protein marker associated
with DNA damage repair (5,38–40),
the γH2AX and RAD51 levels were examined by western blot analysis.
As expected, the results revealed that, compared to the TMZ-treated
cells, DSF plus TMZ treatment resulted in increased γH2AX and
decreased RAD51 levels (Fig. 7).
These results indicated that the reduction of MGMT levels by DSF
inhibits the MGMT-mediated repair of TMZ-induced DSBs.
Discussion
TMZ, an orally administered alkylating agent, was
the first chemotherapeutic agent showing anti-tumor activity
against aggressive pituitary adenomas (11,12,16).
TMZ has been widely used as salvage therapy against aggressive
pituitary adenomas resistant to conventional treatment, including
surgery, dopamine agonists, somatostatin analogues and radiotherapy
(7,11–14,16,17,35,41,42).
A previous study by our group confirmed that the DNA repair protein
MGMT in human pituitary adenomas is closely associated with the
tumor resistance to TMZ (19).
DSF, which has been approved by the Food and Drug Administration
for the treatment of alcoholism since 1951, was recently reported
to inhibit MGMT protein expression and sensitize glioblastoma cells
to TMZ in vitro and in vivo (20). Compared to other potential MGMT
inhibitors, DSF has numerous advantages: Oral administration, the
ability to cross the blood-brain barrier as well as established
pharmacokinetics and drug safety (31–34,43,44),
all of which imply patient compliance and feasibility of fast
clinical application. Based on these features, the present study
hypothesizes that DSF may be used to inhibit MGMT activity in human
pituitary adenomas in the clinic. In this case DSF has the
potential to be used as an adjuvant therapy to chemotherapy with
TMZ. This is likely to improve the efficacy of the treatment of
aggressive pituitary adenomas resistant to conventional treatment,
which is of key importance for patients with these tumors, as they
are usually treated with TMZ as the last line therapy in clinical
practice.
As there is no well-established human pituitary
adenoma cell line (45,46), the present study established
primary cultures of human pituitary adenoma cells from tumor
samples. It was found that DSF inhibited MGMT expression in these
human pituitary adenoma cells in primary culture. It is known that
mitotic catastrophe, apoptosis and G2/M cell cycle arrest are the
main anti-tumor mechanisms of TMZ (28,30).
In the present study, compared to TMZ alone, DSF plus TMZ treatment
resulted in increased occurrence of mitotic catastrophe and
apoptosis in human pituitary adenoma cells in primary culture.
Treatment with DSF plus TMZ increased the levels of γH2AX while
decreasing protein expression levels of RAD51 and MGMT, indicating
that DSF inhibited the DNA repair ability of MGMT protein in
pituitary adenoma cells. However, treatment with DSF alone had no
significant anti-tumor effects on pituitary adenoma cells. All
these results suggested that DSF is able to improve the anti-tumor
efficacy of TMZ in human pituitary adenoma cells in primary
culture.
It is known that classic chemotherapeutic agents can
only eliminate highly differentiated and rapidly dividing tumor
cells, while less differentiated and slower proliferating tumor
stem cells are predisposed to be spared by chemotherapeutic agents
(47–49). Due to this premise, classical
chemotherapeutic agents are likely fail to obtain successful
long-term disease remission (47–49).
Recently, putative pituitary adenoma stem-like cells highly
expressing nestin and CD133 were obtained from human pituitary
adenomas; these cells also displayed increased resistance to
chemotherapeutic drugs (21). In
reference to the abovementioned study (21), the present study also isolated
seemingly pituitary adenoma stem-like cells using CD133- and
nestin-specific magnetic microbeads. The isolated cells were shown
to be able to generate spheres, differentiate into normal pituitary
adenoma cells and form tumor xenografts in the right hemisphere of
nude mice, indicating the self-renewal ability, multilineage
differentiation and in vivo tumorigenicity of these cells.
The present study also found that DSF can inhibit the expression of
MGMT and sensitize pituitary adenoma stem like cells to TMZ.
However, in the present study, numerous attempts to isolate nestin-
and CD133-positive cells failed. These failures may be due to the
rarity of the aggressive MGMT-positive pituitary adenoma tissue in
the clinic, immaturity of the primary culture and the stem cell
isolation technology; associated experiments are still ongoing in
our laboratory.
It is known that DSF inactivate MGMT activity
through eliminating MGMT protein via the ub-proteolysis pathway
(20,31–34,43,44).
The mode of elimination of DSF-modified MGMT protein in human
pituitary adenoma cells was further investigated in the present
study. In line with a previous study (20), the proteasome inhibitor PS-341
pretreatment attenuated the DSF induced MGMT reduction. These
results present evidence that DSF can eliminate the MGMT protein
through the ub-proteolysis pathway in pituitary adenomas.
Limitations of the present study include the fact
that the cells were derived from a small number of human pituitary
adenomas, and more human pituitary adenomas are required to be
analyzed in future studies. The diversity in primary cultured human
pituitary adenoma cells also undermined the significance of the
results of the present study. A mature human-derived pituitary cell
line should be developed to better evaluate the effects of
therapeutic agents in human pituitary adenomas. Due to the low
abundance of human pituitary adenoma tissues as well as the
requirement for development of primary culture and stem cell
isolation technology, the isolation for human pituitary adenoma
stem-like cells is currently difficult. During the establishment of
cultures, a large quantity of valuable but limited human aggressive
pituitary adenoma tissues was wasted in the isolation process. As
the numbers of acquired human pituitary adenoma stem-like cells are
generally low, only a small number of studies using human pituitary
adenoma stem-like cells have been performed to date. Further
studies are required to improve the primary culture and stem cell
isolation technology. Finally, due to the immature cellar
implantation technology and the rarity of the primarily cultured
cells, the tumor formation rate was low. The present study only
verified the tumor initiation ability of the isolated stem-like
cells in the right cerebral hemisphere of nude mice. The cells were
implanted subcutaneously into the lower rear flank of nude mice in
the other in vivo experiments, while intracranial
implantation of cells in the right cerebral hemisphere of nude mice
may have been able to improve the significance of the in
vivo experiment in the present study, by better mimicking
pathological and physiological behavior of pituitary adenoma cells.
Further studies using the intracranial implantation of pituitary
adenoma cells are required.
In spite of these limitations, the present study
presented the first evidence that DSF can inhibit MGMT levels and
sensitize human pituitary adenoma cells and stem like cells to the
anti-tumor drug TMZ. Although these results remain to be translated
into clinical applications with caution, the sensitizing effect of
DSF in the setting of TMZ therapy may offer added benefit. Further
characterization of the benefit-to-risk profile of TMZ plus DSF in
patients with aggressive pituitary adenomas refractory to
conventional treatments is required to justify this combined
treatment.
Acknowledgments
The current study was supported by a joint training
program foundation for talents in Henan Province, China (grant no.
U1404822).
References
|
1
|
Beckers A: Higher prevalence of clinically
relevant pituitary adenomas confirmed. Clin Endocrinol (Oxf).
72:290–291. 2010. View Article : Google Scholar
|
|
2
|
Tamer G, Telci A, Mert M, Uzum AK, Aral F,
Tanakol R, Yarman S, Boztepe H, Colak N and Alagöl F: Prevalence of
pituitary adenomas in macroprolactinemic patients may be higher
than it is presumed. Endocrine. 41:138–143. 2012. View Article : Google Scholar
|
|
3
|
Karavitaki N: Prevalence and incidence of
pituitary adenomas. Ann Endocrinol (Paris). 73:79–80. 2012.
View Article : Google Scholar
|
|
4
|
Ezzat S, Asa SL, Couldwell WT, Barr CE,
Dodge WE, Vance ML and McCutcheon IE: The prevalence of pituitary
adenomas: a systematic review. Cancer. 101:613–619. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Selman WR, Laws ER Jr, Scheithauer BW and
Carpenter SM: The occurrence of dural invasion in pituitary
adenomas. J Neurosurg. 64:402–407. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scheithauer BW, Kovacs KT, Laws ER Jr and
Randall RV: Pathology of invasive pituitary tumors with special
reference to functional classification. J Neurosurg. 65:733–744.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zemmoura I, Wierinckx A, Vasiljevic A, Jan
M, Trouillas J and François P: Aggressive and malignant prolactin
pituitary tumors: pathological diagnosis and patient management.
Pituitary. 16:515–522. 2013. View Article : Google Scholar
|
|
8
|
Levy A: Pituitary disease: presentation,
diagnosis and management. J Neurol Neurosurg Psychiatry. 75(Suppl
3): iii47–52. 2004. View Article : Google Scholar
|
|
9
|
Buchfelder M: Management of aggressive
pituitary adenomas: current treatment strategies. Pituitary.
12:256–260. 2009. View Article : Google Scholar
|
|
10
|
Maïza JC and Caron P: Pituitary carcinomas
and aggressive adenomas: an overview and new therapeutic options.
Ann Endocrinol (Paris). 70(Suppl 1): 12–19. 2009.In French.
View Article : Google Scholar
|
|
11
|
Syro LV, Uribe H, Penagos LC, Ortiz LD,
Fadul CE, Horvath E and Kovacs K: Antitumour effects of
temozolomide in a man with a large, invasive prolactin-producing
pituitary neoplasm. Clin Endocrinol (Oxf). 65:552–553. 2006.
View Article : Google Scholar
|
|
12
|
Neff LM, Weil M, Cole A, Hedges TR,
Shucart W, Lawrence D, Zhu JJ, Tischler AS and Lechan RM:
Temozolomide in the treatment of an invasive prolactinoma resistant
to dopamine agonists. Pituitary. 10:81–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mohammed S, Kovacs K, Mason W, Smyth H and
Cusimano MD: Use of temozolomide in aggressive pituitary tumors:
case report. Neurosurgery. 64:E773–774; discussion E774. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaltsas GA, Mukherjee JJ, Plowman PN,
Monson JP, Grossman AB and Besser GM: The role of cytotoxic
chemotherapy in the management of aggressive and malignant
pituitary tumors. J Clin Endocrinol Metab. 83:4233–4238. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou PZ, Ma WC, Wang XJ, Cheng SW and
Jiang S: Isolation and preliminary identification of stem-like
cells in pituitary adenoma. Sichuan Da Xue Xue Bao Yi Xue Ban.
44:466–469. 2013.In Chinese. PubMed/NCBI
|
|
16
|
Raverot G, Sturm N and de Fraipont F:
Temozolomide treatment in aggressive pituitary tumors and pituitary
carcinomas: a French multicenter experience. J Clin Endocrinol
Metab. 95:4592–4599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bush ZM, Longtine JA, Cunningham T, Schiff
D, Jane JA Jr, Vance ML, Thorner MO, Laws ER Jr and Lopes MB:
Temozolomide treatment for aggressive pituitary tumors: correlation
of clinical outcome with O (6)-methylguanine meth-yltransferase
(MGMT) promoter methylation and expression. J Clin Endocrinol
Metab. 95:E280–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kovacs K, Scheithauer BW, Lombardero M,
McLendon RE, Syro LV, Uribe H, Ortiz LD and Penagos LC: MGMT
immunoexpression predicts responsiveness of pituitary tumors to
temozolomide therapy. Acta Neuropathol. 115:261–262. 2008.
View Article : Google Scholar
|
|
19
|
Chen W, Xiao Z, Zhao Y, Huang L and Du G:
HIF-1α inhibition sensitizes pituitary adenoma cells to
temozolomide by regulating MGMT expression. Oncol Rep.
30:2495–2501. 2013.PubMed/NCBI
|
|
20
|
Paranjpe A, Zhang R, Ali-Osman F, Bobustuc
GC and Srivenugopal KS: Disulfiram is a direct and potent inhibitor
of human O6-methylguanine-DNA methyltransferase (MGMT) in brain
tumor cells and mouse brain and markedly increases the alkylating
DNA damage. Carcinogenesis. 35:692–702. 2014. View Article : Google Scholar :
|
|
21
|
Xu Q, Yuan X, Tunici P, et al: Isolation
of tumour stem-like cells from benign tumours. Br J Cancer.
101:303–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Ma Z, Xiao Z, Liu H, Dou Z, Feng X
and Shi H: Chk1 knockdown confers radiosensitization in prostate
cancer stem cells. Oncol Rep. 28:2247–2254. 2012.PubMed/NCBI
|
|
23
|
Xiao Z, Liu Q, Mao F, Wu J and Lei T:
TNF-α-induced VEGF and MMP-9 expression promotes hemorrhagic
transformation in pituitary adenomas. Int J Mol Sci. 12:4165–4179.
2011. View Article : Google Scholar
|
|
24
|
Xiao Z, Liu Q, Zhao B, Wu J and Lei T:
Hypoxia induces hemorrhagic transformation in pituitary adenomas
via the HIF-1α signaling pathway. Oncol Rep. 26:1457–1464.
2011.PubMed/NCBI
|
|
25
|
Yang J, Xiao Z, Li T, Gu X and Fan B:
Erythropoietin promotes the growth of pituitary adenomas by
enhancing angiogenesis. Int J Oncol. 40:1230–1237. 2012.
|
|
26
|
Wang X, Ma Z, Xiao Z, Liu H, Dou Z, Feng X
and Shi H: Chk1 knockdown confers radiosensitization in prostate
cancer stem cells. Oncol Rep. 28:2247–2254. 2012.PubMed/NCBI
|
|
27
|
Chen W, Xiao Z, Zhao Y, Huang L and Du G:
HIF-1α inhibition sensitizes pituitary adenoma cells to
temozolomide by regulating MGMT expression. Oncol Rep.
30:2495–2501. 2013.PubMed/NCBI
|
|
28
|
O’Reilly SM, Newlands ES, Glaser MG, et
al: Temozolomide: a new oral cytotoxic chemotherapeutic agent with
promising activity against primary brain tumours. Eur J Cancer.
29A:940–942. 1993. View Article : Google Scholar
|
|
29
|
Castedo M, Perfettini JL, Roumier T,
Andreau K, Medema R and Kroemer G: Cell death by mitotic
catastrophe: a molecular definition. Oncogene. 23:2825–2837. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bocangel DB, Finkelstein S, Schold SC,
Bhakat KK, Mitra S and Kokkinakis DM: Multifaceted resistance of
gliomas to temozolomide. Clin Cancer Res. 8:2725–2734.
2002.PubMed/NCBI
|
|
31
|
Cen D, Brayton D, Shahandeh B, Meyskens FL
Jr and Farmer PJ: Disulfiram facilitates intracellular Cu uptake
and induces apoptosis in human melanoma cells. J Med Chem.
47:6914–6920. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cvek B: Targeting malignancies with
disulfiram (Antabuse): multidrug resistance, angiogenesis and
proteasome. Curr Cancer Drug Targets. 11:332–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Triscott J, Lee C, Hu K, et al:
Disulfiram, a drug widely used to control alcoholism, suppresses
the self-renewal of glioblastoma and over-rides resistance to
temozolomide. Oncotarget. 3:1112–1123. 2012.PubMed/NCBI
|
|
34
|
Johansson B: A review of the
pharmacokinetics and pharmacodynamics of disulfiram and its
metabolites. Acta Psychiatr Scand Suppl. 369:15–26. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McCormack AI, McDonald KL, Gill AJ, et al:
Low O6-methylguanine-DNA methyltransferase (MGMT) expression and
response to temozolomide in aggressive pituitary tumours. Clin
Endocrinol (Oxf). 71:226–233. 2009. View Article : Google Scholar
|
|
36
|
Gerson SL: MGMT: its role in cancer
aetiology and cancer therapeutics. Nat Rev Cancer. 4:296–307. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Widhalm G, Wolfsberger S, Preusser M,
Woehrer A, Kotter MR, Czech T, Marosi C and Knosp E: O
(6)-methylguanine DNA methyltransferase immunoexpression in
nonfunctioning pituitary adenomas: are progressive tumors potential
candidates for temozolomide treatment. Cancer. 115:1070–1080. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Syljuåsen RG, Sørensen CS, Hansen LT,
Fugger K, Lundin C, Johansson F, Helleday T, Sehested M, Lukas J
and Bartek J: Inhibition of human Chk1 causes increased initiation
of DNA replication, phosphorylation of ATR targets and DNA
breakage. Mol Cell Biol. 25:3553–3562. 2005. View Article : Google Scholar
|
|
39
|
Sorensen CS, Hansen LT, Dziegielewski J,
Syljuåsen RG, Lundin C, Bartek J and Helleday T: The cell-cycle
checkpoint kinase Chk1 is required for mammalian homologous
recombination repair. Nat Cell Biol. 7:195–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bahassi EM, Ovesen JL, Riesenberg AL,
Bernstein WZ, Hasty PE and Stambrook PJ: The checkpoint kinases
Chk1 and Chk2 regulate the functional associations between hBRCA2
and Rad51 in response to DNA damage. Oncogene. 27:3977–3985. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Beck-Peccoz P, Lania A, Beckers A,
Chatterjee K and Wemeau JL: 2013 European thyroid association
guidelines for the diagnosis and treatment of thyrotropin-secreting
pituitary tumors. Eur Thyroid J. 2:76–82. 2013.2013. View Article : Google Scholar
|
|
42
|
Kovacs K, Horvath E, Syro LV, Uribe H,
Penagos LC, Ortiz LD and Fadul CE: Temozolomide therapy in a man
with an aggressive prolactin-secreting pituitary neoplasm:
Morphological findings. Hum Pathol. 38:185–189. 2007. View Article : Google Scholar
|
|
43
|
Wickström M, Danielsson K, Rickardson L,
Gullbo J, Nygren P, Isaksson A, Larsson R and Lövborg H:
Pharmacological profiling of disulfiram using human tumor cell
lines and human tumor cells from patients. Biochem Pharmacol.
73:25–33. 2007. View Article : Google Scholar
|
|
44
|
Chen D, Cui QC, Yang H and Dou QP:
Disulfiram, a clinically used anti-alcoholism drug and
copper-binding agent, induces apoptotic cell death in breast cancer
cultures and xenografts via inhibition of the proteasome activity.
Cancer Res. 66:10425–10433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rizzoti K: Adult pituitary
progenitors/stem cells: from in vitro characterization to in vivo
function. Eur J Neurosci. 32:2053–2062. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Florio T: Adult pituitary stem cells: from
pituitary plasticity to adenoma development. Neuroendocrinology.
94:265–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Eyler CE and Rich JN: Survival of the
fittest: cancer stem cells in therapeutic resistance and
angiogenesis. J Clin Oncol. 26:2839–2845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fernandez A, Karavitaki N and Wass JA:
Prevalence of pituitary adenomas: a community-based,
cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol
(Oxf). 72:377–382. 2010. View Article : Google Scholar
|