Introduction
Increasing evidence supports the hypothesis that
inflammation serves a function in the initiation, progression and
plaque rupture of the acute coronary syndrome, atherosclerosis
(1,2). A number of inflammatory biomarkers
have been identified (3), thereby
facilitating the development of several novel therapeutic targets
and intervention methods for atherosclerosis by targeting
inflammation and immune-associated factors (4). The Canakinumab Anti-inflammatory
Thrombosis Outcome (5) and
Cardiovascular Inflammation Reduction Trials (6), were initiated to obtain direct
evidence on the reduction of cardiovascular risks through
intervention of the inflammatory reaction. The results of these
studies may initiate a new era for the treatment of coronary heart
diseases (7).
The inflammatory reactions, mediated by the
interactions between cells in the vascular wall and leukocytes, are
involved in the progression of atherosclerosis, tumour development,
allergic reactions and other diseases (8). The co-culture of endothelial cells
(ECs) and smooth muscle cells (SMCs) is a frequently used method in
investigations of atherosclerosis (9). EC-SMC co-culture enables the
involvement of the growth factors, cytokines and other soluble
mediators secreted by these two types of cells in intercellular
communication (10–13). These mediators can mutually affect
functions through receptor mediation, myoendothelial bridges
between ECs and SMCs, establishment of gap junctions and changes in
the extracellular matrix components (14–16).
The EC, SMC and mononuclear cell (MC) co-culture system,
established on a polyethylene microporous membrane has revealed
that the direct contact between ECs and SMCs serves a key function
in MC adhesion and infiltration, and also accelerates the adhesion
dynamics of THP-1 cells with ECs (17). MC infiltration, foam cell
formation, the expression of interleukin (IL)-8 in SMCs and
collagen deposition have been observed in the EC-SMC co-culture
system, using fibrin gel as the scaffold (18). In the EC-SMC-MC co-culture system,
the advanced glycation endoproducts can upregulate the expression
levels of IL-6, monocyte chemoattractant protein-1 (MCP-1) and
other factors in the SMC (19). In
these in vitro co-culture models, the primary focus was on
MC adhesion, foam cell formation and associated aspects under
oxidized low-density lipoprotein (ox-LDL) stimulation. Cellular
inflammatory reactions are not the focus of these invetigations.
Changes in ECs and MCs were examined, however minimal reference was
made to corresponding changes in SMCs, particularly under
inflammatory conditions.
Atherosclerosis is commonly understood as an
inflammatory vascular disease, and targeting key inflammatory
mediators through the inhibition of cytokine activities is a
successful approach for preventing or slowing the progression of
atherosclerosis (20). Previous
studies have started to use inflammatory cytokines directly, as
inflammatory inducers, to stimulate cells in the vascular wall to
initiate the inflammatory process of atherosclerosis. This
simulation may assist in evaluating drugs with potential
anti-atherosclerotic and anti-inflammatory actions, with the most
important stimulating factor being tumour necrosis factor (TNF)-α
(20–22). In our previous study, we
demonstrated that TNF-α exhibits a number of effects as a
stimulator, however, TNF-α was not an ideal stimulator in this
EC-SMC-MC model.
IL-1 is associated with the inflammatory
environment, oxidative stress and the formation of atherosclerosis
(7). An IL-1 gene-knockout
(23) and the IL-1β monoclonal
antibody (24) significantly
reduce the formation of atherosclerotic plaques and suppress
hypertension in mice (25).
Cholesterol crystals can activate the NOD-like receptor family,
pyrin domain containing 3 inflammasome in macrophages and stimulate
the secretion of IL-1 and IL-1β, resulting in a chronic low-level
inflammatory state, which increases the formation and progression
of atherosclerosis (26). Certain
previous studies have reported that six genes, including basic
leucine zipper transcription factor, the BH3 interacting-domain
death agonist apoptosis-associated gene, IL-1RN, complement
receptor C3aR1, SEC61B and SLC43A3, are associated with the
inflammation of atherosclerosis (27). IL-1 has been described as a
promising novel target for future anti-athero-sclerotic drugs
(25).
This present study hypothesized inflammatory
reactions as the primary mechanism underlying atherosclerosis.
IL-1β was used as the key stimulating factor, along with oxLDL, and
was added into the EC-SMC-MC co-culture system. This set-up
simulated atherosclerosis-induced changes in the cell functions and
inflammatory microenvironment of three types of cell. This aimed to
provide a novel method for the identification of drugs for use in
atherosclerosis intervention from the inflammatory perspective, and
to investigate the underlying mechanisms. The present study also
investigated whether tanshinone IIA and andrographolide affected
the early processes of atherosclerosis, including the inhibition of
inflammatory markers, which are important for the EC-SMC-MC
interaction and for plaque destabilization.
Materials and methods
EC monocultures and preparation of
EC-SMC-MC co-cultures
Human umbilical vein smooth muscle cells (HUSMC;
Sciencell Research Laboratories, Inc., Carlsbad, CA, USA) and human
umbilical artery endothelial cells (HUAEC; Sciencell Research
Laboratories, Inc.) were first incubated in separate culture dishes
with smooth muscle conditioned medium and enriched culture medium
(Sciencell Research Laboratories, Inc.), containing 5 (v/v) fetal
calf serum (FCS; Gibco Life Technologies, Carlsbad, CA, USA), 100
IU/ml penicillin and 100 µg/ml streptomycin (Beyotime
Institute of Biotechnology, Jiangsu, China) at 37°C in a 5%
CO2 and 95% air-humidified atmosphere. Cells between
passages three and six were used to prepare the EC monocultures and
EC-SMC co-cultures. On obtaining a sufficient number of HUAECs and
HUASMCs (2–3×106 cells), the cells were detached from
the cell culture dish using trypsin-ethylene diamine tetra-acetic
acid solution.
THP-1 cells (Sciencell Research Laboratories, Inc.)
were cultured in 90% RPMI-1640 (Gibco Life Technologies), 10% FCS,
2 mM glutamine (Beyotime Institute of Biotechnology, Jiangsu,
China), non-essential amino acids (Beyotime Institute of
Biotechnology, Jiangsu, China), 1 mM sodium pyruvate (Beyotime
Institute of Biotechnology, Jiangsu, China), 10 µg/ml human
insulin (Beyotime Institute of Biotechnology, Jiangsu, China) and 1
mM oxalacetate (Beyotime Institute of Biotechnology, Jiangsu,
China) at 37°C and 5% CO2. The cells collected and
adjusted to at density of 1×106 cells/ml. The THP-1
cells were fluorescently labelled with 1 mmol/l
3′,6′-Di(O-acetyl)-4′,
5′-bis[N,N-bis(carboxymethyl)aminomethyl]fluorescein,
tetraacetoxy-methyl ester (Calcein AM; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) for 30 min at 37°C and 5%
CO2. Following washing the cells with Hank’s Balanced
Salt Solution (Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China), 1×105 THP-1 cells/well were added to
the upper chamber of the insert and incubated for 30 min at 37°C
and 5% CO2.
To prepare an EC-SMC co-culture for use as a model
of the arterial wall, Millicell insert units (PIHP01250; Millicell
Millipore, Bedford, MA, USA) were placed in sterile tissue culture
dishes and inverted, so that the outside of the membrane faced
upward. The SMCs were seeded onto this outer membrane surface at a
density of 1×105 cells/cm2. The SMCs became
adherent following 6 h of culture, and the insert units were placed
in a 24-well culture plate in an upright position at 37°C and 5%
CO2. The ECs were plated onto the inner surface of the
membrane at a density of 1×105 cells/well. These dishes
were co-incubated for different durations at 37°C and 5%
CO2 to form an EC-SMC co-culture system, according to
the different requirements for the respective experiments, the
EC-SMCs were co-cultured for 3–6 days). In addition, monocultures
of ECs or SMCs were also prepared using the same method as the
control. The supernatants from the upper and lower chambers of the
insert were obtained, and the phosphatidylethanolamines (PE)
membrane was cut to detect the markers and determine the EC-SMC
co-culture condition.
The EC-SMC were co-cultured for 6 days and incubated
with 100 µg/ml oxLDL (Peking Union-Biology Co., Ltd.,
Beijing, China) or 100 µg/ml oxLDL and 10 ng/ml IL-1β
(PeproTech, Inc,. Rocky Hill, NJ, USA) for 4 h at 37°C and 5%
CO2. The MCs were subsequently added into the upper
chamber of the insert at a density of 1×105
cells/cm2, and the mixture was co-cultured for another
20 h. The EC-SMCs were co-cultured for 6 days and the MCs were
directly added to continue the co-culture for 20 h at 37°C and 5%
CO2, which was used as a control to determine the
inducer and sensitive marker of the inflammatory reaction in this
model of atherosclerosis.
The EC-SMCs were co-cultured for 6 days and
incubated with 100 µg/ml oxLDL and 10 ng/ml IL-1β or
different samples, including atorvastatin, indomethacin, tanshinone
IIA and andrographolide, for 4 h at 37°C and 5% CO2. The
MCs were subsequently added and co-cultured for another 20 h at
37°C and 5% CO2 to assess the effect of the drug on the
inflammatory reaction in atherosclerosis.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of TNF-α, MCP-1, ICAM-1, IL-10,
endothelian 1 (ET-1) and nitric oxide (NO) released from the ECs,
and the levels of IL-6, IL-8, matrix metalloproteinase (MMP)-2,
MMP-9, transforming growth factor (TGF) β-1 and malondialdehyde
(MDA) released from the SMCs were determined using a commercially
available ELISA kit, according to the manufacturer’s instructions
(RapidBio, West Hills, CA, USA). The co-culture incubation media
(400–600 µl) were collected following the removal of
floating cells through centrifugation at 300 × g for 5 min at 4°C,
using an Eppendorf 5424R centrifuge (Eppendorf, Hamburg, Gemany).
The absorbance was measured at 450 nm using a multi-plate
spectrophotometer (Bio-Tek Instruments, Inc., Winooski, VT, USA),
according to the manufacturer’s instructions.
Immunofluorescence staining and laser
scanning confocal microscopy
The growth of cells in the inner PE membrane was
assessed using an FV1000 laser scanning confocal microscope
(Olympus, Tokyo, Japan). The PE membranes, which were cut from the
insert, were washed three times with phosphate-buffered saline
(PBS; Zhongshan Golden Bridge Biotechnology Co., Ltd.), fixed with
4% paraformaldehyde (Sigma-Aldrich) for 10 min and labelled with 1
mmol/l 3′-O-Acetyl-2′, 7′-bis(carboxyethyl)-4 or
5-carboxyfluorescein, diacetoxy-methyl ester (BCECF-AM; Dojindo
Molecular Technologies, Inc.) for 10 min. Images were then captured
and analysed using an FV1000 laser scanning confocal
microscope.
The THP-1 cells were washed three times with PBS and
the number of THP-1 cells bound to the ECs were measured by
counting the number of adherent fluorescence-labelled THP-1 cells
observed under a TE2000S fluorescence microscope (Nikon, Tokyo,
Japan). This microscope encompassed a surface area of 0.314
mm2. A total of six areas were measured, with the
results expressed as the number of THP-1 cells/mm2.
The expression of connexin-43 on the surface of the
ECs was estimated by subtracting the mean fluorescence intensity of
the cells labelled with the non-specific antibody from that of the
connexin-43 antibody-labeled cells. All experiments were performed
at least three times. Alexa Fluor 647-conjugated connexin-43
staining (Invitrogen Life Technologies, Carlsbad, CA, USA) was
performed, according to the manufacturer’s instructions. Images
were captured and analysed using an FV1000 laser scanning confocal
microscope, equipped with an FV10-ASW viewer 2.0 image processing
system (Olympus).
RNA extraction and estimation of mRNA
levels
The total RNA was isolated from agonist-stimulated
or quiescent cells using TRIzol reagent (Invitrogen Life
Technologies) and was reverse transcribed using a reverse
transcription system (Promega, Madison, WI, USA), according to the
manufacturer’s instructions (Invitrogen Life Technologies).
Quantitative polymerase chain reaction (qPCR) was performed using a
7500 real-time PCR system (Applied Biosystems, Foster City, CA,
USA) with SYBR Green PCR Master mix (Applied Biosystems), according
to the manufacturer’s instructions. The mRNA expression levels of
nuclear factor (NF)-κB p65 and peroxisome proliferator-activated
receptor (PPAR)γ were normalized against that of
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and were
subsequently quantified. The following sequences of the forward and
reverse primer pairs were used: NF-κB p65, forward
5′-GTTCACAGACCTGGCATCCGT-3′ and reverse 5′-AGAAGTCCATGTCCGCAATG-3′;
PPARγ, forward 5′-ATGCTTGTGAAGGATGCAA G-3′ and reverse
5′-GATGGCATTATGAGACATCCC-3′ and GAPDH, forward
5′-ACCACAGTCCATGCCATCAC-3′ and reverse 5′-TCCACCAC CCTGTT G
CTGTA-3′. All primers were synthesized by Taihe Biotechnology Co.,
Ltd., (Beijing, China). The following conditions were used:
Denaturation at 95°C for 2 min; 45 cycles of 95°C for 20 sec, 58°C
for 25 sec and 72°C for 30 sec, with a final fluorescence
measurement. The data were normalized against the control and
fold-changes were calculated using the 2−∆∆Ct method.
All reactions were performed in triplicate, using samples derived
from three independent experiments.
Statistical analysis
The data are expressed as the mean ± standard
deviation. The experimental groups were compared by one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell growth and expression levels of
inflammatory markers in EC or SMC monoculture and EC-SMC
co-culture
To systematically investigate the changing patterns
of inflammation-associated factors during the co-culture of cells
in the vascular system, the cell growth and the expression levels
of inflammatory markers were compared in the EC or SMC monoculture
and co-culture for 3 days. The results demonstrated that the cells
grew significantly faster in the EC-SMC co-culture system compared
with the EC and SMC monoculture (Fig.
1A and B). The levels of TNF-α and NO in the EC supernatant
(Fig. 1B), and IL-6 and MMP2 in
the SMC supernatant (Fig. 1D) were
significantly higher in the co-culture system compared with the EC
or SMC monoculture. However, these differences were not
statistically significant.
Cell growth and the level of
atherosclerosis-associated inflammatory markers in cell supernatant
in EC-SMC co-culture for different durations
To understand the changing patterns of
atherosclerosis-associated inflammatory markers at different
time-points of EC-SMC co-culture, confocal microscopy (FV1000;
Olympus; BCECF-labeled; excitation, 488 nm and emmission, 525 nm)
and electron microscopy (Hitcahi S-3400N scanning electron
microscope; were used to observe the growth of the ECs and SMCs
following EC-SMC co-culture for 3, 6, 9 and 12 days. Based on these
observations, the changes in atherosclerosis-associated
inflammatory markers in the EC and SMC cell culture supernatants
were further determined following EC-SMC co-culture for 1, 3, 6 and
9 days. The results demonstrated that the cells grew more
efficiently following the EC-SMC co-culture compared with thte EC
or SMC monoculture between 3 and 9 days, EC formed a dense
monolayer between 6 and 9 days, and the SMCs exhibited multilayer
growth and formed a lamellar structure, which was detected by
electron microscopy. Following co-culture for 12 days, the cell
conditions deteriorated, demonstrating significant exfoliation and
widened intercellular space (Fig.
2A). Following 1 day of co-culture, the levels of ET-1, NO and
TNF-α in the EC cell supernatant increased between 3 and 9 days. In
addition, the increased levels of TNF-α observed at 6 and 9 days
was statistically significant (Fig.
2B). The levels of MMP2 and IL-6 in the SMC supernatant
exhibited an increasing trend between 3 and 9 days in the
co-culture system, and the increase in MMP2 after 6 days was
statistically significant (Fig.
2C). Based on the comprehensive analysis of the results, the
subsequent experiments were performed following EC-SMC co-culture
for 6 days.
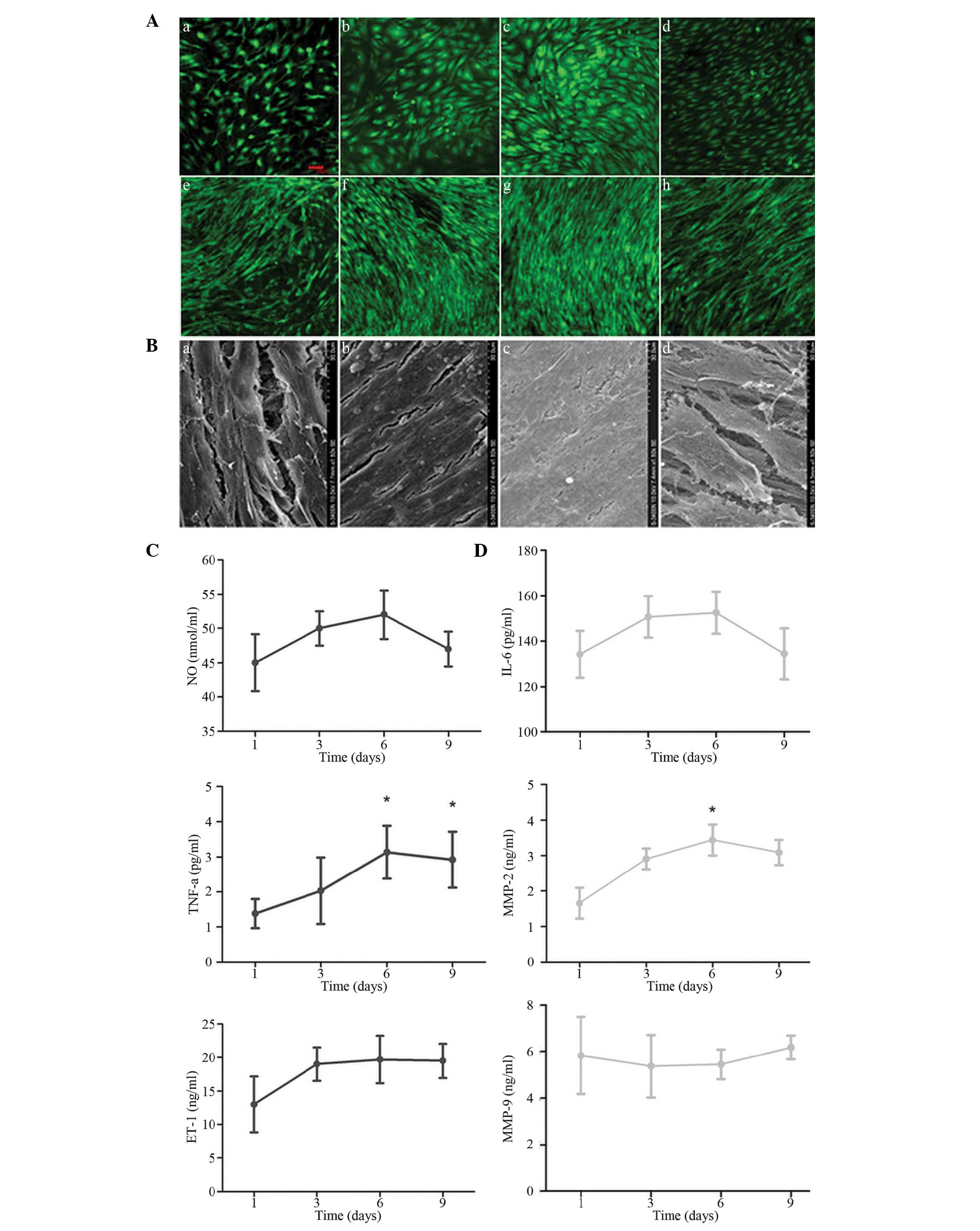 | Figure 2Comparison of the cell growth
conditions and atherosclerosis-associated inflammatory markers in
the cell supernatant at different time-points of EC-SMC co-culture.
(A) Confocal laser scanning microscopy results. (a and e) EC and
SMC growth conditions following EC-SMC co-culture for 3 days; (c
and f) EC and SMC growth conditions following co-culture for 6
days; (c and g) EC and SMC growth conditions following co-culture
for 9 days. (d and h) EC and SMC growth conditions following
co-culture for 12 days, respectively (magnification, ×100; scale
bar=50 µm). (B) Electron microscopy of the SMC growth
conditions following co-culture for 3, 6, 9 and 12 days
(magnification, ×1,500). The concentrations of
atherosclerosis-associated inflammatory markers in the cell
supernatant of the (C) ECs and (D) SMCs following EC-SMC co-culture
for 1–9 days, determined using ELISA. The data are expressed as the
mean ± standard deviation (*P<0.05, vs. 1 day). EC,
endothelial cell; SMC, smooth muscle cell; TNF, tumour necrosis
factor; ET, endothelian; NO, nitric oxide; IL, interleukin; MMP,
matrix metalloproteinase. |
Level of atherosclerosis-associated
inflammatory markers in the IL-1β-induced inflammation-activated
EC-SMC-MC co-culture model
To simulate the inflammatory reaction process of the
cells in the vascular wall during atherosclerosis, a series of
atherosclerosis-associated reactions and the expression levels of
inflammatory markers were compared. These markers included the
EC-surface adherent MC counts, expression levels of EC-surface
connexin-43, concentrations of TNF-α, MCP-1, ET-1, NO, ICAM-1 and
IL-10 in the EC supernatant, concentrations of IL-6, IL-8, MMP-2,
MMP-9, TGFβ-1 and MDA in the SMC supernatant, and the mRNA
expression levels of NF-κB and PPARγ in the cells of the EC layer.
This was performed under four culture conditions, including EC-SMC
co-culture, EC-SMC-MC co-culture, MC co-culture with
oxLDL-activated EC-SMC and MC co-culture with oxLDL and
IL-1β-activated EC-SMC. The results demonstrated that the EC
surface adherent MC count and the expression of EC-surface
connexin-43 were significantly higher following co-culture of MC
with oxLDL and IL-1β-activated EC-SMC (EC-SMC-MC+O+I group)
compared with the other groups (Fig.
3A). The levels of TNF-α and MCP-1 in the EC supernatant
(Fig. 3B); IL-6, MMP-2, TGFβ-1 and
MDA in the SMC supernatant (Fig.
3C); and of NF-κB in the EC layer increased significantly in
this group (Fig. 3D). Therefore,
the conditions required for the establishment of this model were as
follows: EC-SMC co-culture for 6 days, followed by incubation with
100 µg/ml oxLDL and 10 ng/ml IL-1β for 4 h, followed by MC
addition and co-culture for another 20 h.
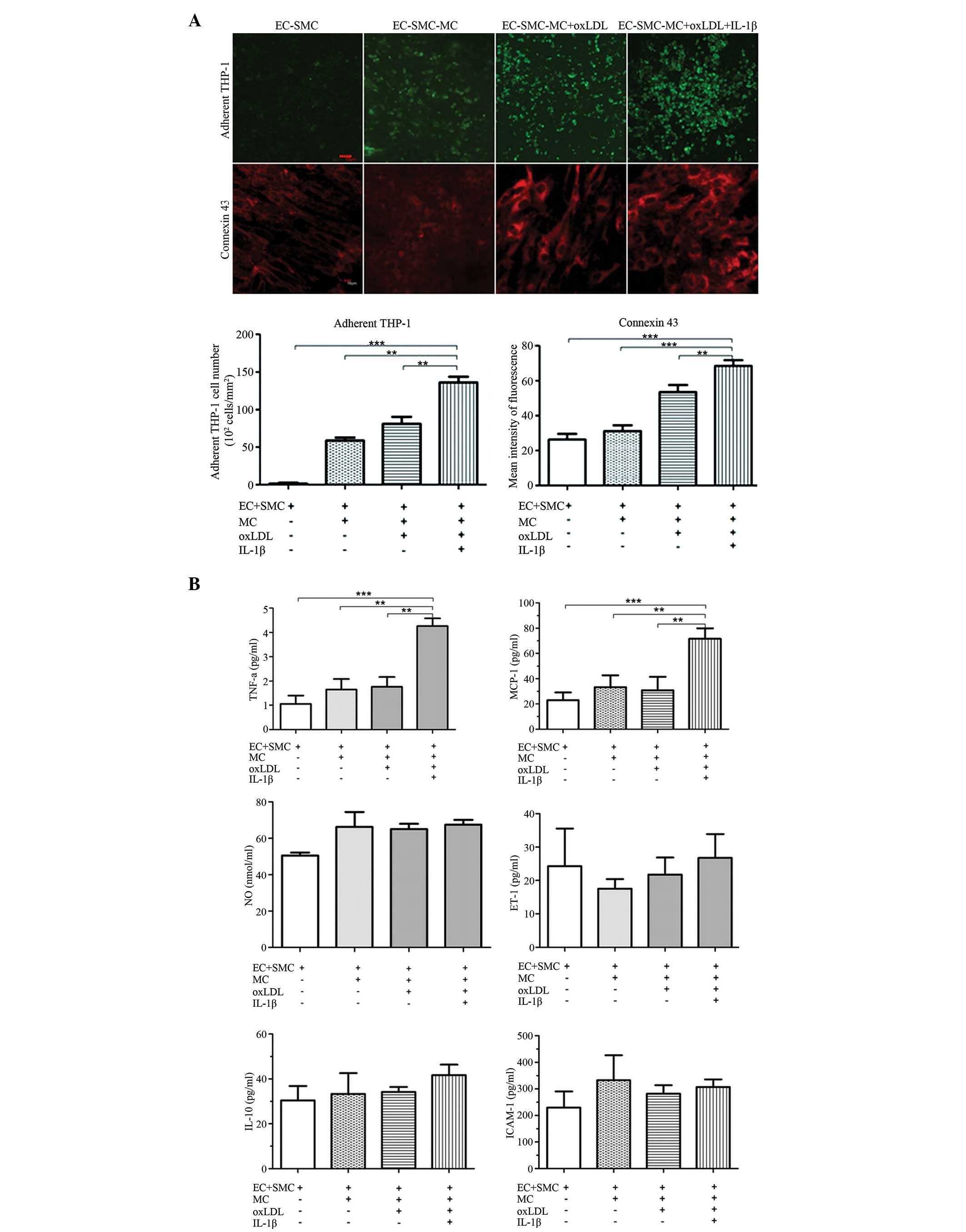 | Figure 3Comparison of the expression levels
of atherosclerosis-associated inflammatory markers in EC-SMC
co-culture, with or without MCs, oxLDL and IL-1β. (A) Confocal
laser scanning microscopy images of EC-surface adhered MCs (scale
bar=50 µm) and the expression of connexin 43 (scale bar=10
µm). (B) Concentrations of atherosclerosis-associated
inflammatory markers in the EC supernatant in EC-SMC co-culture,
with or without MCs, oxLDL and IL-1βs. The data are expressed as
the mean ± standard deviation (**P<0.01;
***P<0.001, vs. EC-SMC-MC co-culture with oxLDL and
IL-1β groups). EC, endothelial cell; SMC, smooth muscle cell; MC,
monocyte; TNF, tumour necrosis factor; ET, endothelian; NO, nitric
oxide; IL, interleukin; MMP, matrix metalloproteinase; MCP,
monocyte chemoattractant protein; ICAM, intercellular adhesion
molecule; TGF, transforming growth factor; oxLDL, oxidative low
density lipoprotein; NF, nuclear factor; PPAR, peroxisome
proliferator-activated receptor. (C) Expression levels of
atherosclerosis-associated inflammatory markers in the SMC
supernatant, with or without MCs, oxLDL and IL-1β, measured by
enzyme-linked immunosorbent assay. (D) mRNA expression levels of
NF-κB and PPARγ in the cells of EC layer were subjected to reverse
transcription-quantitative polymerase chain reaction. The data are
expressed as the mean ± standard deviation (*P<0.05;
**P<0.01, vs. EC-SMC-MC co-culture with oxLDL and
IL-1β). EC, endothelial cell; SMC, smooth muscle cell; MC,
monocyte; TNF, tumour necrosis factor; ET, endothelian; NO, nitric
oxide; IL, interleukin; MMP, matrix metalloproteinase; MCP,
monocyte chemoattractant protein; ICAM, intercellular adhesion
molecule; TGF, transforming growth factor; oxLDL, oxidative low
density lipoprotein; NF, nuclear factor; PPAR, peroxisome
proliferator-activated receptor. |
Validation of the IL-1β-induced
inflammation-activated EC-SMC-MC co-culture model
To determine the reliability of the co-culture
model, the model was validated using anti-atherosclerotic agent,
atorvastatin, which has known lipid-lowering and anti-inflammatory
effects, and the non-steroidal anti-inflammatory drug,
indomethacin. The experimental results demonstrated that treatment
with atorvastatin resulted in an effective reduction of the
EC-surface adhered cell count and inhibition of the expression of
connexin-43 (Fig. 4A), decreased
levels of TNF-α and MCP-1 in the EC supernatant (Fig. 4B) and MMP-2, IL-6, and MDA in SMC
supernatant (Fig. 4C), and
downregulation of the mRNA expression of NF-κB and upregulation of
the mRNA expression of PPARγ in the ECs (Fig. 4D). By contrast, indomethacin
reduced the EC surface-adhered cell count, inhibited the secretion
of TNF-α and MCP-1 by the ECs, and suppressed the mRNA expression
of NF-κB. However, treatment with indomethacin revealed no
significant effects on the other indices. These results suggested
that this model effectively reflected the efficacy of
anti-athero-sclerotic agents with an anti-inflammatory effect, and
may be used to identify drugs with potential anti-inflammatory and
anti-atherosclerotic activities, and to assess their efficacy.
 | Figure 4Effects of atorvastatin and
indomethacin on atherosclerosis-associated inflammatory markers in
the IL-1β-induced inflammation-activated EC-SMC-MC co-culture
model. (A) EC-surface adhered MC count and the expression of
Connexin 43. Concentrations of atherosclerosis-associated
inflammatory markers in the (B) EC and (C) SMC supernatant were
measured by ELISA. (D) The mRNA expression levels of NF-κB and
PPARγ in cells from the EC layer were subjected to reverse
transcription quantitative polymerase chain reaction. The data are
expressed as the mean ± standard deviation (*P<0.05,
**P<0.01, ***P<0.001, vs. Model). E,
endothelial cell; S, smooth muscle cell; M, monocyte; O, oxidated
low density lipoprotein; I, interleukin-1β induced; TNF, tumour
necrosis factor; IL, interleukin; MMP, matrix metalloproteinase;
MCP, monocyte chemoattractant protein; TGF, transforming growth
factor; NF, nuclear factor; PPAR, peroxisome proliferator-activated
receptor. |
Evaluation of the anti-atherosclerotic
effects of atorvastatin and indomethacin in the IL-1β-induced
inflammation-activated EC-SMC-MC co-culture model
Based on the IL-1β-induced inflammation-activated
co-culture model, the anti-athero-sclerotic efficacy of tanshinone
IIA and andrographolide in exhibiting anti-inflammatory or
anti-atheroscleroticactivity was comprehensively assessed. The
present study investigated whether tanshinone IIA and
andrographolide affected the early stages of atherosclerosis,
including the inhibition of inflammatory markers. The results
demonstrated that tanshinone IIA in the co-culture model inhibited
the EC surface-adhered MC count and the expression of connexin-43,
decreased the secretion of TNF-α and MCP-1 by the ECs, and TGFβ-1,
MMP-2 and MDA in the SMC supernatant, and affected the mRNA
expression levels of NF-κB and PPARγ in EC layer cells. Therefore,
tanshinone IIA demonstrated significant anti-atherosclerotic and
anti-inflammatory effects (Fig.
5). Andrographolide also inhibited certain indices in this
model, which were principally associated with inflammation,
demonstrating a significant anti-inflammatory effect, consistent
with previous studies (28,29).
The experimental results also suggested that tanshinone IIA
affected the expression of inflammatory markers in atherosclerosis
and may serve an important interventional function in the formation
of atherosclerosis and plaque stabilization by inhibiting
inflammatory reactions.
 | Figure 5Effects of tanshinone IIA and
andrographolide on atherosclerosis-associated inflammatory markers
in the IL-1β-induced inflammation-activated EC-SMC-MC co-culture
model. (A) EC-surface adhered MC count and the expression of
Connexin 43. Concentrations of atherosclerosis-associated
inflammatory markers in the (B) EC and (C) SMC supernatant were
measured by ELISA. (D) The mRNA expression levels of NF-κB and
PPARγ in cells from the EC layer were subjected to reverse
transcription quantitative polymerase chain reaction. The data are
expressed as the mean ± standard deviation (*P<0.05,
**P<0.01, ***P<0.001, vs. Model). EC,
endothelial cell; SMC, smooth muscle cell; MC, monocyte; TNF,
tumour necrosis factor; IL, interleukin; MMP, matrix
metalloproteinase; MCP, monocyte chemoattractant protein; TGF,
transforming growth factor; NF, nuclear factor; PPAR, peroxisome
proliferator-activated receptor. |
Discussion
The interactions between ECs, SMCs and MCs
contribute to the normal function of the vessel wall and to the
pathogenesis of certain diseases, including atherosclerosis.
Previous studies used the EC-SMC co-culture system in
investigations of atherosclerosis (30–32).
Takaku et al (31) observed
the migration and differentiation of MCs and the formation of foam
cells under oxLDL in the EC-SMC culture system in 1999, and
subsequent studies have predominantly used oxLDL as a primary
stimulant for the atherosclerotic process. EC-SMC co-culture with
oxLDL can upregulate the expression levels of intercellular
adhesion molecule (ICAM)-1, vascular cell adhesion protein-1 and
other factors by ECs, and the release of TNF-α (33,34).
Furthermore, the migratory function of ECs can vary, which
variation is associated with the activation of histone deacety-lase
6 and downregulation of the expression of acetylated tubulin in ECs
(35). In the EC-SMC co-culture
system, SMCs can secrete vascular endothelial growth factor to
stimulate the ECs to produce a series of changes, including cell
proliferation, differentiation, migration and the deposition of
extracellular matrix proteins (36). Co-cultured SMCs promote the
adhesion of ECs by modulating the microtubule cytoskeleton
polymerization state, which in turn activates the extracellular
regulated kinase pathway and upregulates the expression of
phosphorylated paxillin to accelerate focal adhesion formation
(37). In the present study, to
investigate the atherosclerosis-associated inflammatory reaction in
the co-culture model, changes in a series of atherosclerosis
inflammatory markers at different time-points and under different
culture conditions were examined. The results demonstrated that the
levels of TNF-α in the EC culture supernatant increased
significantly following EC-SMC co-culture for 6 days (Fig. 2B), which was consistent with a
previous report (35), and the
levels of MMP-2 in the SMC culture supernatant increased
significantly (Fig. 2C). Based on
the overall evaluation of the cell growth conditions, EC-SMC
co-culture for 6 days was determined as the suitable condition for
subsequent experiments. In addition, differences in the expression
of atherosclerosis-associated inflammatory factors under several
co-culture conditions and stimulations were systematically observed
and compared (Fig. 3). These
results confirmed that the inflammatory changes were the most
evident following co-culture of EC-SMC-MC combined with stimulation
of ox-LDL and IL-1β. The extensive preliminary experiments enabled
the screening of certain stable atherosclerosis-associated
inflammation indicators, including the expression of the EC-surface
connexin-43, the number of adherent MCs, changes in the series of
inflammatory markers secreted by ECs and SMCs, and the changes in
the inflammatory signalling molecules (Fig. 3).
Connexin-43 is a member of the connexin family,
which forms intercellular gap junctions and is closely associated
with inflammation. Connexin-43-knockdown alleviates the brain
inflammation and glia activation induced by peripheral
lipopolysaccharide injection (38). Connexin-43 is upregulated in the
neointimal SMCs at an early stage of atherosclerosis in rabbits
(39). The results of the present
study demonstrated that connexin-43 is expressed in ECs for the
entire duration of culture. This finding provides evidence of EC
inflammatory stimulation and of ongoing intercellular communication
between ECs and SMCs in the model, similar to previous studies
(40,41), acting as an indicator of
atherosclerosis-associated inflammation.
The atherosclerosis-associated factors in the SMC
supernatant under inflammatory conditions were also observed. MMP-2
contributes to the development of atherosclerosis and, activated
MMP-2 has been observed in human carotid endarterectomy specimens
(42). A significant reduction in
atherosclerotic plaques in the aortic sinus and arch were observed
with the decrease in smooth muscle cell-positive area in MMP-2(−/−)
in mice (43). The increased
expression of MMP-2 was also a significant feature of the model in
the present study. SMC proliferation and lipid peroxide product
accumulation-associated indices, including TGFβ-1 and MDA, were
also observed in the SMC supernatant, therefore this model can be
used to evaluate the effects of drugs, focus-sing on pathological
processes associated with atherosclerosis.
Danshen (DS) is a traditional Chinese medicine,
which is commonly used for the treatment of cardiovascular and
cerebrovascular diseases, and tanshinone is one of its active
ingredients. Tanshinone IIA exhibits certain anti-atherosclerotic
effects, protects cells from being injured by hydrogen peroxide
(44), suppresses cholesterol
accumulation and affects the formation of foam cell (45,46).
Stumpf et al (46) reported
that DS and its major ingredients significantly inhibited
TNF-α-induced expression, the release of adhesion molecules,
cytokines and chemokines, and the adenosine diphosphate-induced
expression of platelet P-selectin. Andrographolide exhibits an
anti-inflammatory effect by downregulating the p38 mitogen
activated protein kinase, signal transducer and activator of
transcription 3 and NF-κB pathways (47,48).
The present study assessed whether tanshinone IIA and
andrographolide exhibited the potential anti-inflammatory effects
of atherosclerosis in the established experimental system. The
results demonstrated that tanshi-none IIA exhibited significant
efficacy against atherosclerosis and its inflammatory reactions.
Tanshinone IIA inhibited the MMP-2 and NF-κB signalling pathways in
the IL-1β-induced inflammation-activated co-culture model, as
confirmed previously (49).
The IL-1β-induced inflammation-activated EC-SMC-MC
co-culture model in the present study demonstrated that changes in
the expression levels of a series of atherosclerosis inflammatory
markers secreted by ECs, SMCs, levels of EC connexin, MC adhesion
and changes in the inflammatory signalling molecules can be used as
evaluation indices for the inflammatory microenvironment of
atherosclerosis. This model was able to simulate a series of
relevant changes in the cell structure and function of the arterial
wall, character-ized by inflammatory reactions of inflammatory
cytokines with oxLDL, a risk factor for atherosclerosis, during the
atherosclerotic process. The establishment of this model system
offers a reliable method for identifying drugs with potential
anti-atherosclerotic activity, and for investigating the mechanisms
of action to improve the inflammatory state and increase plaque
stability. Based on this model, the present study revealed that
tanshinone IIA affected the early stages of atherosclerosis through
inflammatory reactions and plaque destabilization.
Acknowledgments
This study was funded by grants from the Natural
Sciences Foundation of China (no. 30973901), the International
Science and Technology Cooperation Program of the People’s Republic
of China (no. 2011DFA30870) and the Joint Research Project of The
Twentieth Session of China-Thailand Joint Committee On Science and
Technology Cooperation (no. 20–602 J).
References
|
1
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong BW, Meredith A, Lin D and McManus BM:
The biological role of inflammation in atherosclerosis. Can J
Cardiol. 28:631–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel D, Devaraj S, Mitra A, Raychaudhuri
SP, Raychaudhuri SK and Jialal I: Inflammation, atherosclerosis and
psoriasis. Clin Rev Allergy Immunol. 44:194–204. 2013. View Article : Google Scholar
|
|
4
|
Charo IF and Taub R: Anti-inflammatory
therapeutics for the treatment of atherosclerosis. Nat Rev Drug
Discov. 10:365–376. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ridker PM, Thuren T, Zalewski A and Libby
P: Interleukin-1β inhibition and the prevention of recurrent
cardiovascular events: rationale and design of the canakinumab
anti-inflammatory thrombosis outcomes study (CANTOS). Am Heart J.
162:597–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ridker PM: Testing the inflammatory
hypothesis of athero-thrombosis: scientific rationale for the
cardiovascular inflammation reduction trial (CIRT). J Thromb
Haemost. 7:332–339. 2009. View Article : Google Scholar
|
|
7
|
Verma S, Gupta M and Ridker PM:
Therapeutic targeting of inflammation in atherosclerosis: we are
getting closer. Can J Cardiol. 28:619–622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davies PF: Vascular cell interactions with
special reference to the pathogenesis of atherosclerosis. Lab
Invest. 55:5–24. 1986.PubMed/NCBI
|
|
10
|
Campbell JH and Campbell GR: Endothelial
cell influences on vascular smooth muscle phenotype. Annu Rev
Physiol. 48:295–306. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Powell RJ, Hydowski J, Frank O, Bhargava J
and Sumpio BE: Endothelial cell effect on smooth muscle cell
collagen synthesis. J Surg Res. 69:113–118. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Powell RJ, Bhargava J, Basson MD and
Sumpio BE: Coculture conditions alter endothelial modulation of
TGF-beta 1 activation and smooth muscle growth morphology. Am J
Physiol. 274:H642–H649. 1998.PubMed/NCBI
|
|
13
|
Lyle AN and Griendling KK: Modulation of
vascular smooth muscle signaling by reactive oxygen species.
Physiology (Bethesda). 21:269–280. 2006. View Article : Google Scholar
|
|
14
|
Spagnoli LG, Villaschi S, Neri L and
Palmieri G: Gap junctions in myo-endothelial bridges of rabbit
carotid arteries. Experientia. 38:124–125. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fillinger MF, O’Connor SE, Wagner RJ and
Cronenwett JL: The effect of endothelial cell coculture on smooth
muscle cell proliferation. J Vasc Surg. 17:1058–1067; discussion
1067–1068. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Powell RJ, Carruth JA, Basson MD,
Bloodgood R and Sumpio BE: Matrix-specific effect of endothelial
control of smooth muscle cell migration. J Vasc Surg. 24:51–57.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kinard F, Jaworski K, Sergent-Engelen T,
et al: Smooth muscle cells influence monocyte response to LDL as
well as their adhesion and transmigration in a coculture model of
the arterial wall. J Vasc Res. 38:479–491. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dorweiler B, Torzewski M, Dahm M, et al: A
novel in vitro model for the study of plaque development in
atherosclerosis. Thromb Haemost. 95:182–189. 2006.PubMed/NCBI
|
|
19
|
Nam MH, Lee HS, Seomun Y, Lee Y and Lee
KW: Monocyte-endothelium-smooth muscle cell interaction in
co-culture: proliferation and cytokine productions in response to
advanced glycation end products. Biochim Biophys Acta.
1810:907–912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Desai A, Darland G, Bland JS, Tripp Ml and
Konda VR: META060 attenuates TNF-alpha-activated inf lam-mation,
endothelial-monocyte interactions and matrix metalloproteinase-9
expression and inhibits NF-kappaB and AP-1 in THP-1 monocytes.
Atherosclerosis. 223:130–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moon MK, Lee YJ, Kim JS, Kang DG and Lee
HS: Effect of caffeic acid on tumour necrosis factor-alpha-induced
vascular inflammation in human umbilical vein endothelial cells.
Biol Pharm Bull. 32:1371–1377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deuell KA, Callegari A, Giachelli CM,
Rosenfeld ME and Scatena M: RANKL enhances macrophage paracrine
pro-calcific activity in high phosphate-treated smooth muscle
cells: dependence on IL-6 and TNF-alpha. J Vasc Res. 49:510–521.
2012. View Article : Google Scholar
|
|
23
|
Hoge M and Amar S: Role of interleukin-1
in bacterial athero-genesis. Drugs Today (Barc). 42:683–688. 2006.
View Article : Google Scholar
|
|
24
|
Bhaskar V, Yin J, Mirza AM, et al:
Monoclonal antibodies targeting IL-1 beta reduce biomarkers of
atherosclerosis in vitro and inhibit atherosclerotic plaque
formation in apolipoprotein E-deficient mice. Atherosclerosis.
216:313–320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chamberlain J, Francis S, Brookes Z, et
al: Interleukin-1 regulates multiple atherogenic mechanisms in
response to fat feeding. PLoS One. 4:e50732009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang Y, Wang M, Huang K, et al: Oxidized
low-density lipo-protein induces secretion of interleukin-1beta by
macrophages via reactive oxygen species-dependent NLRP3
inflammasome activation. Biochem Biophys Res Commun. 425:121–126.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sivapalaratnam S, Farrugia R, Nieuwdorp M,
et al: Identification of candidate genes linking systemic
inflammation to atherosclerosis; results of a human in vivo LPS
infusion study. BMC Med Genomics. 4:642011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Navab M, Hough GP, Stevenson LW,
Drinkwater DC, Laks H and Fogelman AM: Monocyte migration into the
subendo-thelial space of a coculture of adult human aortic
endothelial and smooth muscle cells. J Clin Invest. 82:1853–1863.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van Lenten BJ, Hama SY, de Beer FC, et al:
Anti-inflammatory HDL becomes pro-inflammatory during the acute
phase response. Loss of protective effect of HDL against LDL
oxidation in aortic wall cell cocultures. J Clin Invest.
96:2758–2767. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishikawa K, Navab M, Leitinger N, Fogelman
AM and Lusis AJ: Induction of heme oxygenase-1 inhibits the
monocyte transmigration induced by mildly oxidized LDL. J Clin
Invest. 100:1209–1216. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takaku M, Wada Y, Jinnouchi K, et al: An
in vitro coculture model of transmigrant monocytes and foam cell
formation. Arterioscler Thromb Vasc Biol. 19:2330–2339. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang JC, Ruan Q, Paucz L, Fabry A, Binder
BR and Wojta J: Stimulation of tissue factor expression in human
microvascular and macrovascular endothelial cells by cultured
vascular smooth muscle cells in vitro. J Vasc Res. 36:126–132.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rainger GE, Stone P, Morland CM and Nash
GB: A novel system for investigating the ability of smooth muscle
cells and fibroblasts to regulate adhesion of flowing leukocytes to
endothelial cells. J Immunol Methods. 255:73–82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chao CY, Lii CK, Tsai IT, Li CC, Liu KL,
Tsai CW and Chen HW: Andrographolide inhibits ICAM-1 expression and
NF-κB activation in TNF-α-treated EA.hy926 cells. J Agric Food
Chem. 59:5263–5271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang YH, Yan ZQ, Qi YX, et al: Normal
shear stress and vascular smooth muscle cells modulate migration of
endothelial cells through histone deacetylase 6 activation and
tubulin acetylation. Ann Biomed Eng. 38:729–737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Evensen L, Micklem DR, Blois A, et al:
Mural cell associated VEGF is required for organotypic vessel
formation. PLoS One. 4:e57982009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang YH, Yan ZQ, Shen BR, Zhang L, Zhang P
and Jiang ZL: Vascular smooth muscle cells promote endothelial cell
adhesion via microtubule dynamics and activation of paxillin and
the extracellular signal-regulated kinase (ERK) pathway in a
co-culture system. Eur J Cell Biol. 88:701–709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ruan LM, Cai W, Chen JZ and Duan JF:
Effects of losartan on expression of connexins at the early stage
of atherosclerosis in rabbits. Int J Med Sci. 7:82–89. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Larson DM, Haudenschild CC and Beyer EC:
Gap junction messenger RNA expression by vascular wall cells. Circ
Res. 66:1074–1080. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Navab M, Liao F, Hough GP, et al:
Interaction of monocytes with cocultures of human aortic wall cells
involves interleukins 1 and 6 with marked increases in connexin43
message. J Clin Invest. 87:1763–1772. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Leclercq A, Houard X, Loyau S, et al:
Topology of protease activities reflects atherothrombotic plaque
complexity. Atherosclerosis. 191:1–10. 2007. View Article : Google Scholar
|
|
42
|
Kuzuya M, Nakamura K, Sasaki T, Cheng XW,
Itohara S and Iguchi A: Effect of MMP-2 deficiency on
atherosclerotic lesion formation in apoE-deficient mice.
Arterioscler Thromb Vasc Biol. 26:1120–1125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lin R, Wang WR, Liu JT, Yang GD and Han
CJ: Protective effect of tanshinone IIA on human umbilical vein
endothelial cell injured by hydrogen peroxide and its mechanism. J
Ethnopharmacol. 108:217–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang WL, Xiao Y, Liu JP, et al: Structure
and remodeling behavior of drug-loaded high density lipoproteins
and their atherosclerotic plaque targeting mechanism in foam cell
model. Int J Pharm. 419:314–321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu Z, Wang J, Huang E, et al: Tanshinone
IIA suppresses cholesterol accumulation in human macrophages: role
of haem oxygenase-1. J Lipid Res. 55:201–213. 2014. View Article : Google Scholar :
|
|
46
|
Stumpf C, Fan Q, Hintermann C, et al:
Anti-inflammatory effects of danshen on human vascular endothelial
cells in culture. Am J Chin Med. 41:1065–1077. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee KC, Chang HH, Chung YH and Lee TY:
Andrographolide acts as an anti-inflammatory agent in
LPS-stimulated RAW264.7 macrophages by inhibiting STAT3-mediated
suppression of the NF-kappaB pathway. J Ethnopharmacol.
135:678–684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guo W, Liu W, Chen G, et al: Water-soluble
androgra-pholide sulfonate exerts anti-sepsis action in mice
through down-regulating p38 MAPK, STAT3 and NF-κB pathways. Int
Immunopharmacol. 14:613–619. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fang ZY, Lin R, Yuan BX, Yang GD, Liu Y
and Zhang H: Tanshinone IIA downregulates the CD40 expression and
decreases MMP-2 activity on atherosclerosis induced by high fatty
diet in rabbit. J Ethnopharmacol. 115:217–222. 2008. View Article : Google Scholar
|



















