Introduction
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) is widely used to quantify RNA expression levels
(1). RT-qPCR is highly sensitive,
allowing the quantification of rare transcripts (2). However, the accuracy of this
technique may be affected at multiple stages throughout the
experimental process and by several factors, including the quantity
of the samples analyzed, the quality of the RNA, the efficiencies
of RT and the PCR itself (3,4). The
stability of the reference gene is important for an appropriate
normalization standard (5,6). An ideal reference gene requires it
not to be regulated or affected in any sample under different
experimental treatment conditions (7). However, no single universal and
completely constant reference gene has been reported (8). Accumulating evidence has indicated
that the expression levels of widely used reference genes vary
significantly in different independent investigations (7,9,10).
Several housekeeping genes, commonly used as reference genes, can
be dynamically expressed in response to treatment (11). Therefore, the identification and
evaluation of the expression stabilities of reference genes is
required to obtain accurate profiling of gene expression
levels.
The plasticity of BMSCs offers potential in the
progress of investigation into regeneration in neural tissues, and
offers a foundation for the application of stem cells in diseases
of the nervous system (12). It
has been reported that BMSCs can be induced to differentiate into
neuron-like cells in vitro and in vivo, which can be
induced by various factors and differentiated into several specific
neuronal phenotypes (13,14). Woodbury et al, was the first
to induce rat and human BMSCs to differentiate into neuron-like and
glial-like cells in vitro (13). Our previous study demonstrated that
the retinal tissue offered an inductive microenvironment to promote
stem cell differentiation into neural-like cells with morphological
characteristics of nerve cells, and promoted the expression of
nestin and nuclear factor (15).
Several types of cytokines have also been used to induce BMSCs to
differentiate into neuron-like cells in vitro. These cells
can promote functional recovery and neural protection following
spinal cord injury (12). During
development, BMSCs undergo complex differentiation procedures and
phenotypic changes. To realize the potential of these cells, it is
necessary to understand the processes, which govern their
differentiation (16).
In the present study, the expression stabilities of
11 commonly used reference genes, ACTB, ARBP, B2M, CYCA, GAPDH,
GUSB, HPRT, PPIA, RPL13A, TBP and PGK1, were investigated. The
commonly used statistical algorithms, geNorm (17), NormFinder (18) and BestKeeper (19) were used to evaluate their
expression stabilities.
The results of the present study may assist in
determining the optimal number of reference genes required for
reliable normalization of gene expression data.
Materials and methods
Isolation, culture and identification of
BMSCs
Neonatal (8-day-old; 3 rats, 15–25 g) Wistar rats,
provided by the Animal Center of Jilin University (Jilin, China),
were housed together at 19–29°C with a 12 h light/dark cycle, and
were placed in 75% alcohol (Beijing Sinopharm Chemical Reagent Co.,
Ltd., Beijing, China) for sterilization for 5 min. The BMSCs were
harvested from the bone marrow of the femur of neonatal female
Wistar rats under 10% chloral hydrate anesthetic (Tianjin Huadong
Chemical Reagent Co., Ltd., Tianjin, China; 1 ml; intraperitoneal
injection), by inserting a needle into the bone shaft and
repeatedly flushing it with 5 ml Dulbecco’s modified Eagle’s medium
(DMEM)/F12 medium (GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA). The rinsed solution was transferred slowly into a centrifuge
tube, which contained equal quantities of 1.073 g/l percol
separation liquid (Sigma-Aldrich, St. Louis, MO, USA), and
centrifuged (Legend Micro 17R; Thermo Fisher Scientific, Waltham,
MA, USA) at 350 × g for 20 min at room temperature. The middle
monolayer was then collected and washed twice with
phosphate-buffered saline (PBS; Wuhan Boster Biological Technology,
Ltd., Wuhan, China). The cells were maintained in a humidified
incubator (Sanyo, Osaka, Japan) in an atmosphere of 5%
CO2 at 37°C with DMEM/F12 medium and 5% fetal bovine
serum (FBS; Gibco Life Technologies, Carslbad, CA, USA). After 24
h, the non-adherent cells were removed by medium replacement, and
the medium was replaced every 3 days. The cells were passaged when
they reached 90% confluence to maintain exponential growth.
Third-generation BMSCs were used for the subsequent
experiments.
Following three passages, the expression levels of
the CD90, CD71, CD29 and CD45 cell surface markers (BD Biosciences,
Franklin Lakes, NJ, USA) were detected by flow cytometry (Epics XL;
Beckman Coulter, Brea, CA, USA).
Differentiation of BMSCs
The third passage BMSCs were seeded into six-well
plates at a density of 20,000 cells/cm2, and were
cultured at ~4×104 cells/cm2 in growth medium
until confluent, prior to being divided into four groups. In the
blank group, BMSCs contained no inducer. In the chemical group, the
BMSCs were maintained in β-mercaptoethanol (BME; Beijing Dingguo
Company, Beijing, China) in six-well plates at 1 mmol/l for 24 h
pre-induction at 37°C, to induce neuronal differentiation, the
cells were then transferred into serum-free medium containing 5
mmol/l BME (13). In the cytokine
group, the BMSCs were maintained in basic fibroblast growth factor
(Peprotech company), induced at 37°C for 24 h at 20 ng/ml, and the
medium was replaced with serum-free medium containing 10 ng/ml
brain-derived neurotrophic factor (Peprotech company) (20). In the co-culture group, the
eyeballs of six 3-day-old Wistar rats were removed under sterile
conditions. The eyeball was cut from the limbus, the lens and
vitreous were removed under a microscope (CKX41; Olympus, Tokyo,
Japan) and the retina was separated. For digestion, 1 ml 0.125%
trypsin/EDTA (GE Healthcare Life Sciences) was added to the retinal
tissue and incubated at 37°C for 10 min until the retinal tissue
became chyle. This was followed by the addition of 2 ml DMEM/F12
medium containing 10% FBS to terminate the digestion. The cells
were then centrifuged at 60 × g for 5 min at room temperature, and
the cells were resuspended and maintained in a humidified incubator
in 5% CO2 at 37°C. The medium was replaced every 3 days
and the medium was collected, The differentiation medium was mixed
with the collected medium and fresh medium (2:3). The
differentiation medium was used for the third passage BMSCs.
Immunohistochemistry was performed to detect the
expression levels of the nestin, neuron-specific enolase (NSE) and
neurofilament (NF) neuronal markers (Abcam, Cambridge, MA, USA) in
the induced groups. The frozen cell sections were hydrated with
distilled water for 30 min, then 0.2% Triton X-100 (Beijing Dingguo
Company) was added for 20 min prior to washing (with 0.2 M PBS, 3
times, 5 min each time). The cells were blocked with methanol and
30% hydrogen peroxide (50:1) (both from Beijing Sinopharm Chemical
Reagent Co., Ltd.) at room temperature for 30 min, then 5% bovine
serum albumin (Sigma-Aldrich) blocking buffer was added at room
temperature for 20 min. Excess buffer was then removed. The cells
were incubated with the primary antibody at 4°C overnight, were
washed 3 times with 0.2 M PBS (5 min each time), then were
incubated with the secondary antibody at room temperature for 1 h.
Washing was then conducted (with 0.2 M PBS, 3 times, 5 min each
time). Streptavidin-biotin complex (Wuhan Boster Biological
Technology, Ltd.) was added at room temperature for 20 min, then
the cells were washed (with 0.2 M PBS, 3 times, 5 min each time).
The DAB kit (Wuhan Boster Biological Technology, Ltd.) was then
used in accordance with the manufacturer’s instructions, allowing
color to develop for 30 min prior to washing with water.
Hematoxylin (Nanchang Yvlu Chemical Reagent Co., Ltd., Jiangxi,
China) staining was conducted for 5 min, the cells were dehydrated,
cleared using xylene and fixed with 40 µl neutral gum
(Nanchang Yvlu Chemical Reagent Co., Ltd.), then imaged under the
microscope, the positive results appearing brown.
RNA extraction and cDNA synthesis
Total RNA was isolated from the independent
biological replicates, each group containing two samples, and were
isolated using TRIzol reagent (Invitrogen Life Technologies),
according to the manufacturer’s instructions. The quantity and
purity of the total RNA extracted was estimated by monitoring the
absorbance at the ratio 260/280 using a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The
260/280 ratio did not vary significantly among the groups. The
integrity of the 28S and 18S ribosomal RNA was confirmed using 1%
agarose gel electrophoresis (Sigma-Aldrich) with 500 ng of the
total RNA. The ethidium-bromide (Beijing Dingguo Company) stained
gels were exposed to ultraviolet light (FS-312; Shanghai Fusheng
Institute of Biotechnology, Shanghai, China), to determine the
quality of the RNA.
For cDNA synthesis, 1 µg total RNA from each
pooled sample was reverse transcribed into cDNA using a PrimeScript
RT Reagent kit (Takara Bio, Inc., Dalian, China), according to the
manufacturer’s instructions, in a 20 µl reaction volume. All
the cDNA samples were stored at −20°C until qPCR analyses.
Quantification of mRNA
The primers of the ACTB, ARBP, B2M, CYCA, GAPDH,
GUSB, HPRT, PPIA, RPL13A, TBP and PGK1 reference genes were
synthesized by Sangon Company (Shanghai, China; Table I). The primers were designed using
the following criteria: A temperature between 58–62°C and optimal
temperature of 60°C; a PCR product size between 75 and 200 base
pairs, a length of between 18 and 24 nucleotides and optimal length
of 20 nucleotides; and a GC content between 40 and 60%. The primers
were assessed using BLAST analysis to verify the specificity and
selective amplification of the target gene, as primers can obtain
amplification of cDNA and not genomic DNA.
 | Table IReference genes for gene expression
normalization in induced bone mesenchymal stem cells. |
Table I
Reference genes for gene expression
normalization in induced bone mesenchymal stem cells.
| Gene | Accession no | Amplicon. length
(bp) | Sequence
(5′-3′) | E | R2 |
|---|
| TBP | NM 001004198.1 | 111 | Sense
TAATCCCAAGCGGTTTGCTG | 2.12 | 0.998 |
| | | Antisense
TTCTTCACTCTTGGCTCCTGTG | | |
| B2M | NM 012512.1 | 114 | Sense
CGAGACCGATGTATATGCTTGC | 1.94 | 0.986 |
| | | Antisense
GTCCAGATGATTCAGAGCTCCA | | |
| HPRT | NM 012583.2 | 152 | Sense
TCCCAGCGTCGTGATTAGTGA | 2.08 | 0.975 |
| | | Antisense
CCTTCATGACATCTCGAGCAAG | | |
| RPL13A | NM 173340.2 | 145 | Sense
TCTCCGAAAGCGGATGAACAC | 1.92 | 0.991 |
| | | Antisense
CAACACCTTGAGGCGTTCCA | | |
| ARPB | NM 022402.1 | 165 | Sense
CCCTTCTCCTTCGGGCTGAT | 1.90 | 0.985 |
| | | Antisense
TGAGGCAACAGTCGGGTAGC | | |
| ACTB | NM 031144.2 | 86 | Sense
CTGACTGACTACCTCATGAAGATCCT | 1.83 | 0.946 |
| | | Antisense
CTTAATGTCACGCACGATTTCC | | |
| PGK1 | NM 053291.3 | 113 | Sense
GCAGATTGTTTGGAACGGTCC | 2.05 | 0.943 |
| | | Antisense
TAGTGATGCAGCCCCTAGACGT | | |
| PPIA | NM 017101.1 | 135 | Sense
CCAAACACAAATGGTTCCCAGT | 1.88 | 0.990 |
| | | Antisense
ATTCCTGGACCCAAAACGCT | | |
| GUSB | NM 017015.2 | 101 | Sense
CCTTTCTACTTCCAAGGCGTCA | 1.74 | 0.993 |
| | | Antisense
CAACGGAGGAGGTTGAAATCC | | |
| CYCA | NM 017101.1 | 126 | Sense
TATCTGCACTGCAAGACTGAGTG | 1.98 | 0.986 |
| | | Antisense
CTTCTTGCTGGTCTTGCCATTCC | | |
| GAPDH | NM 17008.4 | 223 | Sense
GGCATTGCTCTCAATGACAA | 1.90 | 0.981 |
| | | Antisense
TGTGAGGGAGATGCTCAGTG | | |
The mRNA expression levels were analyzed by RT-qPCR.
Equal quantities of DNA-free RNA from each sample were used. The
PCRs were performed using SGExcelFastSYBR mix, containing ROX
(SK2954B; Sangon Company). The PCR mix in each well contained 5
µl of 2X SGExcelFastSYBR mix with ROX, 4.2 µl
dH2O, 0.4 µl each of the forward and reverse
primers (10 pmol/µl) and 0.4 µl single strand cDNA
(50 ng/µl) in a final reaction volume of 10 µl.
Amplification was performed on a LightCycler 480 (Roche
Diagnostics, Basel, Switzerland) using the following program: 72°C
for 5 min; 94°C for 3 min, stage 3: 94°C for 20 sec, 57°C for 20
sec and 72°C for 20 sec for 40 cycles; and 72°C for 3 min. To
minimize variation in the RT reaction, all the RNA samples from a
single experimental setup were reverse transcribed simultaneously.
The qPCR products were subjected to 3% agarose gel
electrophoresis.
To estimate the efficiencies of amplification, a
standard curve was generated for each primer pair, based on four
points of a serial 2-fold dilution of cDNA (40.0, 4.0, 0.4 and 0.04
ng). The amplification efficiencies and the correlation coefficient
(R2) values were calculated using the slope of the
calibration curve, using the following equation: E =
2−1/slope.
The threshold cycle values were collected for export
using the PCR system software (LightCycler 480 software, version
1.5.0) for further analysis.
Statistical analysis
To analyze and confirm the results, the geNorm
(17), NormFinder (18) and BestKeeper (19) applications were used. These
bioinformatics packages calculate stability values. The genes were
then ranked in order of stability, according to these obtained gene
expression stability values.
GeNorm-based analysis of candidate
reference genes
GeNorm software is designed to determine an accurate
selection for a set of genes, which exhibit minimal variation
across different biological conditions (17). It uses the average pair-wise
variation between a particular gene and all other reference genes,
and calculates the optimal number of genes necessary for
normalization. This analysis provides a ranking of the assessed
genes based on their stability measure (M), determining the most
stable reference genes or a combination of multiple stable genes
for normalization.
The M value denotes the mean pair-wise variation for
a specific gene compared with other assessed genes, The gene with
the highest M value is then excluded from the analysis, and
recalculated in order to select the two most stable genes. The
reference genes are ranked according to their M value, between the
lowest and the highest. Lower M values represent higher expression
stabilities.
NormFinder-based analysis of candidate
reference genes
NormFinder computes the expression stability values
using an analysis of variance-based model (18). NormFinder is a mathematical
approach for identifying the optimal gene to use as reference genes
among a set of candidates. It uses all candidate genes and provides
a ranking order based on the estimated intragroup stability of the
candidate genes, based on an estimate of the variation in each
subgroup, and intergroup stability, based on the combination of all
subgroups. The two results are then combined, to produce a
stability value for each gene investigated. The program ranks genes
based on a stability value, with the lowest value indicating the
most stably expressed gene.
BestKeeper analysis of candidate
reference genes
BestKeeper analysis is based on the threshold cycle
(Ct) values and is presented as the standard deviation (SD) and
coefficient of correlation (R2) (19). The genes with an SD>1.00 are
considered unreliable as a stable reference gene. The remaining
genes are ranked according to their r values, higher r values
indicating higher stability. A novel feature of BestKeeper is the
calculation of the intrinsic variance of expression for a single
sample, which detects outliers. Feng et al previously
described that the lowest ranking genes in geNorm and NormFinder
were the same (21), however
BestKeeper was not included in their analysis.
Results
Culture and identification of BMSCs
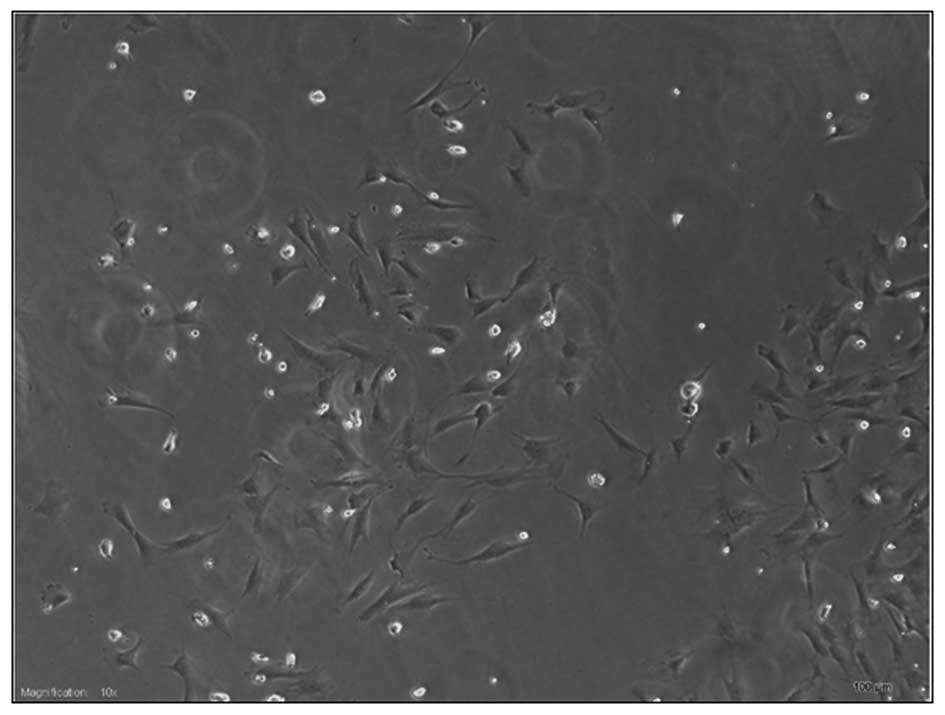
The majority of the cells grew adherently following
inoculation for 24 h, were spindly with adequate refraction
(Fig. 1), were mixed with other
types of cells, and certain cells were suspended in the culture
medium. The non-adherent cells were removed by replacing the
medium. The cells reached a confluence of up to 90% after 5–7 days,
following which the growth rate accelerated. The cells were
passaged once after 3–5 days and, at the third passage, the cells
exhibited a spindle-shaped fibroblastic morphology. Fluorescent
cell sorting at passage three demonstrated that the cells were
negative for CD45, and positive for CD90, CD71 and CD29 (Fig. 2).
Differentiation of BMSCs
Following the addition of inducers, the cell bodies
became increasingly spherical and refractive, exhibiting a typical
neuronal appearance. The processes continued to grow, exhibiting
primary and secondary branches, growth cone-like terminal
expansions and putative filopodial extensions. To further
characterize the neuronal differentiation, the cells were stained
for the neuronal markers, nestin, NSE and NF (Fig. 3).
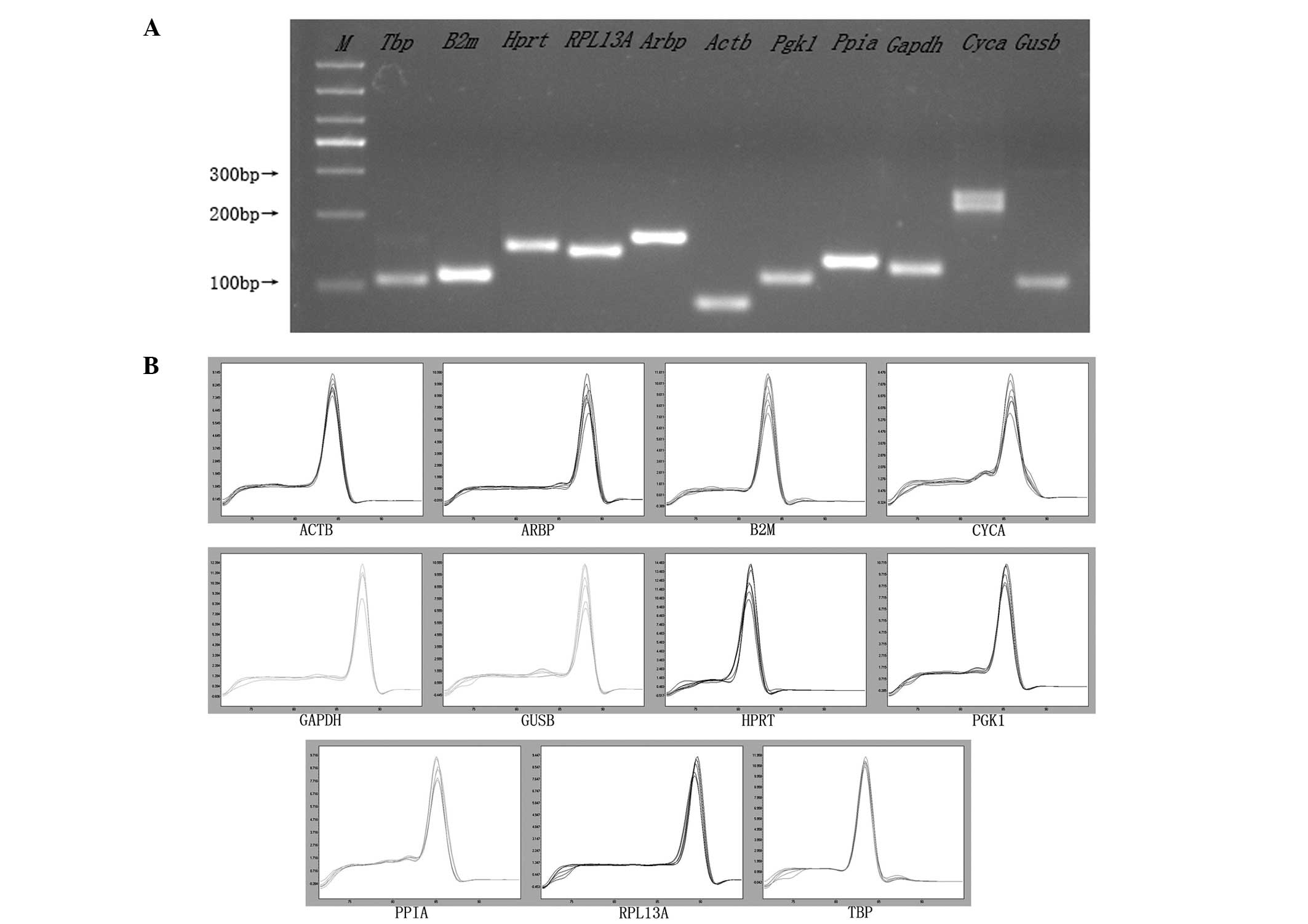
Amplification performance of the
primers
The qPCR amplification product was detected using 3%
agarose gel electrophoresis with the expected size and no primer
dimmers, (Fig. 4A). For relative
quantification, a standard curve was generated in each individual
run by serially diluting the pool of cDNA samples (40.0, 4.0, 0.4
and 0.04 ng) with high expression values. One single peak was
obtained in each amplification reaction on analyzing the melting
curves, which confirmed the specific amplification of The primers
(Fig. 4B).
For all 11 genes, the amplification efficiencies
were between 1.74 and 2.11, and the correlation coefficients
(R2) were >0.94.
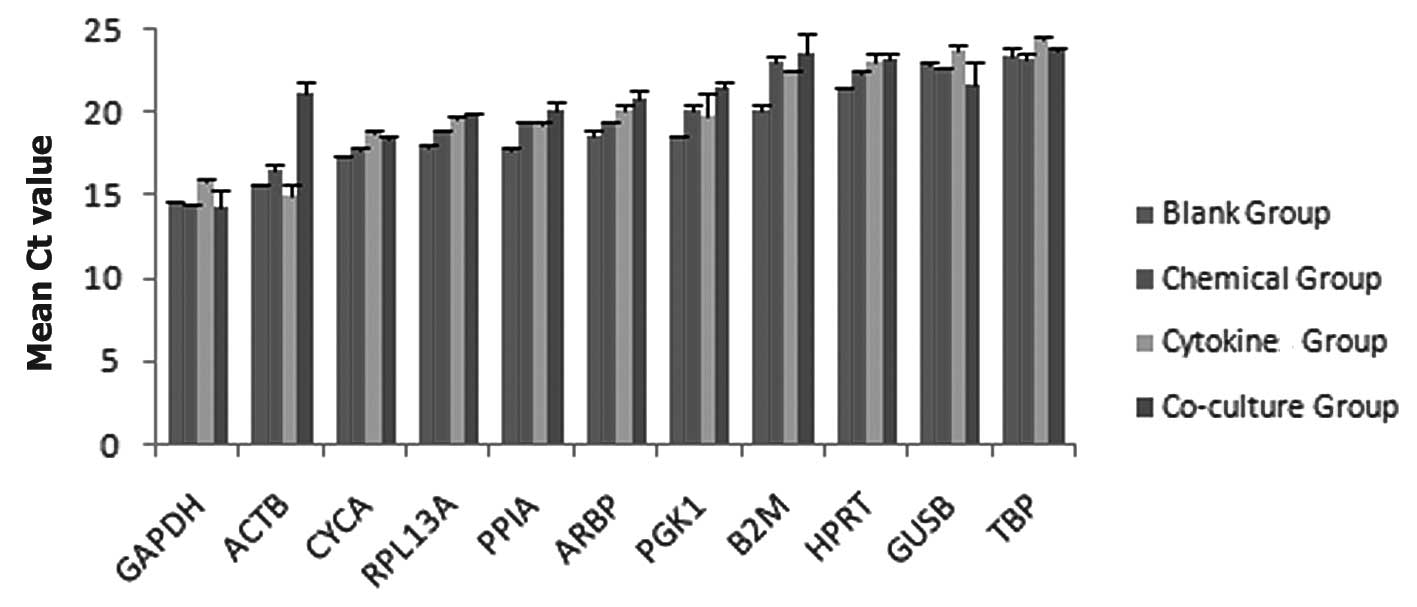
Expression profile of the reference
genes
Each sample was analyzed in duplicate, and the
average Ct value for each sample and the coefficient of variation
(CV) for each groups was obtained. The CV, expressed as percentage
and calculated as the SD/mean Ct, was used to compare the degree of
variation among the 11 reference genes. The Ct values for each of
the 11 candidate reference genes ranged between 13 and 25 cycles.
Figure 5 shows the mean Ct values
of each gene in the four groups of treated cells.
The reference genes exhibited Ct values between
13.63 for GAPDH and 24.53 for TBP. These genes exhibited different
Ct values, GAPDH exhibited the lowest Ct values, between 13.63 and
15.94, while TBP exhibited the highest Ct values, between 22.93 and
24.53. ACTB exhibited the highest variation in levels of RNA
expression (SD=2.59). The lowest variation i levels of RNA
expression was observed for TBP (SD=0.58), followed by CYCA
(SD=0.60)
Expression stability of candidate
reference genes using GeNorm
The geNorm analysis software was used to select the
optimal reference genes. The parameters, which were considered to
quantify reference gene stability were the M value and pair-wise
variation. The software eliminates genes with the highest M-value,
and repeats the process until there only two genes remain. An M
value <1.5 indicates that no genes are considered unreliable as
a stable reference gene. Among the 11 genes, no M value were
>1.5. RPL13A and HPRT were the most stable genes, exhibiting the
lowest M values, and ACTB was the least stable gene, exhibiting the
highest M value (Fig. 6A).
To determine the optimal number of required
reference genes for each group, the Vn/n+1 was evaluated using
geNorm. For all the Vn/n+1 values, including V2/3, indicating the
number of further reference genes to add on the normalization
factor, a threshold of 0.15 was designed as the cut-off value for
pair-wise variation. The inclusion of the third reference gene was
not necessary (Fig. 6B).
NormFinder analysis
NormFinder was used to confirm the most stably
expressed gene. As shown in Table
II, a low stability value represents a low expression variance.
The results demonstrated that RPL13A was the most stablly expressed
gene, with a stability value of 0.158, and ACTB was the least
stably expressed gene, with a stability value of 1.186. These
results were consistent with those of the geNorm analysis. The most
suitable combination of genes was CYCA and PPIA, with a combined
stability value of 0.119, and their stability value was lower than
RPL13A, therefore, this combination was confirmed as the most
stable genes, providing a higher level of stability compared with
using only one (Fig. 7).
 | Table IIAnalysis of the expression
stabilities of the 11 reference genes using NormFinder
software. |
Table II
Analysis of the expression
stabilities of the 11 reference genes using NormFinder
software.
| Reference gene | Stability
value | Rank |
|---|
| RPL13A | 0.158 | 1 |
| PPIA | 0.205 | 2 |
| HPRT | 0.230 | 3 |
| CYCA | 0.280 | 4 |
| ARBP | 0.319 | 5 |
| TBP | 0.559 | 6 |
| GAPDH | 0.618 | 7 |
| PGK1 | 0.625 | 8 |
| B2M | 0.687 | 9 |
| GUSB | 0.784 | 10 |
| ACTB | 1.186 | 11 |
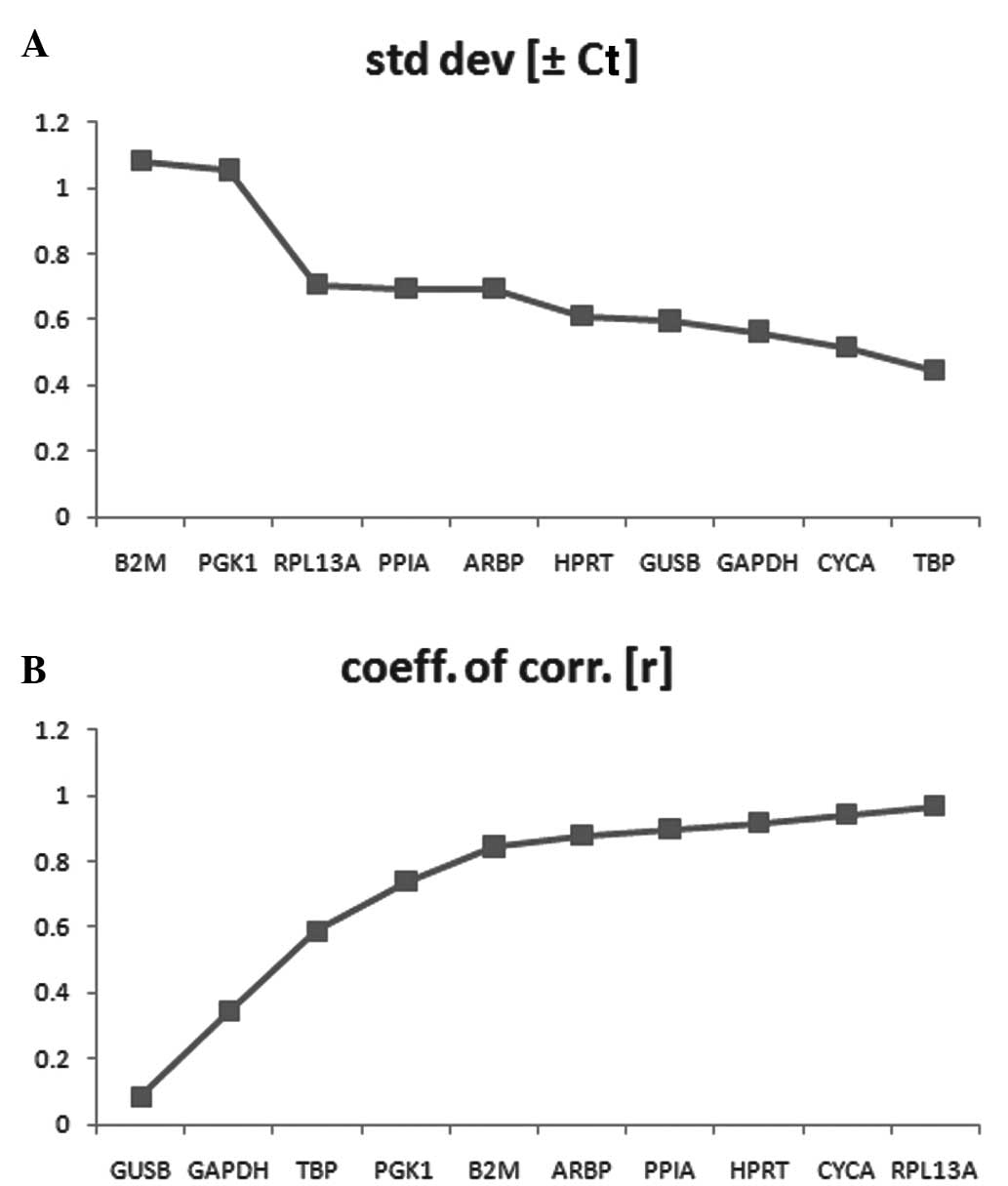
BestKeeper analysis
BestKeeper was also used to identify the most stably
expressed genes, by comparing the r values and SD. The number of
candidate reference genes was limited by this software tool,
therefore, 10 of the candidate reference genes were analyzed. As
geNorm and NormFinder identified ACTB with the most variance, ACTB
was eliminated. The results revealed that the SD value of B2 M and
PGK1 were >1.00 and were, therefore, considered unacceptable and
eliminated (Fig. 8A). Among the
remaining genes, according r values of the candidate reference
genes, the higher stability values implied increased stability, of
which RPL13A exhibited the highest stability (Fig. 8B). The descriptive statistics of
the reference genes based on their Ct values, analyzed using
BestKeeper, are shown in Table
III.
 | Table IIIDescriptive statistics of the 11
reference genes based on threshold cycle values, analyzed using
BestKeeper software. |
Table III
Descriptive statistics of the 11
reference genes based on threshold cycle values, analyzed using
BestKeeper software.
| Value | GAPDH | CYCA | RPL13A | PPIA | ARBP | PGK1 | B2M | HPRT | GUSB | TBP |
|---|
| Geo mean Ct | 14.80 | 18.06 | 19.05 | 19.16 | 19.76 | 19.93 | 22.21 | 22.51 | 22.67 | 23.66 |
| Ar mean Ct | 14.82 | 18.06 | 19.07 | 19.18 | 19.78 | 19.97 | 22.25 | 22.52 | 22.69 | 23.67 |
| Min Ct | 13.63 | 17.25 | 17.77 | 17.78 | 18.49 | 18.51 | 19.88 | 21.35 | 20.58 | 22.93 |
| Max Ct | 15.94 | 18.91 | 19.99 | 20.45 | 21.20 | 21.67 | 24.36 | 23.43 | 23.97 | 24.53 |
| SD of Ct | 0.56 | 0.52 | 0.70 | 0.69 | 0.69 | 1.06 | 1.08 | 0.61 | 0.60 | 0.45 |
| CV (%) | 3.80 | 2.86 | 3.69 | 3.61 | 3.50 | 5.28 | 4.86 | 2.70 | 2.62 | 1.88 |
| Min (-fold) | −2.25 | −1.75 | −2.44 | −2.61 | −2.40 | −2.68 | −5.02 | −2.23 | −4.24 | −1.66 |
| Max(-fold) | 2.20 | 1.81 | 1.92 | 2.44 | 2.72 | 3.34 | 4.45 | 1.90 | 2.47 | 1.82 |
| SD (±-fold) | 1.48 | 1.43 | 1.63 | 1.62 | 1.62 | 2.08 | 2.12 | 1.52 | 1.51 | 1.36 |
| CC (r) | 0.344 | 0.944 | 0.968 | 0.899 | 0.881 | 0.741 | 0.845 | 0.918 | −0.086 | 0.588 |
Ranking of the reference genes, using the results of
the geNorm, NormFinder and BestKeeper analyses, are shown in
Table IV.
 | Table IVRanking of reference genes using
geNorm, NormFinder and BestKeeper analyses. |
Table IV
Ranking of reference genes using
geNorm, NormFinder and BestKeeper analyses.
| Rank | geNorm | NormFinder | BestKeeper |
|---|
| 1 | RPL13A/HPRT | RPL13A | RPL13A |
| 2 | PPIA | CYCA | |
| 3 | PIPA | HPRT | HPRT |
| 4 | ARBP | CYCA, | PPIA |
| 5 | CYCA | ARBP | ARBP |
| 6 | TBP | TBP | TBP |
| 7 | PGK1 | GAPDH | GAPDH |
| 8 | GAPDH | PGK1 | GUSB |
| 9 | B2M | B2M | |
| 10 | GUSB | GUSB | |
| 11 | ACTB | ACTB | |
Discussion
BMSCs are a type of stem cell, which are different
from hematopoietic stem cells in bone marrow. They are a type of
early mesodermal cell with multiple differentiation potentials. As
the availability of pluripotent retinal stem cells is not limited
(22), and are not rejected by the
host immune system in either allogeneic or xenogenic host species
recipients (23), BMSCs have
become an area of interest. Several studies have demonstrated that
BMSCs differentiate into chondrocytes, fibroblasts, adipocytes,
cardiomyocytes and skeletal muscle cells in vitro (24–26).
The differentiation of BMSCs into neuronal cells has been a focus
of investigations. Studies have reported that they can be induced
to differentiate into neuron-like cells in vitro and in
vivo (13,15,27).
In vivo investigations have demonstrated that BMSCs can
differentiate into neuron-like cells, and the neurogenesis,
maturation and migration of the cells were the same as those
observed in neural stem cells. The understanding of these cells and
their differentiation potentials is important for their use to
achieve desired functions in vivo. To evaluate the
differentiation of the cells, determination of the mRNA levels of
specific genes is necessary.
At present, qPCR is the most fundamental, accurate
and commonly used method to investigate mRNA levels Due to its low
cost and ease of operation, qPCR is consistently used to assess the
expression levels of specific genes. As a crucial step requires
that the RT-qPCR data is accurately normalized by use of
appropriate reference genes, as the use of non-validated reference
genes can lead to erroneous results, which are biologically
meaningless (28). However, there
are currently no universally applicable reference genes. The same
reference genes in different species, and different reference genes
in the same species, can have markedly different expression levels
under diverse experimental conditions (7,29).
For example, during rat MSC differentiation into osteocyte,
adipocyte and chondrocyte lineages, the GUSB and B2M reference
genes were among the least stable genes in the MSC and adipocyte
lineages, but were the most stable in the chondrocyte and osteocyte
lineages (30).
To the best of our knowledge, there are no previous
reports regarding the validation of reference genes for gene
expression assays of rat BMSCs during neuronal differentiation. In
the present study, the expression stabilities of 11 commonly
accepted candidate reference genes was analyzed. The public
programs, geNorm, NormFinder and BestKeeper, were used for the
evaluation of gene stability (31). In each of the experimental
differentiation procedures, the BMSCs group was defined as the
calibrator sample, and the data of each differentiated lineage was
combined with the BMSC group (30).
The resulting Ct values demonstrated that GAPDH
exhibited the lowest mean Ct values, followed by ACTB and CYCA.
ACTB was observed to exhibit the highest variation in RNA
expression levels. The lowest level of RNA expression variation was
observed in TBP, followed by CYCA. This revealed that the
quantities of the reference genes varied markedly. The specificity
of the 18 s rRNA designed primer easily amplifies nonspecific
bands, and in vivo data has revealed that 18S rRNA is unfit
as a reference gene in neurons (31). A possible explanation for this may
be that, when 18S rRNA is used as a reference gene, it may be
associated with an imbalance between mRNA and rRNA. Therefore, 18S
rRNA was not included in the presents study.
GeNorm, NormFinder and BestKeeper software were used
to rank the analyzed reference genes according to their expression
stabilities. This rank order differed marginally between these
tools, possibly due to the fact they are based on different
mathematical models (32). All the
software indicated that RPL13A exhibited the highest stability,
and, ACTB was affirmed as the least stable gene by all three
algorithms. This suggested that it was not possible to estimate the
stability of a reference gene using the Ct value alone. These
results are the same as those of a previous study. Bonefeld et
al validated eight reference genes in rat hippocampal tissue
neurons in vivo and also identified CYCA and RPL13A as the
most stably expressed genes, and ACTB and 18S rRNA as the least
stable genes (33). In a previous
study investigating suitable reference genes for qPCR in the
dentate gyrus following experimental febrile seizures, geNorm and
Normfinder also indicated CYCA, RPL13A and TBP as the most stable
genes, whereas 18S rRNA and ACTB were found to be the least stably
expressed genes (31).
The optimal number of reference genes for
normalization is suggested by a V value below the cut-off of 0.15
in geNorm (17). The results of
the present study indicated that the normalization factor requires
two reference genes. As indicated in Fig. 6, the combination of RPL13A and HPRT
were identified as the optimal pair for evaluating gene stability,
however, according to NormFinder (Fig.
7), the results indicated that CYCA and PIPA were the optimal
pair of reference genes. Based on the above results, the present
study recommended that the pair of RPL13A and HPRT were optimal for
the evaluation of gene stability, according to ranking of the genes
from 1–12 for stability. According to Fig. 5, a pair-wise variation of 0.118 was
observed following the addition of the second most stable gene.
In conclusion, the present study investigated the
expression stabilities of 11 reference genes, which are commonly
used for normalization in gene analysis, in three types of
differentiated neuron cell from BMSCs and normal BMSCs. The results
demonstrated that RPL13A, and the combination of CYCA and PPIA
provided a reliable interpretation of the mRNA expression data in
the experimental set-up, while ACTB appeared unsuitable as a
reference gene. These results can be consulted for further qPCR
investigations, and also present an appropriate strategy for the
evaluation of candidate reference genes for any BMSC
differentiation procedure.
Acknowledgments
This study was supported by the Project (GAP-43 gene
modified bone marrow mesenchymal stem cells can differentiate into
ganglion-like cells and promote nerve regeneration) supported by
the National Natural Science Foundation of China (grant. no.
30973265).
References
|
1
|
Bustin SA, Benes V, Garson JA, et al: The
MIQE guidelines: minimum information for publication of
quantitative real-time PCR experiments. Clin Chem. 55:611–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huggett J, Dheda K, Bustin S and Zumla A:
Real-time RT-PCR normalisation; strategies and considerations.
Genes Immun. 6:279–284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bustin SA and Nolan T: Pitfalls of
quantitative real-time reverse-transcription polymerase chain
reaction. J Biomol Tech. 15:155–166. 2004.PubMed/NCBI
|
|
4
|
Zhong Q, Zhang Q, Wang Z, et al:
Expression profiling and validation of potential reference genes
during Paralichthys olivaceus embryogenesis. Mar Biotechnol (NY).
10:310–318. 2008. View Article : Google Scholar
|
|
5
|
Bustin SA, Benes V, Nolan T and Pfaffl MW:
Quantitative real-time RT-PCR-a perspective. J Mol Endocrinol.
34:597–601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dheda K, Huggett JF, Bustin SA, Johnson
MA, Rook G and Zumla A: Validation of housekeeping genes for
normalizing RNA expression in real-time PCR. Biotechniques.
37:112–114. 116118–119. 2004.PubMed/NCBI
|
|
7
|
Radonic A, Thulke S, Mackay IM, Landt O,
Siegert W and Nitsche A: Guideline to reference gene selection for
quantitative real-time PCR. Biochem Biophys Res Commun.
313:856–862. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki T, Kobayashi K and Sasaki O:
Real-time displacement measurement with a two-wavelength sinusoidal
phase-modulating laser diode interferometer. Appl Opt.
39:2646–2652. 2000. View Article : Google Scholar
|
|
9
|
Cordoba EM, Die JV, González-Verdejo CI,
Nadal S and Román B: Selection of reference genes in Hedysarum
coronarium under various stresses and stages of development. Anal
Biochem. 409:236–243. 2011. View Article : Google Scholar
|
|
10
|
Schmittgen TD and Zakrajsek BA: Effect of
experimental treatment on housekeeping gene expression: validation
by real-time, quantitative RT-PCR. J Biochem Biophys Methods.
46:69–81. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santos AR and Duarte CB: Validation of
internal control genes for expression studies: effects of the
neurotrophin BDNF on hippocampal neurons. J Neurosci Res.
86:3684–3692. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun C, Shao J, Su L, et al: Cholinergic
neuron-like cells derived from bone marrow stromal cells induced by
tricyclodecane-9-yl-xanthogenate promote functional recovery and
neural protection after spinal cord injury. Cell Transplant.
22:961–975. 2013. View Article : Google Scholar
|
|
13
|
Woodbury D, Schwarz EJ, Prockop DJ and
Black IB: Adult rat and human bone marrow stromal cells
differentiate into neurons. J Neurosci Res. 61:364–370. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sanchez-Ramos J, Song S, Cardozo-Pelaez F,
et al: Adult bone marrow stromal cells differentiate into neural
cells in vitro. Exp Neurol. 164:247–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu C, Su G and Mu D: Retinal cells of rat
neonates inducing the bone marrow mesenchymal cells into RGC-like
cells. Chin Ophthal Res. 26:174–178. 2008.
|
|
16
|
Wang C, Su G, Qi S, et al: Study of GAP-43
expression on differentiation of bone mesenchymal stem cells to
neuron-like cells. Chin J Lab Diagn. 12:1206–1209. 2008.
|
|
17
|
Vandesompele J, De Preter K, Pattyn F, et
al: Accurate normalization of real-time quantitative RT-PCR data by
geometric averaging of multiple internal control genes. Genome
Biol. 3:RESEARCH00342002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andersen CL, Jensen JL and Ørntoft TF:
Normalization of real-time quantitative reverse transcription-PCR
data: a model-based variance estimation approach to identify genes
suited for normalization, applied to bladder and colon cancer data
sets. Cancer Res. 64:5245–5250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pfaffl MW, Tichopad A, Prgomet C and
Neuvians TP: Determination of stable housekeeping genes,
differentially regulated target genes and sample integrity:
BestKeeper-excel-based tool using pair-wise correlations.
Biotechnol Lett. 26:509–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Long X, Olszewski M, Huang W and Kletzel
M: Neural cell differentiation in vitro from adult human bone
marrow mesenchymal stem cells. Stem Cells Dev. 14:65–69. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng X, Xiong Y, Qian H, Lei M, Xu D and
Ren Z: Selection of reference genes for gene expression studies in
porcine skeletal muscle using SYBR green qPCR. J Biotechnol.
150:288–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aramant RB, Seiler MJ and Ball SL:
Successful cotransplantation of intact sheets of fetal retina with
retinal pigment epithelium. Invest Ophthalmol Vis Sci.
40:1557–1564. 1999.PubMed/NCBI
|
|
23
|
Gouras P, Flood MT, Kjedbye H, Bilek MK
and Eggers H: Transplantation of cultured human retinal epithelium
to Bruch’s membrane of the owl monkey’s eye. Curr Eye Res.
4:253–265. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gimble JM, Zvonic S, Floyd ZE, Kassem M
and Nuttall ME: Playing with bone and fat. J Cell Biochem.
98:251–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dezawa M, Kanno H, Hoshino M, et al:
Specific induction of neuronal cells from bone marrow stromal cells
and application for autologous transplantation. J Clin Invest.
113:1701–1710. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gregory CA, Prockop DJ and Spees JL:
Non-hematopoietic bone marrow stem cells: molecular control of
expansion and differentiation. Exp Cell Res. 306:330–335. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu S, Xing C, Han J, Tso MO and Hong J:
Differentiation of rabbit bone marrow mesenchymal stemcells into
corneal epithelial cells in vivo and ex vivo. Mol Vis. 15:99–107.
2009.
|
|
28
|
Liu C, Xin N, Zhai Y, et al: Reference
gene selection for quantitative real-time RT-PCR normalization in
the half-smooth tongue sole (cynoglossus semilaevis) at different
developmental stages, in various tissue types and on exposure to
chemicals. PLoS One. 9:e917152014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McCurley AT and Callard GV:
Characterization of housekeeping genes in zebrafish: male-female
differences and effects of tissue type, developmental stage and
chemical treatment. BMC Mol Biol. 9:1022008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Farrokhi A, Eslaminejad MB, Nazarian H,
Moradmand A, Samadian A and Akhlaghi A: Appropriate reference gene
selection for real-time Pcr Data normalization during rat
mesenchymal stem cell differentiation. Cell Mol Biol
(Noisy-le-grand). 58(Suppl): OL1660–1670. 2012.
|
|
31
|
Swijsen A, Nelissen K, Janssen D, Rigo JM
and Hoogland G: Validation of reference genes for quantitative
real-time PCR studies in the dentate gyrus after experimental
febrile seizures. BMC Res Notes. 5:6852012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang E, Shi S, Liu J, et al: Selection of
reference genes for quantitative gene expression studies in
Platycladus orientalis (Cupressaceae) using real-time PCR. PLoS
One. 7:e332782012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bonefeld BE, Elfving B and Wegener G:
Reference genes for normalization: a study of rat brain tissue.
Synapse. 62:302–309. 2008. View Article : Google Scholar : PubMed/NCBI
|