Introduction
Ovarian cancer, also termed epithelial ovarian
cancer, is the sixth most common type of cancer affecting female
individuals worldwide, with a mortality rate of ~125,000 annually
(1). Ovarian cancer occurs as four
major histological subtypes, serous, mucinous, endometrioid and
clear cell, with serous being the most common (2). According to previous studies, 5-year
survival is observed in only 30% of patients with advanced-stage
ovarian cancer, however, only 19% of all cases of ovarian cancer
are diagnosed at an early stage (2,3),
therefore, additional therapeutics strategies for ovarian cancer
are required. Quercetin is one of the most abundant flavonoids in
plants, fruits and vegetables, and possess several pharmacological
properties that are closely associated with those of existing
therapeutic agents, including cardioprotective, antiviral,
anti-inflammatory and anti-aging properties, and capacities to
extend lifespan (4). Previous
studies have demonstrated that quercetin not only induces tumor
cell apoptosis, but also acts as a chemosensitizer in anticancer
therapy via a different cell signaling pathway (5–7).
Furthermore, quercetin can inhibit the growth of OVCAR-3 human
ovarian cancer cells, associated with expression of vascular
endothelial growth factor (VEGF) (8). However, no data is available
regarding the antitumoral activity of quercetin on the SKOV-3 and
A2780 human ovarian cancer cell lines.
MicroRNAs (miRNAs/miR) are a novel class of
endogenous, non-coding RNAs involved in post-transcriptional gene
regulation by binding to a target site in the 3′-untranslated
region of target mRNAs (9,10). miRNAs can function as regulatory
molecules, which act as tumor suppressors or oncogenes and are
involved in the development of human cancer (11,12).
miR-145 is located on chromosome 5q32-33 within a 4.09 kb region
(13). miR-145 has frequently been
reported to be downregulated in certain types of cancer, including
bladder (13) and colon cancer,
and acts as a tumor suppressive miRNA, which inhibits the growth,
invasion and migration of cancer cells (14). In a previous study, miR-145 was
downregulated in ovarian cancer cells compared with normal groups
of cells (15,16). Notably, the overexpression of
miR-145 has been observed to suppress MCF-7 cell growth and induce
apoptosis in vitro (17).
Another study suggested that dietary quercetin supplementation can
increase the concentrations of hepatic miR-122 and miR-125b, which
contribute to the gene-regulatory activity of quercetin in
vivo, suggesting that quercetin regulates the expression of
miRNAs (18). However, the exact
mechanisms underlying the action of miR-145 in ovarian cancer
remain to be elucidated and require further examination. The aim of
the present study was to clarify the role of miR-145 in human
ovarian cancer and elucidate whether quercetin induces the
apoptosis of human ovarian cancer cell lines (SKOV-3 and A2780) via
miR-145.
Materials and methods
Cell culture
The SKOV-3 and A2780 human ovarian cancer cell lines
were purchased from Shanghai Institutes for Biological Sciences,
Chinese Academy of Cell Resource Center (Shanghai, China) and
maintained in Dulbecco’s modified Eagle’s medium (Hyclone, Logan,
UT, USA) with 10% fetal bovine growth serum (Hyclone), 100 U/ml
penicillin and 100 μg/ml streptomycin (BD Pharmingen, San
Jose, CA, USA). The cells were maintained at 37°C in humidified
conditions containing 5% CO2.
MTT assay
The cell viability was determined using an MTT
assay. Briefly, the cells were seeded in 96-well plates at
1×104 cells/well and treated without (control) or with
different concentrations of quercetin (25, 50 or 100 μm/ml;
Sigma-Aldrich, St. Louis, MO, USA) for 12, 24, 48 or 72 h at 37°C.
Subsequently, 10 ml MTT (Sigma-Aldrich) was added to each well,
followed by incubation for 4 h at 37°C. The medium was then removed
and 150 ml dimethyl sulfoxide (Sigma-Aldrich) was added to
solubilise the formazan produced. The optical densities of the
cells were read at 490 nm using a Synergy™ HTX Multi-Mode
Microplate Reader (BioTek, Winooski, VT, USA).
miRNA-145 extraction and
purification
For extraction and purification, 5×106
cells were treated without (control) or with quercetin for 24 h at
37°C. Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) was used to determine the expression levels of miR-145 in
the cells with a Roche Lightcycler 480 (Roche Diagnostics,
Mannheim, Germany). Initially, total RNAs were extracted from the
cultured cells using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). The RT-qPCR analysis of the levels of miR-145
was performed using a TaqMan Reverse Transcription kit and TaqMan
microRNA Assay kit (Applied Biosystems, Carlsbad, CA, USA),
according to the manufacturers’ instructions. Primers were
purchased from Invitrogen Life Technologies and the sequences were
as follows: miR145 forward, 5′-ACA CTCCAGCTGGGCAGGTCAAAAGGGTCC-3′
and reverse, 5′-TGTGAGGTCGACCCGTCCAGTTTTCCCAGG-3′. and U6 forward,
5′-TGCGGGTGCTCGCTTCGGCAGC-3′, which could ensure the specificity of
the PCR products and reverse 5′-GGTGTCGTGGAGTCG-3′, which was
universal. The PCR cycling conditions were set as follows: Initial
denaturation at 95°C for 5 min, followed by 30 cycles at 94°C for
0.5 min, 56°C for 1 min and 72°C for 0.5 min, and a final extension
at 72°C for 10 min. The expression levels of miR-145 were
calculated by calculating the threshold cycle (Ct) values and using
the 2−ΔΔCt method, with U6 as an internal control
(14–16).
Transfection of anti-miR-145
The human ovarian cancer cell lines SKOV-3 and A2780
were transfected with 100 nmol/l of the miR inhibitor,
anti-miR-199a (Ambion, Austin, TX, USA). At 24 h after
transfection, the cells were treated with or without quercetin.
Western blotting
Following harvesting, the cells were washed in
phosphate-buffered saline (PBS) and lysed in 1X sodium
dodecylsulfate (SDS) loading buffer (Sigma-Aldrich). The lysates
were then clarified via centrifugation at 15,000 × g for 10 min at
4°C and collected. The protein concentrations were determined using
a Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA).
Equivalent quantities of protein (20–50 μg) were separated
using SDS-PAGE. Briefly, the proteins were resolved using 12%
SDS-polyacrylamide gels and transferred onto polyvinylidene
difluoride membranes (Millipore, Bedford, MA, USA). The membranes
were incubated with PBS containing 0.05% Tween 20 (Abcam,
Cambridge, MA, USA) and 5% non-fat dry milk to block non-specific
binding. The membranes were then incubated with the appropriate
rabbit monoclonal antibodies against cleaved caspase-3 (cat. no.
9661) and β-actin (cat. no. 8457) (1:2,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) at 4°C overnight. Subsequently,
the blots were washed with iced PBS and incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:2,000;
cat. no. 7074; Cell Signaling Technology, Inc.) at 4°C for 2 h,
washed again, and immunoreactivity was detected by
chemiluminescence. For all immunoblots, β-actin immunoreactivity
was used as a loading control. All antibodies were used at a
dilution of 1:2,000. Western blot analyses were performed in three
independent experiments. The blots were then exposed to
radiographic film (China Lucky Film Corp., Baoding, China) to
visualize the immunoreactive signals. Signals were quantified using
Multi Gauge Image Analysis version 3.0 software (FujiFilm, Tokyo,
Japan).
Statistical analysis
The results are expressed as the mean ± standard
error of the mean. Statistical analysis was performed using
commercially available SPSS version 13.0 (SPSS, Inc., Chicago, IL,
USA). Comparisons among groups were performed using a Student’s
t-test, as appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibitory effect of quercetin on SKOV-3
and A2780 cell growth
Following treatment with quercetin at different
concentrations, the growth of the SKOV-3 and A2780 cells were
inhibited in a dose- and time-dependent manner, as determined by
the MTT assay (Fig. 1A and B).
Furthermore, the half maximal inhibitory concentration
(IC50) value of the two cell lines at 48 h was 50
μm/ml.
Quercetin stimulates the expression of
miR-145
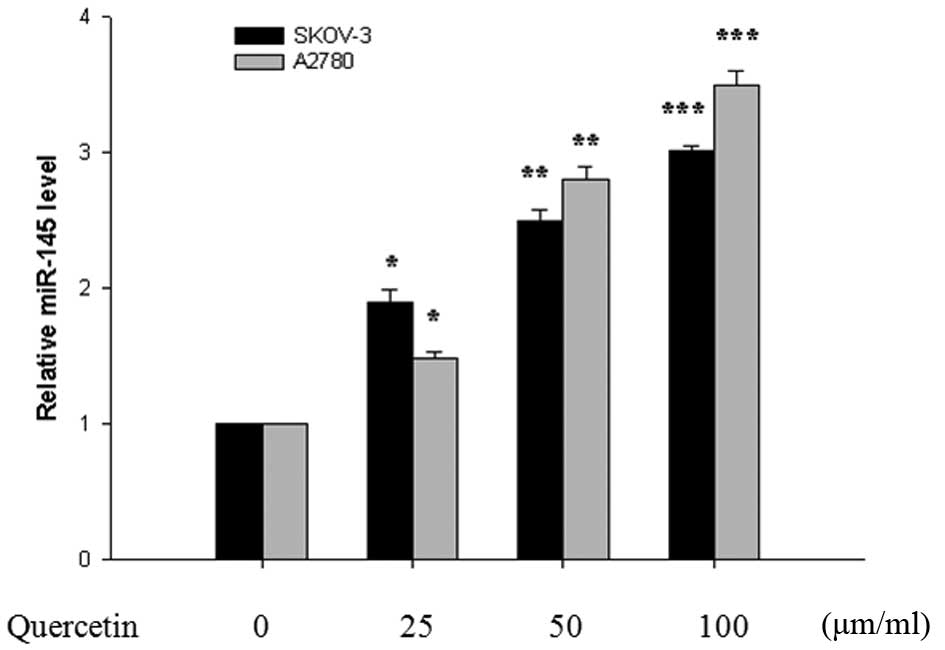
To determine whether quercetin induces the
expression of miR-145 in the SKOV-3 and A2780 cells, RT-qPCR was
performed. Following quercetin treatment at different
concentrations, the expression levels of miR-145 in the SKOV-3 and
A2780 cells increased in a dose-dependent manner compared with the
untreated control group, at 24 h (Fig.
2). Therefore, this change suggested that miR-145 may be
important in the inhibitory effect of quercetin on SKOV-3 and A2780
cell growth.
Anti-miR-145 reverses the effect of
quercetin
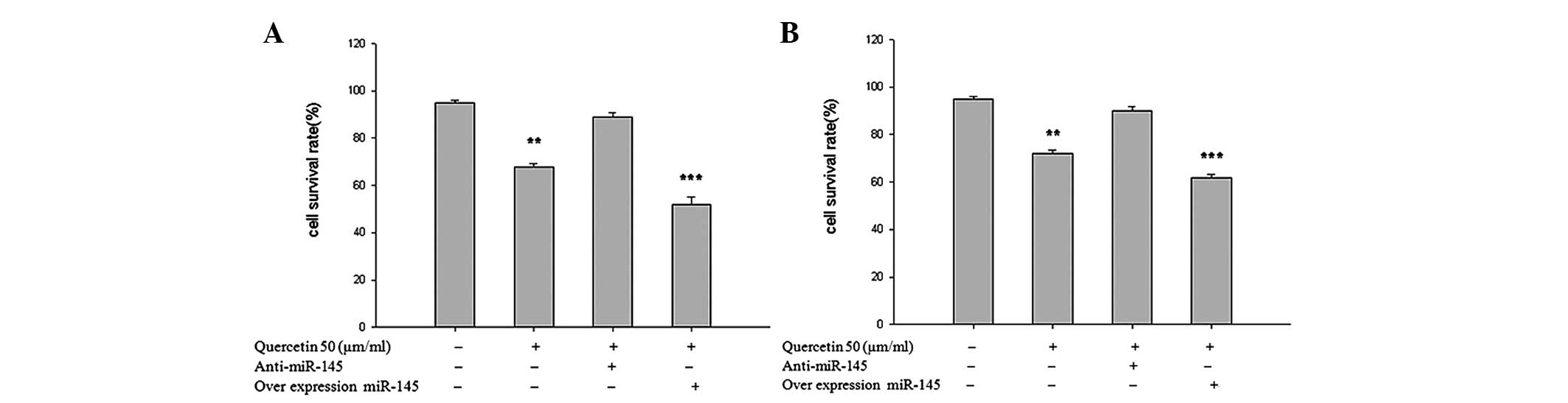
To confirm the role of miR-145 in the growth
inhibition of SKOV-3 and A2780 human ovarian cancer cell lines by
quercetin, miR-145 was either overexpressed or the cells were
transfected with anti-miR-145 to inhibit its expression. As shown
in Fig. 3A and B, the
overexpression of miR-145 reduced SKOV-3 and A2780 proliferation at
24 h compared with the untreated cells and the cells treated with
anti-miR-145, demonstrating that anti-miR-145 entered the SKOV-3
and A2780 cells and knocked down miR-145.
Quercetin stimulates the expression of
caspase-3 via upregulating the expression of miR-145
To confirm the mechanism underlying the upregulated
expression of miR-145 by quercetin on the growth of the SKOV-3 and
A2780 human ovarian cancer cell lines, the expression of cleaved
caspase-3, a cell apoptosis marker, was examined. This was assessed
in the SKOV-3 and A2780 human ovarian cancer cell lines, with or
without the overexpression of miR-145 or following transfection
with anti-miR-145 and with 50 μm/ml quercetin for 24 h. As
shown in Fig 4A and B, the
expression levels of cleaved caspase-3 in the SKOV-3 and A2780
cells were significantly increased when miR-145 was overexpressed
compared with those treated with quercitin alone (P<0.01).
However, the expression of cleaved caspase-3 in the anti-miR-145
group was markedly decreased compared with the overexpressed
miR-145 group (P<0.01). Therefore, these results suggested that
the extrinsic death receptor-mediated and intrinsic mitochondrial
pathways were involved in quercetin-induced apoptosis.
Discussion
The present study is the first, to the best of our
knowledge, to demonstrate that quercetin induced apoptosis in the
SKOV-3 and A2780 human ovarian carcinoma cell lines, via
upregulation of the expression of miR-145, which significantly
induced the activity of caspase-8 and -9, and the expression of
cleaved caspase-3. These results suggested that quercetin, a common
constituent in food, may be used as an antitumoral therapy in human
ovarian carcinoma by inducing apoptosis.
Quercetin is present in certain fruits and
vegetables and has been observed to possess antitumoral properties
in cancer, including lung cancer (6), leukemia (19) and breast cancer (20). Furthermore, quercetin inhibits the
growth of OVCAR-3 human ovarian cancer cells, associated with the
expression of VEGF (8). In the
present study, quercetin was administered at different doses
resulting in the inhibition of SKOV-3 and A2780 cell growth in a
dose- and time-dependent manner (Fig.
1A and B), and the IC50 value of the two cell lines
at 48 h was 50 μm/ml. These results suggested that quercetin
may inhibit the growth of SKOV-3 and A2780 cells.
miRNAs may function as regulatory molecules, which
act as tumor suppressors or oncogenes and are involved in the
development of human cancer (11,12).
Several studies have reported that the expression of miR-145 is
downregulated in human ovarian cancer (15,16).
By contrast, dietary quercetin supplementation may increase the
hepatic expression levels of miR-122 and miR-125b, which contribute
to the gene-regulatory activity of quercetin in vivo, and
suggest that quercetin regulates the expression of miRNA (18). The exact mechanisms underlying the
effect of miR-145 in ovarian cancer have not been previously
reported and, therefore required further examination. The present
study demonstrated that quercetin increased the expression levels
of miR-145 in the SKOV-3 and A2780 cells in a dose- and
time-dependent manner compared with the control group at 24 h
(Fig. 2), and these changes were
reversed by anti-miR-145 (Fig. 3A and
B). Furthermore, the expression of cleaved caspase-3, a cell
apoptosis marker, which induces cell death (21,22)
was examined. Following overexpression of miR-145 or transfection
with anti-miR-145 prior to treatment with 50 μm/ml quercetin
for 24 h, the expression levels of cleaved caspase-3 in the SKOV-3
and A2780 cells were significantly increased when treated with
overexpressed miR-145 compared with quercetin treatment alone
(P<0.01). However, the expression of cleaved caspase-3 in the
anti-miR-145 group was markedly decreased compared with the
overexpressed miR-145 group (P<0.01). Therefore, these results
suggested the involvement of the extrinsic death receptor-mediated
and intrinsic mitochondrial pathways in quercetin-induced
apoptosis. In conclusion, the present study indicated that miR-145
may be important in quercetin-induced apoptosis in ovarian cancer
cells. However, the role of quercetin in the modulation of miR-145
requires further clarification.
Acknowledgments
The present study was supported by grants from the
Scientific Research Fund of Zhejiang Provincial Education
Department (grant no. Y201224795).
References
|
1
|
Maier-Lenz H, Hauns B, Haering B, et al:
Phase I study of paclitaxel administered as a 1-hour infusion:
toxicity and pharmacokinetics. Semin Oncol. 24:16–19. 1997.
|
|
2
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramos C, Coin-de-Carvalho JE and
Corrêa-Mangiacavalli MA: Impact and (im)mobilization: a study of
cancer prevention campaigns. Cien Saude Colet. 12:1387–1396.
2007.In Portuguese. View Article : Google Scholar
|
|
5
|
Lin CW, Hou WC, Shen SC, et al: Quercetin
inhibition of tumor invasion via suppressing PKC
delta/ERK/AP-1-dependent matrix metalloproteinase-9 activation in
breast carcinoma cells. Carcinogenesis. 29:1807–1815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murakami A, Ashida H and Terao J:
Multitargeted cancer prevention by quercetin. Cancer Lett.
269:315–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun ZJ, Chen G, Hu X, et al: Activation of
PI3K/Akt/IKK-alpha/NF-kappaB signaling pathway is required for the
apoptosis-evasion in human salivary adenoid cystic carcinoma: its
inhibition by quercetin. Apoptosis. 15:850–863. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo H, Jiang BH, King SM and Chen YC:
Inhibition of cell growth and VEGF expression in ovarian cancer
cells by flavonoids. Nutr Cancer. 60:800–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li YJ, Zhang ZY, Mao YY, et al: A genetic
variant in miR-146a modifies digestive system cancer risk: a
meta-analysis. Asian Pac J Cancer Prev. 15:145–150. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji T, Zheng ZG, Wang FM, et al:
Differential microRNA expression by Solexa sequencing in the sera
of ovarian cancer patients. Asian Pac J Cancer Prev. 15:1739–1743.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiyomaru T, Enokida H, Tatarano, et al:
miR-145 and miR-133a function as tumour suppressors and directly
regulate FSCN1 expression in bladder cancer. Br J Cancer.
102:883–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arndt GM, Dossey L, Cullen LM, et al:
Characterization of global microRNA expression reveals oncogenic
potential of miR-145 in metastatic colorectal cancer. BMC Cancer.
9:3742009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iorio MV, Visone R, Di-Leva G, et al:
MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Q, Liu LZ, Qian X, et al: MiR-145
directly targets p70S6K1 in cancer cells to inhibit tumor growth
and angiogenesis. Nucleic Acids Res. 40:761–774. 2012. View Article : Google Scholar :
|
|
17
|
Wang S, Bian C, Yang Z, et al: miR-145
inhibits breast cancer cell growth through RTKN. Int J Oncol.
34:1461–1466. 2009.PubMed/NCBI
|
|
18
|
Boesch-Saadatmandi C, Wagner AE, Wolffram
S and Rimbach G: Effect of quercetin on inflammatory gene
expression in mice liver in vivo-role of redox factor 1, miRNA-122
and miRNA-125b. Pharmacol Res. 65:523–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spagnuolo C, Russo M, Bilotto S, Tedesco
I, Laratta B and Russo GL: Dietary polyphenols in cancer
prevention: the example of the flavonoid quercetin in leukemia. Ann
NY Acad Sci. 1259:95–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duo J, Ying GG, Wang GW and Zhang L:
Quercetin inhibits human breast cancer cell proliferation and
induces apoptosis via Bcl-2 and Bax regulation. Mol Med Rep.
5:1453–1456. 2012.PubMed/NCBI
|
|
21
|
Yang MY, Wang CJ, Chen NF, Ho WH, Lu FJ
and Tseng TH: Luteolin enhances paclitaxel-induced apoptosis in
human breast cancer MDA-MB-231 cells by blocking STAT3. Chem Biol
Interact. 213:60–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith MA and Schnellmann RG: Calpains,
mitochondria, and apoptosis. Cardiovasc Res. 96:32–37. 2012.
View Article : Google Scholar : PubMed/NCBI
|