Introduction
Enterovirus 71 (EV71) is a member of the
Picornaviridae family of the enterovirus genus and is one of
the main etiological agents responsible for hand-foot-mouth disease
(HFMD) in humans (1,2). EV71 is a non-enveloped virus with a
single-stranded RNA genome consisting of P1, P2 and P3 regions
(3). The P1 protein is further
cleaved into VP1, VP3 and VP0 by protease 3CD, while the other two
regions encode seven proteins responsible for replication and
virulence (3). VP1, VP3 and VP0
can spontaneously co-assemble into the icosahedral empty procapsid
(2). A portion of VP0 can be
autocleaved to yield VP2 and VP4, which are associated with
infectious EV71 virions (4). EV71
infections can cause more severe neurological complications than
other enteroviruses and can lead to high morbidity rates in
children (5,6). Since its initial identification in
1969, several HFMD epidemics have occurred worldwide, particularly
in Asia-Pacific regions (7,8). In
China, outbreaks of EV71 infection have been reported throughout
the country with increasing prevalence, particularly during the
last 10 years (9). Several
investigations have focused on the prevention of EV71 infections,
and numerous approaches have been tested to develop a safe and
effective EV71 vaccine (10,11).
Virus-like particles (VLPs) have attracted
increasing attention as great potential vaccine candidates, as they
are non-infectious particles consisting of all the major structural
proteins, mimicking the organization and conformations of the
native particle; however, they are devoid of viral nucleic acids
and are non-infectious (12).
VLP-based prophylactic vaccines have been successful against
hepatitis B virus and human papillomavirus and are now commercially
available. Recombinant EV71 VLPs have been shown to be
neutralization antibodies and confer a degree of protection from
EV71 infection in a neonatal mouse model (13–15).
Variable virus proliferation has been demonstrated
in the central nervous system and associated organs during EV71
infections (16,17). Therefore, the generation of
immunoprotective responses in infected animals and indicators of
pathological responses from the protection by the EV71 vaccine also
require an objective assessment. However, to what extent the VLP
vaccine protects susceptible organs against EV71 infection in
vivo remains elusive. Preliminary studies have indicated that
neutralizing anti bodies induced by VLPs may be able to efficiently
neutralize the homologous live EV71 virus and a panel of two C4
strains isolated in China (data not shown). In the present study,
the efficacy of an EV71 vaccine candidate based on VLPs was
evaluated; furthermore, the significance and value of assessing the
immunogenicity and immunoprotection of vaccine candidates in ICR
mice were further elucidated by using a range of methods, including
pathological, etiological and lethal challenge analyses.
Materials and methods
Viruses and VLP vaccine preparation
The human EV71 FY-15 strain (C4 genogroup, isolated
in Fu Yang, Anhui, China, 2008) was used for immunization. Another
highly mouse-adapted virulent EV71 strain (C4 genogroup) supplied
by the National Vaccine and Serum Institute (Beijing, China) was
used in the challenge experiments. The two EV71 viruses were
propagated in rhabdomyosarcoma (RD) cells using minimum essential
medium (MEM; Gibco-BRL, Invitrogen Life Technologies, Grand Island,
NY, USA) supplemented with 2% fetal bovine erum (FBS; Gibco-BRL).
For virus purification, the FY-15 virus was precipitated with 7%
polyethylene glycol 8000 (Amresco, LLC, Solon, OH, USA) and 2% NaCl
(Sinopharm Chemical Reagent Co., Ltd., Beijing, China) and then
centrifuged (110,000 ×g, 3 h) over 15% cesium chloride (CsCl;
Sinopharm Chemical Reagent Co., Ltd.). Virus pellets were
re-suspended in PBS (pH 7.4), sonicated for 30 sec and centrifuged
(10,0000 ×g, 20 h) over a continuous CsCl gradient (10–40%). The
resultant virus bands were dialyzed against phosphate-buffered
saline (PBS). Purified FY-15 virus was examined by eryo-electron
microscopy (Cryo-EM; Tecnai Tf20; FEI, Houston, TX, USA) and
inactivated with 1/4,000 formalin (Sigma-Aldrich, St Louis, MO,
USA) for 96 h at 37°C (18).
Another mouse-adapted EV71 virus was harvested and centrifuged
(5,000 ×g, 10 min) to remove debris. The viral titer was evaluated
using RD cells by microtitration assay and expressed as the 50%
tissue culture-infective dose (TCID50) (19).
EV71 VLPs were produced in serum-free medium as
previously described (20).
Briefly, Sf9 cells (Invitrogen Life Technologies) were infected
with a recombinant baculovirus (AcMNPV-P1-3CD; constructed
previously in the Department of Viral Hepatitis) for co-expression
of P1 and 3CD derived from the EV71 FY-15 strain at a multiplicity
of infection (MOI) of 1 in 1l CellSTACK-10 culture chambers
(Corning-Costar, Corning, NY, USA) at 27°C for 96 h. The infected
cells were harvested with a Tris-NaCl (Invitrogen Life
Technologies) buffer containing 1% NP-40 (Sigma-Aldrich) and cell
debris was removed by centrifugation (9,000 ×g, 20 min). Then the
supernatant was centrifuged over 15% CsCl and a discontinuous
sucrose gradient (10–60%). Purified VLPs were examined by
transmission electron microscopy (TEM; Tecnai 12; FEI). The protein
concentrations were quantified using a Nanodrop (Thermo Fisher
Scientific, Waltham, MA, USA). A solution of the inactivated virus
and VLPs each was then mixed with adjuvant Al(OH)3 (2
mg/ml; Sinovac Biotech Co., Ltd, Beijing, China) at a volumetric
ratio of 1:1 for in vivo studies.
Immunization of adult mice and sampling
procedures
Three groups (n=10/group) of 4-5 week-old female ICR
mice (Beijing HFK Bioscience Co., Ltd., Beijing, China) were
immunized by intramuscular injection with 5 μg (100
μl/mouse) of either purified VLPs, inactivated whole virus
or PBS. The mice were subjected to a 12 h dark/12 h light cycle,
with ad libitum access to food and water. The mice were
housed 5 mice/cage in a room maintained at 19–24°C with 47–63%
relative humidity. Animal treatment and care were provided in
accordance with the guidelines of the National Institute for Viral
Disease Control and Prevention, China Center for Disease Control
and Prevention (Beijing, China). All procedures used in the present
study were approved by the Animal Care and Welfare Committee at the
National Institute for Viral Disease Control and Prevention, China
Center for Disease Control and Prevention. The immunization
schedule consisted of three inoculations administered at two-week
intervals. Blood was sampled from the tail at weeks 0, 2, 4, 6, 8,
10 and 14 for monitoring the immune responses. Mice (3 or 4 per
group) were sacrificed at week 6 and 10 for measurement of the
cellular immune responses. Lung wash, vaginal wash and small
intestine samples were collected at week 6 in order to test
secretory immunoglobulin (Ig)A levels. All these samples were
immediately placed on ice and stored with 100 mM phenylmethyl
sulfonyl fluoride (Sigma-Aldrich) at −20°C until further analysis.
The remaining mice in each group were used to breed neonatal mice
for lethal virus challenge experiments at week eight (week four
post-boost) and sacrificed at week 14.
Measurement of EV71-specific
antibody
Anti-EV71 IgG and IgG subclasses in serum as well as
IgA in mucosal secretions were measured by ELISA. In brief, plates
coated with inactivated native virus (5 μg/ml) were blocked
(PBS containing 0.05% Tween 20 (Sinopharm Chemical Reagent Co.,
Ltd.) and 5% bovine serum albumin (Amresco, LLC) and washed prior
to the addition of the two-fold serially diluted serum samples
(1:22–222) and 1:2-diluted mucosal secretion
samples were added. Plates were incubated for 1 h and washed prior
to addition of 0.15 μg/ml horseradish-peroxidase
(HRP)-conjugated goat anti-mouse IgG (1:30,000 dilution; cat. no.
A9917; Sigma-Aldrich) or 0.125 μg/ml goat anti-mouse IgA
(HRP) secondary antibody (1:8,000 dilution; cat. no. ab97235;
Abcam, Cambridge, MA, USA) was added. The anti-EV71 IgG isotypes in
mouse sera were determined using a mouse antibody isotyping kit
(ISO 2; Sigma-Aldrich) according to the manufacturer’s
instructions. After 30 minutes, color development was initiated by
adding 100 μl tetramethylbenzidine substrate (Sigma-Aldrich)
and terminated by adding 50 μl of
H2SO4 (2M; Sinopharm Chemical Reagent Co.,
Ltd.). The optical density at 40 nm (OD450) was read
using an ELISA reader (FI-01621; Thermo Scientific Multiskan™ GO;
Thermo Fisher Scientific Oy, Vantaa, Finland).
Neutralizing antibody assays
Microneutralization assays were performed against
EV71 in RD cells. Briefly, 50 μl two-fold diluted serum was
mixed with 50 μl 100 TCID50 EV71 in 96-well
plates. RD cells were added after 1 h and incubated for 3–4 days at
37°C. The neutralizing titer was determined as the highest dilution
that gave no cellular cytopathic effects (CPE).
Enzyme-linked immunospot (ELISpot)
assays. interleukin
(IL)-4-, interferon (IFN)-γ- and IgA-secreting
splenocytes were measured using ELISpot for mouse IL-4, IFN-γ
(U-Cytech, Utrecht, the Netherlands) and IgA kits (Mabtech AB,
Nacka Strand, Sweden). Briefly, for detection of virus-specific
IL-4 and IFN-γ, 96-well microplates (Millipore, Billerica, MA, USA)
were pre-coated with anti-IL-4 or anti-IFN-γ monoclonal antibody
(mAb) and incubated overnight at 4°C. Plates were blocked with
RPMI-1640/10% FBS for 1 h at room temperature, followed by addition
of splenocytes (3×105/well). Subsequently, 250
μg/ml inactivated EV71 virus was added (20 μl/well)
to stimulate effector cells. Phytohaemagglutinin (PHA; 9
μg/well, 37°C, 16 h; Gibco-BRL) stimulation was used for the
positive control. For IgA ELISpot, microplates were first coated
overnight with 10 μg/ml anti-IgA antibody or 30 μg/ml
inactivated EV71 virions and blocked with PBS-10% FBS for 30 min.
Then splenocytes (1×105/well) were added. After
incubation of the splenocytes for 16–18 h, following removal of the
cells, plates were processed according to the manufacturer’s
instructions. Spot-forming cells (SFC) were counted using an
automated ELISpot reader (Cellular Technology Ltd, Shaker Heights,
OH, USA).
Lethal viral challenges in neonatal
mice
Four weeks after the final immunization, female
adult mice were allowed to mate. The neonatal mice were challenged
intracerebrally with the EV71 virus attack strain [106
PFUs (plaque-forming units)/ml, 10 μl/mouse] within 72 h
following birth. Mice were monitored for mortality daily over 14
days. In addition, three neonatal mice from each group were used
for histopathological analysis at five days after EV71 challenge.
The neonatal mice were sacrificed using isoflurane (Sigma-Aldrich).
Extracted organs were fixed in 4% paraformaldehyde for 24 h and
processed for paraffin embedding. Tissues sections (5 μm)
were deparaffinized using xylene (Sinopharm Chemical Reagent Co.,
Ltd.) and hydrated with a graded series of alcohol (Sinopharm
Chemical Reagent Co., Ltd.). Sections were stained with hematoxylin
and eosin (Sigma-Aldrich), for morphological evaluation.
Immunohistochemical analyses were performed as described previously
(21). Briefly, hydrated sections
were treated with 0.25% trypsin (Invitrogen Life Technologies)
solution containing 0.5% CaCl2 (Sinopharm Chemical
Reagent Co., Ltd.) in PBS for 30 min and incubated with 3%
H2O2 (Sinopharm Chemical Reagent Co., Ltd,)
in methanol to block endogenous peroxidase activity, followed by
incubation with PBS containing 5% BSA. The treated sections were
then incubated with anti-EV71 mAb (1:300 dilution; cat, no.
05-0001; AbMax Biotechnology Co., Ltd., Beijing, China) at 4°C
overnight. The sections were washed three times with PBS and then
incubated with HRP-conjugated goat anti-mouse IgG (1:1,000
dilution; cat. no. ZB-2305; ZSGB-BIO, Beijing, China) for 1 h at
37°C. The sections were visualized using 3-3′diaminobenzi-dine (10
min) and hematoxylin (30 sec), then examined using a light
microscope (IX51; Olympus Corporation, Tokyo, Japan).
Statistical analysis
All data obtained were processed and analyzed using
SPSS software (version 16.0; SPSS, Inc., Chicago, IL, USA). Figures
were plotted and analyzed using GraphPad Prism software (GraphPad,
La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Characterization of purified VLPs
Purified VLPs and EV71 virus were visualized by TEM
and Cryo-EM. Purified VLPs (Fig.
1A) exhibited icosahedral morphology similar to those of EV71
virions (Fig. 1B) and the size was
~25–27 nm. These EV71 VLPs were used as a vaccine in the subsequent
experiments.
Humoral responses elicited by EV71 VLP
immunization
Neutralization titration of sera of the mice showed
that compared with levels in the control group, high levels of
anti-EV71 IgG antibodies in the serum of mice were present at 2
weeks post-boost immunization with purified VLPs, reaching a
maximum of 212 (Fig.
2A). Compared with the VLPs, inactivated EV71 induced slightly
higher levels of anti-EV71 antibodies with a significant difference
at week six (P<0.05). In the two groups, the time-dependent
changes in antibody levels and magnitudes of the response were
similar. Peak levels of antibodies following the two types of
antigen immunization were detected at week 6 and maintained for at
least 14 weeks. The inactivated EV71-immunized group exhibited
higher levels of all IgG subtypes (Table I); however, high levels of IgG1 and
IgG2b and low levels of IgG2a and IgG3 were also detected in the
serum of the VLP-immunized group, indicating a mixed Th1/Th2
response. The capacity of antibodies generated by VLP immunization
to neutralize the homologous live EV71 virus was investigated
(Fig. 2B). In VLP-immunized mice,
significantly increased neutralization titers were observed
post-boost compared with those pre-vaccination (week 4-8)
(P<0.05); however, these were lower than those in the
formalin-inactivated EV71-immunized mice (P<0.05). While the
neutralization titer profile for the group immunized with VLPs was
similar to that for the group immunized with inactivated EV71,
neutralization titers in the two groups were greatest at week 8 and
persisted for at least 14 weeks.
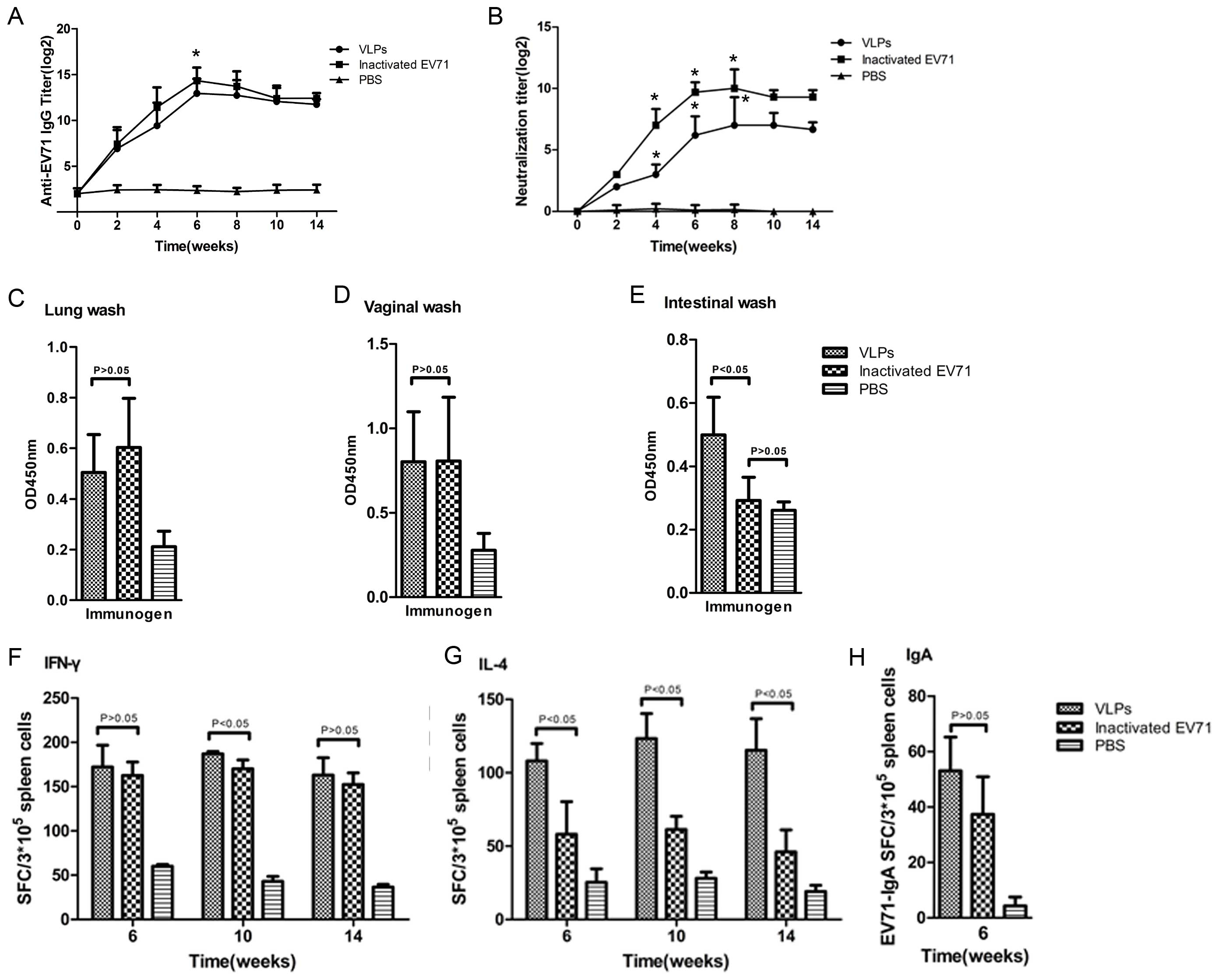 | Figure 2Titer profiles of humoral and cellular
immune responses to EV71 in mouse models. The geometric mean titers
(log2) of (A) total IgG, (B) neutralizing antibody to EV71 and
(C-E) anti-EV71 secretory IgA secreted at mucosal surfaces were
determined using ELISA (for total anti-EV71 IgG titration, the
positive cut-off OD value was defined as 2.1 times that of normal
mouse serum) and neutralization techniques. Enzyme-linked
immunospot detection of specific responses of (F) IFN-γ-secreting T
cells, (G) IL-4-secreting T cells or (H) IgA-secreting spleen cells
to EV71 in spleen lymphocytes from immunized mice. All values are
expressed as the mean ± standard deviation from 3 or 4 mice per
group. EV, enterovirus; VLP, virus-like particle; SFC, spot-forming
cells, PBS, phosphate-buffered saline; OD450, optical
density at 450 nm; IFN, interferon; Ig, immunoglobulin. |
 | Table IIgG subtype profile in mouse
seraa. |
Table I
IgG subtype profile in mouse
seraa.
| Type of
antigen | OD450 of
ELISA for EV71 specific IgG at serum dilution of 1:100
|
|---|
| IgG1 | IgG2a | IgG2b | IgG3 |
|---|
| VLPs (n=10) | 1.644 (0.111) | 0.370 (0.059) | 0.884 (0.042) | 0.248 (0.085) |
| Inactivated EV71
(n=10) | 1.770 (0.154) | 0.958 (0.082) | 1.620 (0.106) | 0.759 (0.054) |
| PBS (n=10) | 0.111 (0.011) | 0.123 (0.014) | 0.114 (0.004) | 0.138 (0.022) |
The specific IgA levels in the lung, vaginal and
intestinal secretions from the vaccinated mice after the third
immunization with EV71 VLPs showed a significant difference from
those in the PBS group (P<0.05; Fig. 2C–E). However, the specific IgA
responses in the intestinal samples from inactivated EV71-immunized
mice were not significantly different from those in the PBS group,
which may be due to incomplete homogenization processing, complex
composition and proteases in the intestinal tract.
Cellular immune responses elicited by VLP
immunization
Compared with splenocytes from mice immunized with
inactivated EV71, splenocytes from VLP-immunized mice produced the
same levels of EV71-specific IFN-γ-producing T-cells (P>0.05,
Fig. 2F) and higher levels of
IL-4-producing T-cells in response to EV71 antigens (P<0.05;
Fig. 2G), confirming the
successful elicitation of Th1 and Th2 cellular immune responses.
The EV71-specific IgA-secreting splenocytes were also quantified at
week 6. Increased numbers of IgA-memory cells in mouse splenocytes
in the VLP group and the inactivated EV71 group were found
following immunization compared with those in the PBS control group
(Fig. 2H; P>0.05). These
results suggested that vaccination with EV71 VLPs leads to the
generation of IgA-memory splenocytes with the capacity to recognize
EV71 virions.
Challenge with EV71 results in mortality
of neonatal mice, which can be prevented by vaccination of their
mothers
To assess the efficacy of passive protection against
the homologous EV71, neonatal ICR mice were challenged
intracerebrally with EV71. As the result, nearly all inoculated
mice from the PBS control group displayed a range of clinical
symptoms (weight loss, hunched back, reduced motility, ruffled coat
and hind limb paralysis) over the course of infection followed by
death from day three (Fig. 3A and
C). By contrast, all of the neonatal mice delivered by
VLP-immunized and inactivated EV71 immunized female adult mice were
able to survive, grew normally and were of good health, and the
immunoprotective efficacy of the vaccines reached nearly 100%
(Fig. 3B). These results
demonstrated that VLP immunization conferred passive protection
that was passed on from the female parental mice to the neonatal
mice.
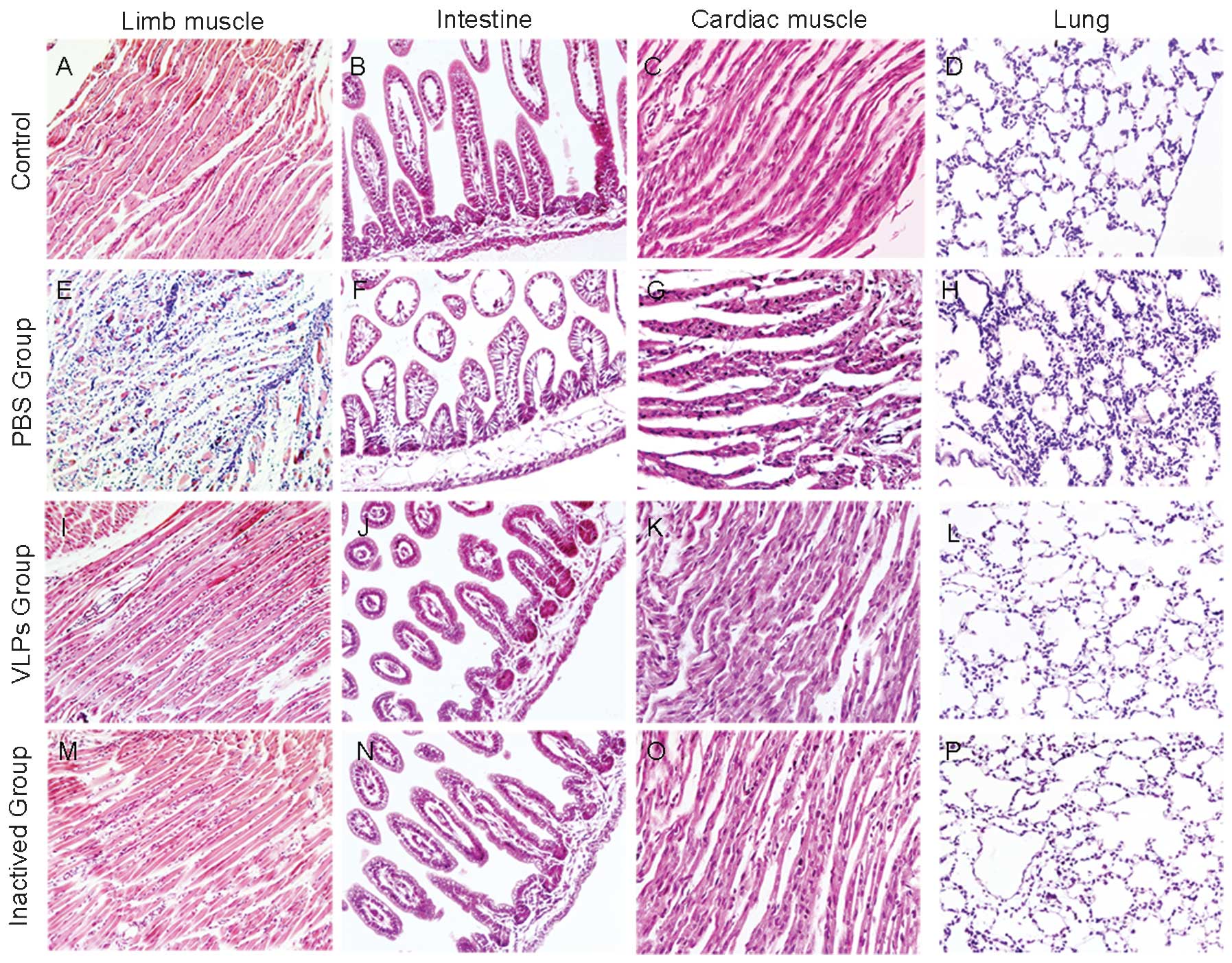
Histopathological analysis shows that
vaccination protects major organs of neonatal mice challenged with
EV71
The pathological changes in the brain and other
major organs of immunized and non-immunized control neonatal mice
were examined in parallel (Fig.
4). Compared with normal control mice, certain pathological
changes were present in the PBS group (Fig. 4A–H). The hind limb muscle was most
severely affected by severe diffuse necrotizing myositis and minor
lymphocytic infiltration, which is likely to be responsible for the
observed hind limb paralysis (Fig.
4E). The intestines revealed dilation of the villi and mild
villous blunting with associated crypt hyperplasia, suggesting
substantial tissue damage in the small intestines (Fig. 4F). A pathological response
comprising minor myocardial interstitial edema and occasional
scattered mononuclear cells invading cardiac muscles were also
observed (Fig. 4G). Prominent
pathological manifestations of pulmonary inflammation in lung
tissue included a widened alveolar wall and extensive inflammatory
cell aggregation (Fig. 4H). By
contrast, under the protection of the VLP vaccines, only mild
muscle atrophy was observed in the limb musculature (Fig. 4I and M). No other pathological
changes were noted.(Fig. 4J–L and
N–P). In addition, the limb musculature of the neonatal mice
immunized with VLPs or inactivated EV71 displayed slight myofiber
regeneration within nuclear rowing, suggesting signs of recovery
from the viral infection.
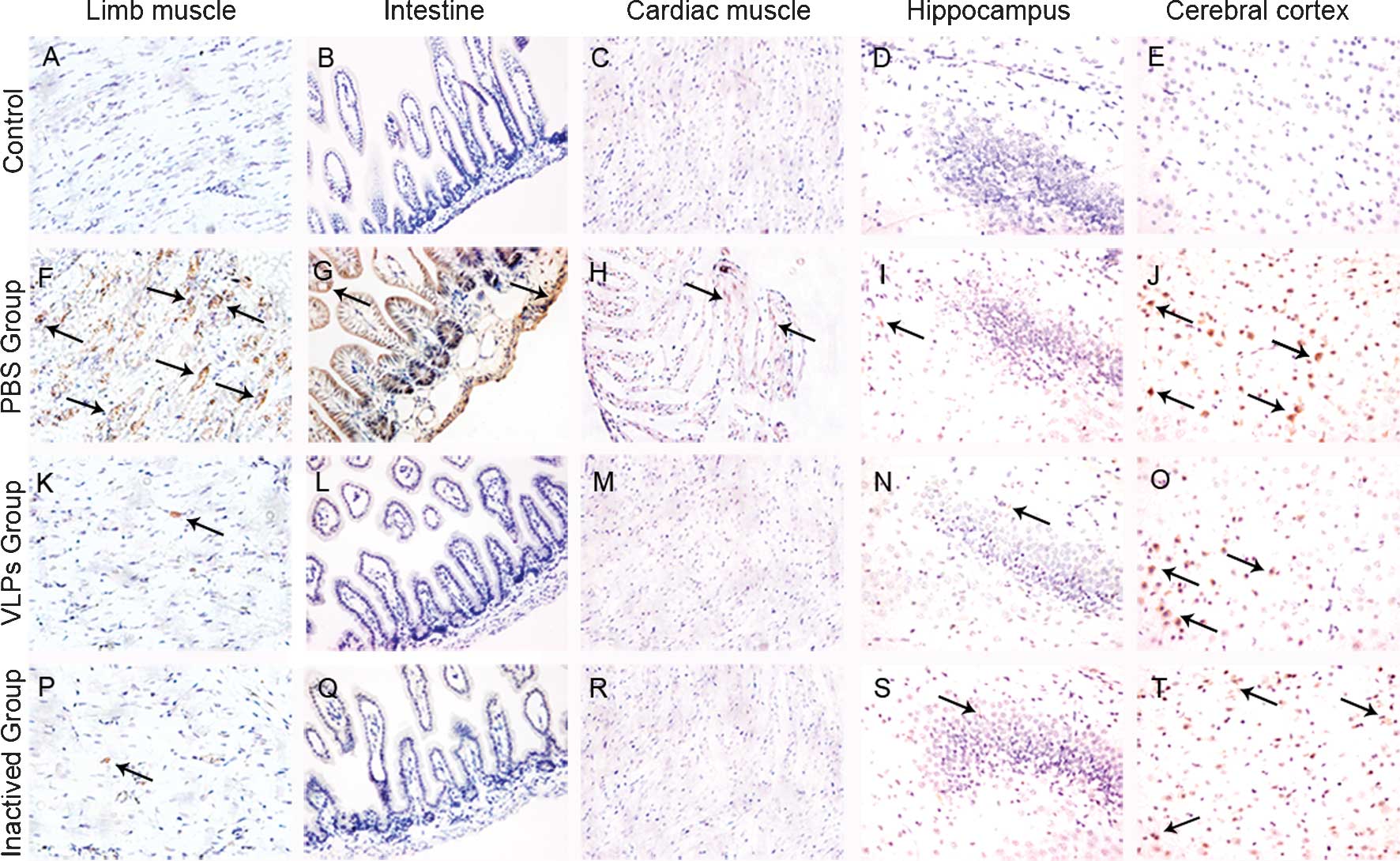
Further immunohistochemical analyses were in
accordance with the results of the pathological analysis (Fig. 5). EV71 infiltration was mainly
observed in the hind limb muscles and to a certain extent in the
small intestines and cardiac muscles in the PBS group (Fig. 5F–H). Low amounts of EV71 were
detected in the hippocampus, while a high level of EV71
infiltration was detected in the cerebral cortex in all groups,
which may be due to the intracerebral injection of the EV71 virus
attack strain in the lethal EV71 challenge experiment (Fig. 5I and J). Following vaccine-mediated
immunization, only very low levels of EV71 antigen were detected in
the hind limb muscles (Fig. 5K–M and
P–R). All these observations demonstrated the marked
immunoprotective efficacy of EV71 VLP immunization against EV71
infection.
Discussion
Preliminary studies have indicated that neutralizing
antibodies induced by VLPs were able to efficiently neutralize the
homologous live EV71 virus and a panel of two C4 strains isolated
in China (data not shown). Although VLPs induced similar levels of
anti-EV71 IgG compared with inactivated EV71, the neutralization
titers in the former case were lower. VP1 is a major antigenic
epitope and has an important role in neutralizing the EV71 virus
(22,23). According to studies by in Lin et
al (11) and Wang et al
(24), residues 210–220 of VP1,
which form part of an important neutralizing epitope of EV71, lie
on the capsid surface, alongside the VP2 EF loop (residues
136–150), to form a single epitope. Disruption in the order of
residues 211–217 upon particle expansion may account for the
reduced immunogenicity (11,24).
The VP1 in the VLPs used in the present study was hypothesized to
be similar with the VP1 in the empty EV71 virus particles
(sedimentation, 80S; 60 copies of VP0, VP1 and VP3) cultivated and
separated by Wang et al (24). Slight conformational changes of VP1
in empty particles may affect their neutralizing capacity.
Although antibody responses are critical for
protection against EV71 infection, certain studies suggested that
cellular immune responses correlate with the clinical severity of
HFMD and have an important role in human immunity (13,15).
Chang et al (13) suggested
that lower EV71-specific cellular responses may be associated with
immunopathogenesis of EV71-associated pulmonary edema. The
different Th1 and Th2 immune response profiles corresponded to the
activation of two distinct major sub-sets of T cells characterized
by their pattern of cytokine production (25). The present study indicated that VLP
vaccine immunization generated good IFN-γ responses and even better
IL-4 responses to the EV71 antigen in mice. The ELISpot assay
further demonstrated the presence of EV71-specific IgG- and
IgA-secreting cells in spleens of the VLP-vaccinated mice, but not
of the PBS-immunized mice. The IgG subtype profile showed that the
EV71 VLP vaccine induced high levels of IgG1 and IgG2b, thus
confirming the generation of a mixed Th1 and Th2 response. These
results were consistent with those obtained following influenza
virus and human papilloma virus VLP-immunization (26,27).
The production of IgA-memory spleen cells detected following VLP
vaccine immunization further confirmed successful priming by
VLPs.
Protection against viral infectivity in animal
models is regarded as the optimal test of vaccine efficacy
(23). EV71 was presumed to infect
the central nervous system (CNS) via peripheral nerves in muscle
tissue followed by active retrograde axonal transport to the CNS
(28). It was also reported that
muscle cells were the main site of proliferation of the EV71 virus
strain found around Fuyang in China (29,30).
Additional studies demonstrated that skeletal muscle was the main
tissue supporting EV71 replication in mice. In addition to CNS
injury, necrotic myositis may also be responsible for the paralysis
and death observed in EV71-infected mice (31). Thus, protection against further
infection of the CNS or skeletal muscle by preventing EV71 from
attacking muscle tissue and neurons is particularly important.
Based on the abovementioned studies, the present study performed
further pathological and etiological analyses on different organs
to understand the specific passive immunoprotective effects in
vivo. It was found that the VLP vaccine was able to inhibit the
transport of EV71 from the CNS to muscle tissue and prevent
infection of muscle tissue along with recovery from the viral
infection. The prevention of pathological injury by effective
resistance to EV71 infection in intestine and cardiac muscle
tissues mediated by VLP vaccine immunization is also of critical
importance. This passive protection offered great immunoprotective
efficacy from the VLP or inactivated EV71 vaccines, reaching nearly
100%. Of note, the results indicated that VLP vaccines not only
prevented the virus from causing serious damage to the body, but
also promoted a quick recovery of the body from minor injuries
resulting from EV71 infection.
It is likely that these results not only confirmed
the immu-noprotective effect of the experimental VLP vaccine
against EV71, but also provided valuable additional insight to aid
in further exploring the detailed mechanism of action of the
vaccine, and supported the protective role for the VLP vaccine in
resistance and resolution following EV71 infection.
Acknowledgments
The authors would like to thank Dr Wuling Xie and Dr
Baoyun Zhang (National Institute for Viral Disease Control and
Prevention, Beijing, China) for technical assistance in tissue
paraffin embedding, paraffin sectioning and immunohistochemical
analysis. The authors also thank Dr Le Sun (AbMax Biotechnology
Co., Ltd, Beijing, China) for critical reading of the manuscript.
The present study was supported by a grant from the National Key
Technology Research and Development Program of the Ministry of
Science and Technology of China (no. 2008BAI69B02).
References
|
1
|
Chan KP, Goh KT, Chong CY, Teo ES, Lau G
and Ling AE: Epidemic hand, foot and mouth disease caused by human
enterovirus 71, Singapore. Emerg Infect Dis. 9:78–85. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McMinn PC: An overview of the evolution of
enterovirus 71 and its clinical and public health significance.
FEMS Microbiol Rev. 26:91–107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown BA and Pallansch MA: Complete
nucleotide sequence of enterovirus 71 is distinct from poliovirus.
Virus Res. 39:195–205. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu CC, Chou AH, Lien SP, Lin HY, Liu SJ,
Chang JY, Guo MS, Chow YH, Yang WS, Chang KH, et al: Identification
and characterization of a cross-neutralization epitope of
enterovirus 71. Vaccine. 29:4362–4372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qiu J: Enterovirus 71 infection: A new
threat to global public health? Lancet Neurol. 7:868–869. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang SM, Lei HY, Huang KJ, Wu JM, Wang JR,
Yu CK, Su IJ and Liu CC: Pathogenesis of enterovirus 71 brainstem
encephalitis in pediatric patients: Roles of cytokines and cellular
immune activation in patients with pulmonary edema. J Infect Dis.
188:564–570. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cardosa MJ, Perera D, Brown BA, Cheon D,
Chan HM, Chan KP, Cho H and McMinn P: Molecular epidemiology of
human enterovirus 71 strains and recent outbreaks in the
Asia-Pacific region: Comparative analysis of the VP1 and VP4 genes.
Emerg Infect Dis. 9:461–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujimoto T, Chikahira M, Yoshida S, Ebira
H, Hasegawa A, Totsuka A and Nishio O: Outbreak of central nervous
system disease associated with hand, foot, and mouth disease in
Japan during the summer of 2000: Detection and molecular
epidemiology of enterovirus 71. Microbiol Immunol. 46:621–627.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yi L, Lu J, Kung HF and He ML: The
virology and developments toward control of human enterovirus 71.
Crit Rev Microbiol. 37:313–327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chong P, Hsieh SY, Liu CC, Chou AH, Chang
JY, Wu SC, Liu SJ, Chow YH, Su IJ and Klein M: Production of EV71
vaccine candidates. Hum Vaccin Immunother. 8:1775–1783. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin YL, Yu CI, Hu YC, Tsai TJ, Kuo YC, Chi
WK, Lin AN and Chiang BL: Enterovirus type 71 neutralizing
antibodies in the serum of macaque monkeys immunized with EV71
virus-like particles. Vaccine. 30:1305–1312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Noad R and Roy P: Virus-like particles as
immunogens. Trends Microbiol. 11:438–444. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang LY, Hsiung CA, Lu CY, Lin TY, Huang
FY, Lai YH, Chiang YP, Chiang BL, Lee CY and Huang LM: Status of
cellular rather than humoral immunity is correlated with clinical
outcome of enterovirus 71. Pediatr Res. 60:466–471. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ku Z, Ye X, Huang X, Cai Y, Liu Q, Li Y,
Su Z and Huang Z: Neutralizing antibodies induced by recombinant
virus-like particles of enterovirus 71 genotype C4 inhibit
infection at pre- and post-attachment steps. PLoS One.
8:e576012013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang LY, Chang IS, Chen WJ, Huang YC,
Chen GW, Shih SR, Juang JL, Shih HM, Hsiung CA, Lin TY, et al:
HLA-A33 is associated with susceptibility to enterovirus 71
infection. Pediatrics. 122:1271–1276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khong WX, Foo DG, Trasti SL, Tan EL and
Alonso S: Sustained high levels of interleukin-6 contribute to the
pathogenesis of enterovirus 71 in a neonate mouse model. J Virol.
85:3067–3076. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Cui W, Liu L, Wang J, Zhao H,
Liao Y, Na R, Dong C, Wang L, Xie Z, et al: Pathogenesis study of
enterovirus 71 infection in rhesus monkeys. Lab Invest.
91:1337–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ong KC and Devi S:
Formaldehyde-inactivated whole-virus vaccine protects a murine
model of enterovirus 71 encephalomyelitis against disease. J Virol.
84:661–665. 2010. View Article : Google Scholar :
|
|
19
|
Hsiung GD: Virus assay, neutralization
test and antiviral assay. Hsiung’s Diagnostic Virology. 4th ed.
Hsiung GD, Fong CKY and Landry ML: Yale University Press; New
Haven: pp. 46–55. 1994
|
|
20
|
Cao L, Yi Y, Song JD, Tian MM, Tian RG,
Meng QL, Qiu F, Jia ZY and Bi SL: The assemblage, purification and
characterization of EV71 VLPs expressed in baculovirus. Bing Du Xue
Bao. 28:201–206. 2012.In Chinese. PubMed/NCBI
|
|
21
|
Jia CS, Liu JN, Li WB, Ma CM, Lin SZ, et
al: The cross-reactivity of the enterovirus 71 to human brain
tissue and identification of the cross-reactivity related
fragments. Virol J. 7:472010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng T, Kolpe AB, Kiener TK, Chow VT and
Kwang J: Display of VP1 on the surface of baculovirus and its
immunogenicity against heterologous human enterovirus 71 strains in
mice. PLoS One. 6:e217572011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Foo DG, Alonso S, Chow VT and Poh CL:
Passive protection against lethal enterovirus 71 infection in
newborn mice by neutralizing antibodies elicited by a synthetic
peptide. Microbes Infect. 9:1299–1306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Peng W, Ren J, Hu Z, Xu J, Lou Z,
Li X, Yin W, Shen X, Porta C, et al: A sensor-adaptor mechanism for
enterovirus uncoating from structures of EV71. Nat Struct Mol Biol.
19:424–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wen ZS, Xu YL, Zou XT and Xu ZR: Chitosan
nanoparticles act as an adjuvant to promote both Th1 and Th2 immune
responses induced by ovalbumin in mice. Mar Drugs. 9:1038–1055.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Emeny RT, Wheeler CM, Jansen KU, Hunt WC,
Fu TM, Smith JF, MacMullen S, Esser MT and Paliard X: Priming of
human papillomavirus type 11-specific humoral and cellular immune
responses in college-aged women with a virus-like particle vaccine.
J Virol. 76:7832–7842. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Quan FS, Huang C, Compans RW and Kang SM:
Virus-like particle vaccine induces protective immunity against
homologous and heterologous strains of influenza virus. J Virol.
81:3514–3524. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu L, Zhao H, Zhang Y, Wang J, Che Y,
Dong C, Zhang X, Na R, Shi H, Jiang L, et al: Neonatal rhesus
monkey is a potential animal model for studying pathogenesis of
EV71 infection. Virology. 412:91–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Duo J, Liu J, Ma C, Zhang L, Wei Q
and Qin C: A mouse muscle-adapted enterovirus 71 strain with
increased virulence in mice. Microbes Infect. 13:862–870. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duo J, Wang W, Tong W, Cong Z, Jiang H, Xu
W, et al: Experimental Studies on ICR Mice with EV71 Infection.
Chin J Comp Med. 19:41–46. 2009.
|
|
31
|
Xiu JH, Zhu H, Xu YF, Liu JN, Xia XZ and
Zhang LF: Necrotizing myositis causes restrictive hypoventilation
in a mouse model for human enterovirus 71 infection. Virol J.
10:2152013. View Article : Google Scholar : PubMed/NCBI
|