Introduction
Chronic obstructive pulmonary disease (COPD) is an
inflammatory airway disease characterized by exacerbations, which
are primarily caused by bacterial or viral infections. Respiratory
viral infections occurring during COPD exacerbations are more
likely to lead to hospitalization as a result of viral infections
leading to a greater airway inflammation and therefore, more severe
exacerbations. Viruses are associated with more severe
exacerbations and with greater morbidity, therefore, require
further research (1).
Human adenovirus, a DNA virus, is associated with
respiratory infections and smoking-induced lung function impairment
(2,3). RSV, which is an enveloped RNA virus,
is one of the most important and frequent viruses for respiratory
tract infections in infants and young children (4). The results of a recent study have
highlighted two seasons that are associated with an increased
incidence of RSV virus infection, which peak during January and
March in Turkey (5). Recently, RSV
has been identified as a pathogen present in adults experiencing
COPD-AEs (4,6,7).
According to previous studies, adenovirus and RSV may result in
chronic infection in the lungs (2,6–10).
However, chronic adenovirus and RSV infection data are currently
unclear and, to the best of our knowledge, few longitudinal studies
have been conducted on the association between chronic infection
and COPD. Furthermore, RSV chronic infection in COPD and its
consequences for local and systemic infection, and functional
status have yet to be fully elucidated.
The aim of the present study was to investigate the
prevalence of adenovirus and RSV A and B among patients with
COPD-AE and those in a stable condition. The present study
evaluated the association between viral infections, functional
status and systemic inflammation in patients experiencing COPD-AEs
and those in a stable condition.
Materials and methods
COPD and criteria
Diagnosis and spirometric assessment of the severity
of COPD was performed according to the global initiative for
chronic obstructive lung disease (GOLD) criteria (11). Patients with a forced expiratory
volume in one second/forced vital capacity (FEV1/FVC)
ratio of <70% and a predicted FEV1 value of <80%
were included in the analyses (stage II–IV according to the GOLD
criteria).
COPD-AE was defined as a change in the patient’s
baseline symptoms beyond day-to-day variability, which was
sufficient to warrant a change in medication. A modified
Anthonisen’s criteria was used in order to describe the infectious
exacerbations, as described in a previous study (12,13).
Patients in a stable condition were defined as those who had been
free of AEs for >4 weeks.
Patients
Forty patients with COPD-AE who had been admitted to
Gazi University Hospital (Ankara, Turkey) were initially included
in the study. Patients provided written informed consent in order
to participate in the study. Gazi University School Of Medicine
Ethical Committee (Ankara, Turkey) approved the methods used in the
present study.
Patients with a history of asthma, FEV1
reversibility (a rise in FEV1 of >15% from the
baseline value, following an inhalation of 400 mcg salbutamol, in
addition to an absolute increase in FEV1 of >200 ml),
bronchiectasis, lung carcinoma, tuberculosis, pneumonia and those
who had received chronic steroid therapy were not included in the
study. Patients with respiratory failure who required mechanical
ventilation were not included in the study.
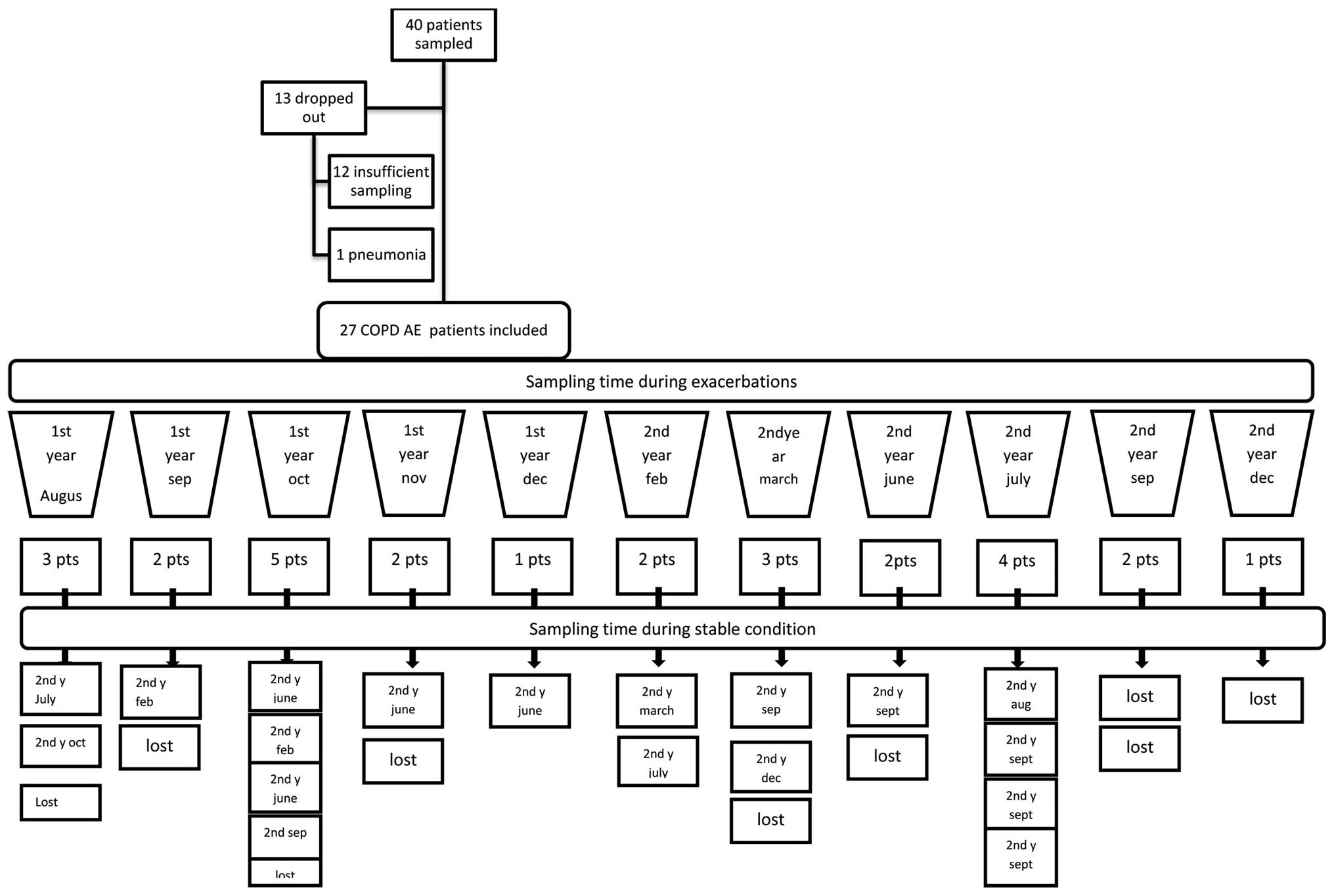
Forty patients with COPD-AE were assessed for 17
months beginning in August and lasting until December in the
following year. Thirteen patiens were not included in the study (12
patients had insuffient sampling, 1 patient had pneumonia).
Eighteen of the twenty seven patients were assessed during COPD-AE
and whilst in a stable condition, and nine patients were not
evaluated during stable condition. The study design and patient
sampling time are provided in Fig.
1.
Following COPD-AE, eighteen patients were also
subsequently evaluated while they were in a stable condition.
Induced sputum, sera and nasal smears were sampled from patients on
the first day of hospitalization for COPD-AE and while they were in
a stable condition. Full blood count, C reactive protein,
fibrinogen, anti streptolysin O, serum biochemistry, arterial blood
gases, FEV1, FVC and FEV1/FVC were recorded
in patients with COPD-AE and while they were in a stable condition.
Patient symptoms, smoking, treatment history and frequency of AEs
in the previous year were recorded.
Pulmonary function tests (PFTs)
PFTs, including basic spirometry and reversibility,
were performed according to the American Thoracic Society criteria,
using Sensor Medics Vmax 20 Spirometer (Sensor Medics Corp., Yorba
Linda, CA, USA) (14).
Oxygen saturation and PFTs were conducted prior to
the sputum induction. The sputum induction procedure was modified
according to the criteria of Pin et al (15). Nebulization using 3% saline was
initiated following inhalation of 400 mcg salbutamol using an
ultrasonic nebulizer (Hikoneb 908 DC Ultrasonic Nebulizer, Kare
Medical and Analytical Devices Ltd. Co., Ankara, Turkey).
Sputum, sera and nasal smears
Nasal smear samples were collected from the subjects
by gently rubbing the nasal turbinates for 5 sec, with a moistened
cotton-tip swab and placing in 1 ml phosphate-buffer saline (PBS).
A baseline serum sample was collected from all subjects.
Sputum samples were macroscopically free of salivary
contamination and were incubated in 1 M dithiothreitol
(Sigma-Aldrich, St. Louis, MO, USA), containing fluid for 35 min at
45°C. Following centrifugation at 3000 rpm the supernatants were
discarded and the sample was washed with PBS three times. The
supernatants were aspirated and pellets were stored at −86°C prior
to DNA and RNA extraction.
Nasal smears obtained from patients with sterile
cotton swabs were placed in 1 ml PBS, vortexed before diluting to
500 μl in eppendorf tubes (Hamburg, Germany) and stored at
−86°C.
DNA extractions
Samples (200 μl) were incubated in lysis
solution (20 mM Tris-HCl, 10 mM EDTA and 0.1% SDS), containing 0.1
mg/ml proteinase K, at 60°C for 1 h. DNA extractions were performed
using phenol-chloroform-isoamyl alcohol followed by precipitation
using pure ethanol, containing 3 M sodium acetate, and incubation
at −20°C overnight. Samples were then mixed with 70% ethyl-alcohol
and dried. Sterile deionized water (50 ml) was added and the
mixture was stored at 55°C for 10 min in order to elute the DNA
(16). Samples were then
immediately subjected to polymerase chain reaction (PCR) and
amplification.
PCR
Invitek RTP DNA/RNA Virus Mini kit (Invitek, Berlin,
Germany) was used to perform RSV RNA isolation, according to the
manufacturer’s instructions. RSV subgroup detection was performed
using subgroup-specific probes following purification and
denaturation. An adenoplex® multiplex PCR kit (Gen-Probe
Prodesse Inc., Waukesha, WI, USA) and a Light Cycler quantitative
PCR (qPCR; Roche Diagnostics GmbH, Mannheim, Germany) device were
used for amplification and subgroup detection of Adenovirus
DNA.
Roboscreen RSV Serotype A and B kits (Leipzig,
Germany) were used in order to amplify RSV RNA Serotype A and B
using an ABI PRISM 7000 (Applied Biosystems, Fostercity, CA, USA).
All PCR devices were used according to the manufacturer’s
instructions with negative and positive controls.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS version 11.5 (SPSS, Inc., Chicago, IL, USA) was used to
perform statistical analysis. Chi-squared and Fisher’s exact
chi-squared tests were performed in order to compare the subgroups.
Wilcoxon Signed Rank and Mann Whitney U tests were performed in
order to conduct group comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
COPD evaluation
Twenty seven patients with moderate to very severe
COPD were evaluated in the present study (Fig. 1). Demographic characteristics and
results of the PFTs of the patients are summarized in Tables I and II. Patients had had a diagnosis of COPD
for a mean of 6.33±5.75 years. The mean duration between visits was
181.66±113.87 (range, 30–420) days. Patients sought medical
attention 6.96±9.81 times in the year prior to admission. The
number of emergency room visits was 2.37±3.22. The number of
patients who received inhaled steroid therapy was 19 (73.1%).
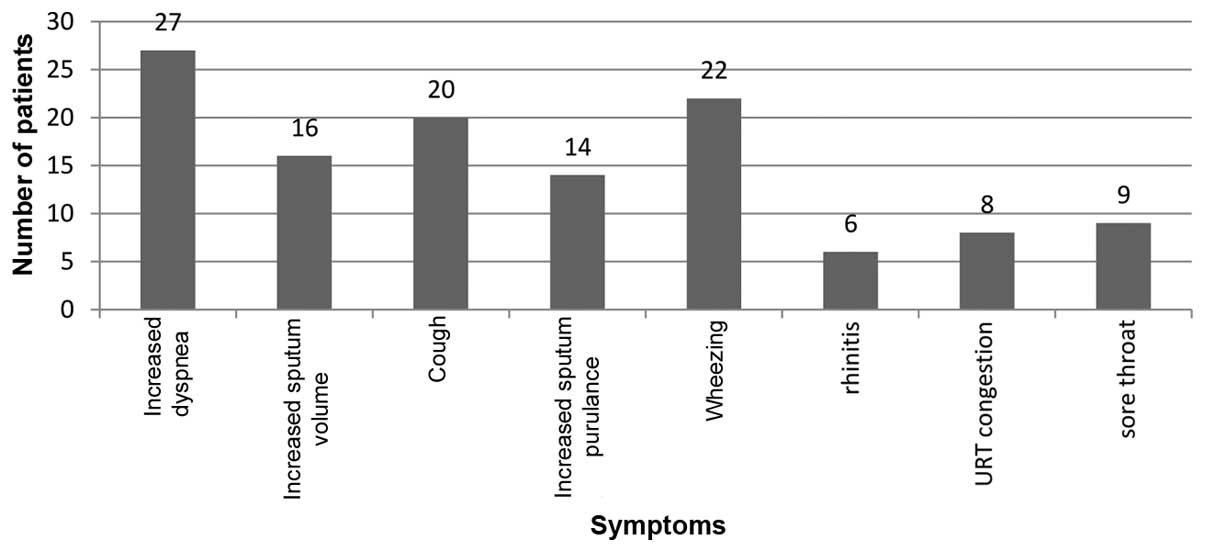
Symptoms associated with AEs observed in patients with COPD-AE are
presented in Fig. 2.
 | Table IDemographic properties of patients
with COPD. |
Table I
Demographic properties of patients
with COPD.
| Variable | Value |
|---|
| Age (range) | 66.93±9.47
(49–82) |
| Gender
(female:male) | 1:26 |
| Smoking status n
(%) | |
| Non-smoker | 1 (3.7) |
| Ex-smoker | 16 (59.3) |
| Current smoker | 10 (37) |
 | Table IIPFT results. |
Table II
PFT results.
| PFT (%) | COPD-AE (n=27) | Stable COPD
(n=18) | P-value |
|---|
| FEV1 | 38.2±17.5 | 43.8±16.9 | 0.015 |
| FVC | 57.6±20.8 | 64.3±21.5 | 0.029 |
|
FEV1/FVC | 52.0±13.13 | 53.83±11.56 | 0.050 |
Five patients had received influenza vaccinations,
16 patients exhibited dyspnea, 8 exhibited cough and 2 exhibited
sore throat in stabile condition. No significant difference in
symptoms was observed between vaccinated and non-vaccinated
patients.
Table III
summarizes the laboratory parameters observed in patients during
COPD-AE and in those a stable condition. The mean percentage of
blood neutrophils observed in patients with COPD-AE was higher than
in patients in a stable condition.
 | Table IIILaboratory parameters in patients with
COPD-AE and those in a stable condition. |
Table III
Laboratory parameters in patients with
COPD-AE and those in a stable condition.
| Parameter | COPD-AE, n=27 | Stable COPD,
n=18 |
|---|
| Hb (g/dl) | 14.66±1.94 | 15.44±1.62 |
| Leukocytes
(/mm3) |
9374.81±3106.55 |
8486.32±2128.15 |
| Blood neutrophils
(%)a | 72.33±10.72 | 64.61±10.76 |
| Blood eosinophils
(%) | 2.50±5.12 | 2.42±1.63 |
| ASO (IU/ml) | 160.65±113.37 | 116.89±85.82 |
| CRP (mg/l) | 17.35±23.11 | 10.21±15.78 |
| Fibrinogen
(mg/dl) | 378.20±133.20 | 422.11±99.64 |
Four patients produced adenovirus-positive sputum
samples during a COPD-AE, and one patient produced a positive
sample while in a stable condition (Table IV). Seventeen/twenty seven (63%)
patients with COPD-AE and ten/eighteen (55.6%) patients in a stable
condition were positive for RSV serotype A. There was no detection
of RSV serotype B in patients with COPD-AE and or a stable
condition.
 | Table IVViral detection in various type of
samples in patients with COPD AE and in stable condition. |
Table IV
Viral detection in various type of
samples in patients with COPD AE and in stable condition.
| COPD type | Blood, n (%) | Nasal aspirate, n
(%) | Sputum, n (%) | Blood + Nasal
Aspirates, n (%) | Blood + Sputum, n
(%) | Nasal Aspirates +
Sputum, n (%) | Blood + Nasal
Aspirates + Sputum, n (%) |
|---|
| COPD-AE, n=27 | | | | | | | |
| Adenovirus | 0 | 0 | 3 (11.1) | 0 | 0 | 0 | 0 |
| RSV serotype
Aa | 3 (11.1) | 9 (33.3) | 12 (44.4) | 3 (11.1) | 1 (3.7) | 4 (14.8) | 1 (3.7) |
| Stable COPD,
n=18 | | | | | | | |
| Adenovirus | 0 | 0 | 1 (5.6) | 0 | 0 | 0 | 0 |
| RSV serotype
Aa | 4 (22.2) | 6 (33.3) | 8 (44.4) | 2 (11.1) | 4 (22.2) | 4 (22.2) | 2 (11.1) |
All specimens were cultivated on blood agar, the
eosine methylene blue (EMB) agar (Merck, Darmstadt, Germany) and
chocolate agar. Inoculated agar plates were incubated for 24 hrs at
37°C. Reproductive examples in cultures were acquried for advanced
histopathological typing and anti-biogram. Routine sputum culture
analyses suggested that Acinetobacter baumannii (1 patient),
Hemophilus influenzae (1 patient), Pseudomonas
aeruginosa (1 patient), Escherichia coli (1 patient) and
Klebsiella pneumoniae (1 patient) were present in the sputum
from samples of separate patients. In patients with A.
baumannii and H. influenza, RSV serotype A infections
were observed during AE periods. E. coli was observed in the
sputum samples from patients in a stable condition who were
RSV-positive. No RSV-negative samples contained E.coli.
Table IV
summarizes virus isolation sites in patients with COPD-AE and in a
stable condition. Adenovirus was only detected in sputum samples
from patients with COPD-AE. By contrast, RSV serotype A was
detected in blood, sputum and nasal aspirate samples. One
adenovirus-positive sputum sample was observed among the samples
analyzed from patients in a stable condition. As in patients with
COPD-AE, RSV serotype A was detected in fewer blood samples than in
sputum and nasal aspirate samples, from patients in a stable
condition. Monthly variation in RSV-positive samples observed in
patients with COPD-AE and in patients in a stable condition is
demonstrated in Fig. 3.
RSV-positive samples were predominantly observed from September to
November in patients with COPD-AE and in those in a stable
condition. Statistical analysis was no performed as a result of an
inadequate number of events.
The mean duration of COPD, and number of AEs
requiring emergency room and doctor visits were not significantly
different between RSV-positive and RSV-negative patients in the
COPD-AE group and in those in a stable condition. The clinical,
laboratory and functional parameters in RSV serotype A-positive and
negative patients with COPD-AE and stable condition are shown in
Table V. Among the acute phase
reactants, fibrinogen levels were significantly higher in RSV
serotype A-positive patients with COPD-AE compared with those who
were RSV serotype A negative.
 | Table VClinical, laboratory and functional
parameters in RSV serotype A positive and negative patients with
COPD AE and stable condition. |
Table V
Clinical, laboratory and functional
parameters in RSV serotype A positive and negative patients with
COPD AE and stable condition.
| Parameter | COPD AE, n=27
| Stable COPD, n=18
|
|---|
| RSV A (+) patients,
n=17 | RSV A (−) patients,
n=10 | P-value | RSV A (+) patients,
n=10 | RSV A (−) patients,
n=8 | P-value |
|---|
| Age (range) | 65.41±10.22 | 69.50±7.83 | 0.286 | 66.40±11.32 | 66.25±8.90 | 0.965 |
| Smoking pack-year
(p/y) | 42.29±31.66 | 68.00±31.99 | 0.059 | 38.60±29.60 | 61.00±34.36 | 0.173 |
| Hb (g/dl) | 15.27±2.02 | 13.63±1.33 | 0.040 | 15.34±1.78 | 15.68±1.57 | 0.965 |
| Leukocytes
(/mm3) |
9680.00±3642.78 |
8856.00±1952.93 | 0.749 |
8936.00±2657.58 |
7988.75±1398.39 | 0.460 |
| Blood neutrophils
(%) | 69.29±11.05 | 77.50±8.26 | 0.059 | 66.00±10.32 | 63.33±12.44 | 0.237 |
| ASO (IU/ml) | 190.44±123.63 | 113.00±78.27 | 0.097 | 74.60±59.94 | 173.00±92.32 | 0.055 |
| CRP (mg/l) | 22.69±26.27 | 8.80±14.33 | 0.286 | 9.40±10.67 | 4.75±8.73 | 0.274 |
| ESR (mm/h) | 24.92±22.35 | 12.77±11.72 | 0.186 | 21.00±22.40 | 14.57±10.73 | 0.694 |
| Fibrinogen
(mg/dl) | 438.60±1126.08 | 287.60±85.92 | 0.003 | 389.50±96.30 | 445.13±92.78 | 0.360 |
| FEV1 (%) | 37.59±13.17 | 39.30±24.11 | 0.570 | 400.78±19.27 | 49.50±13.62 | 0.114 |
| FVC (%) | 56.59±19.52 | 59.50±24.01 | 0.863 | 61.67±24.51 | 68.00±20.37 | 0.481 |
| FEV1/FVC (%) | 52.82±12.19 | 50.60±15.18 | 0.334 | 52.22±11.13 | 57.88±10.75 | 0.321 |
Discussion
Fewer patients with COPD were adenovirus-positive
than RSV serotype A-positive. No significant differences in
laboratory and functional parameters were observed between
RSV-positive and RSV-negative patients with COPD-AE, with the
exception of fibrinogen.
Previous investigations, which have predominantly
used insensitive serological testing, demonstrated that 20% of
cases of COPD-AE were due to viral pathogens (17,18).
However, the frequency of virus-induced COPD-AE cases was found to
be higher than previously assumed, as a result of more sensitive
molecular detection methods such, as PCR (19–21).
In previous studies, adenovirus was detected in ~1% of COPD-AE
cases (19–21). The percentage of patients with
COPD-AE who are infected with RSV varies from 2.3–28% (5–7,20,22–24).
The variation in the results of different studies is likely due to
the following factors: Method sensitivity; study populations
(hospital- or community-based); materials (nasal swabs, sputum or
lung biopsies); study design (cross-sectional or longitudinal); and
the etiology differs according to the geographic areas.
In the present study, adenovirus and RSV infections
were analyzed in a longitudinal study design. To the best of our
knowledge, the present study was the first to analyze a Turkish
population, using a sensitive PCR method for viral
pathogen-associated COPD. Using three different materials (blood,
nasal swab and induced sputum), adenovirus was detected in a low
number of samples in patients with COPD-AE as well as in those in a
stable condition. This is in accordance with the results of a
previous study (21). Regarding
RSV infection, RSV was detected in 63% of patients in the COPD-AE
group. This figure is higher than those observed in previous
studies (6,7). The results of the present study
demonstrated that RSV serotype A was present in patients with
COPD-AE and in those in a stable condition. The mechanisms
underlying these results require further investigation.
Contamination may explain the high levels of RSV
virus detected in the present study. However, freshly prepared
materials were used to prevent technical drawbacks.
The association between latent viral infections and
COPD pathogenesis has received considerable attention since 1990
(25). A previous study suggested
that group C adenovirus E1A protein is expressed in lung tissues
and is associated with COPD severity (9). In guinea pigs, cigarette
smoke-induced emphysema is increased in response to latent
adenoviral infection (8). By
contrast, McManus et al (19) detected the adenovirus hexon gene
and 5 E1A DNA and mRNA in a low number of sputum samples from
patients with COPD-AE and in stable conditions. In accordance with
those of previous studies, the results of the present study
suggested low adenovirus detection in patients with COPD-AE and
those in a stable condition.
In a cross-sectional study using PCR, RSV was
detected in 16/68 (23.5%) patients in a stable condition following
COPD-AE, which may indicate chronic infection with this virus
(7). In a recent study, nasal and
sputum samples were collected every two months from patients with
respiratory illness, which were defined as the presence of any of
the following symptoms: nasal congestion, sore throat, hoarseness,
novel or increased-from-baseline cough, sputum production, dyspnea
and wheezing and in a stable condition (23). In contrast to the results of
previous studies, the authors did not detect RSV in nasal samples
from patients in a stable condition (21,22,26).
Furthermore, of 315 sputum stable samples analyzed, only three were
RSV-positive. RSV detection was found to be associated with
increased airway inflammation and faster FEV1 decline,
which may indicate an association between chronic RSV infection and
the pathogenesis of chronic airflow limitation (26). In the present study, higher RSV
detection rates were observed in samples from patients in a stable
condition (55.6%) following COPD-AE, compared with that observed in
patients experiencing COPD-AEs. However, no associations were
observed between RSV-positive detection and functional parameters
in patients with COPD-AE and in those in a stable condition. The
small sample size used in the present study may explain these
negative results.
In a previous study, virus-associated AEs were found
to be associated with higher plasma fibrinogen and serum
interleukin 6 levels, and fibrinogen levels were shown to increase
during COPD-AE (27). In the
present study higher fibrinogen levels were observed in samples
from patients with COPD-AE who were RSV-positive.
Higher RSV detection rates were observed in samples
from patients with COPD-AE and those in a stable condition,
compared with rates reported in the literature (5–7,19–24).
This may be explained by the different methodologies and specimen
usage. The kit used in the present study revealed a high
sensitivity for RSV detection compared with those used in other
studies. Another explanation for the discrepancies may be
attributed to differences in the sampling sites. It is unclear
which site reflects true infection or persistent asymptomatic
infection. Previous studies have predominantly analyzed RSV
infection of the upper airways. The viral load of RSV RNA in nasal
samples was lower compared with the sputum in almost all instances
(23). In the present study nasal
swab, blood and induced sputum samples were obtained, which may
explain the high detection rates compared with previous studies.
The majority of patients with COPD-AE who were RSV-positive were
also RSV-positive while in a stable condition. This may reflect
chronic asymptomatic infection or recurrent asymptomatic
reinfection.
The present study used a small sample size.
Furthermore, there were no regular patient follow up examinations.
Therefore, although the majority RSV-positive samples were detected
during October and March, the seasonality of infection was not
systematically evaluated. RSV detection in patients in a stable
condition appeared to be randomly distributed throughout the year.
However, sequential sampling was not conducted, therefore, it
cannot be concluded that a persistent infection existed. Recurrent
asymptomatic infection may give the impression of persistent
infection if it was eliminated by the host immunity before it led
to an acute exacerbation (28).
Coexisting bacterial infection was not systematically evaluated in
the present study. Instead, results were obtained from routine
bacterial culture.
In conclusion, the present study suggests that RSV
serotype A is more prevalent than adenovirus in samples from
patients with COPD-AE. In RSV A-positive patients, blood fibrinogen
levels were higher, suggesting systemic inflammation. RSV serotype
A may cause an asymptomatic chronic infection or recurrent
asymptomatic reinfections in patients in a stable condition
following COPD-AE. Further investigations with larger study
populations are required to clarify the results of the present
study.
Acknowledgments
The present study was funded by the Gazi University
Scientific Projects Foundation (Ankara, Turkey).
References
|
1
|
Wedzicha JA: Role of viruses in
exacerbations of chronic obstructive pulmonary disease. Proc Am
Thorac Soc. 1:115–120. 2004. View Article : Google Scholar
|
|
2
|
Hayashi S and Hogg JC: Adenovirus
infections and lung disease. Curr Opin Pharmacol. 7:237–243. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gold DR, Tager IB, Weiss ST, Tosteson TD
and Speizer FE: Acute lower respiratory illness in childhood as a
predictor of lung function and chronic respiratory symptoms. Am Rev
Respir Dis. 140:877–884. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borg I, Rohde G, Löseke S, Bittscheidt J,
Schultze-Werninghaus G, Stephan V and Bufe A: Evaluation of a
quantitative real-time PCR for the detection of respiratory
syncytial virus in pulmonary diseases. Eur Respir J. 21:944–951.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Turkish Neonatal Society: The seasonal
variations of respiratory syncytial virus infections in Turkey: A
2-year epidemiological study. Turk J Pediatr. 54:216–222.
2012.PubMed/NCBI
|
|
6
|
Beckham JD, Cadena A, Lin J, Piedra PA,
Glezen WP, Greenberg SB and Atmar RL: Respiratory viral infections
in patients with chronic, obstructive pulmonary disease. J Infect.
50:322–330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seemungal T, Harper-Owen R, Bhowmik A,
Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries
DJ, Johnston SL and Wedzicha JA: Respiratory viruses, symptoms, and
inflammatory markers in acute exacerbations and stable chronic
obstructive pulmonary disease. Am J Respir Crit Care Med.
164:1618–1623. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hogg JC: Role of latent viral infections
in chronic obstructive pulmonary disease and asthma. Am J Respir
Crit Care Med. 164:S71–S75. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hogg JC: Latent adenoviral infection in
the pathogenesis of emphysema: The Parker B. Francis Lectureship.
Chest. 117(Suppl 1): S282–S285. 2000. View Article : Google Scholar
|
|
10
|
Schwarze J, O’Donnell DR, Rohwedder A and
Openshaw PJ: Latency and persistence of respiratory syncytial virus
despite T cell immunity. Am J Respir Crit Care Med. 169:801–805.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
NHLBI/WHO Workshop Report Global
Initiative for chronic obstructive lung disease: Global Strategy
for the Diagnosis, Management, and Prevention Of Chronic
Obstructive Pulmonary Disease. US Department of Health and Human
Services. Public Health Service, National Institutes of Health,
Lung and Blood Institute; (NIH Publication No. 2701). April.
2001
|
|
12
|
Anthonisen NR, Manfreda J, Warren CP,
Hershfield ES, Harding GK and Nelson NA: Antibiotic therapy in
exacer-bations of chronic obstructive pulmonary disease. Ann Intern
Med. 106:196–204. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhowmik A, Seemungal TA, Sapsford RJ and
Wedzicha JA: Relation of sputum inflammatory markers to symptoms
and lung function changes in COPD exacerbations. Thorax.
55:114–120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
[No authors listed]. Lung function
testing: Selection of reference values and interpretative
strategies. American Thoracic Society. Am Rev Respir Dis.
144:1202–1218. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pin I, Gibson PG, Kolendowicz R,
Girgis-Gabardo A, Denburg JA, Hargreave FE and Dolovich J: Use of
induced sputum cell counts to investigate airway inflammation in
asthma. Thorax. 47:25–29. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maniatis T and Fritsch EF: Moleculer
Cloning: A Laboratory Manual. 3rd edition. Cold Spring Harbor; New
York, NY: 1990
|
|
17
|
Smith CB, Golden CA, Kanner RE and
Renzetti AD Jr: Association of viral and Mycoplasma pneumoniae
infections with acute respiratory illness in patients with chronic
obstructive pulmonary diseases. Am Rev Respir Dis. 121:225–232.
1980.PubMed/NCBI
|
|
18
|
Walsh EE, Falsey AR and Hennessey PA:
Respiratory syncytial and other virus infections in persons with
chronic cardiopulmonary disease. Am J Respir Crit Care Med.
160:791–795. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McManus TE, Marley AM, Baxter N, Christie
SN, Elborn JS, Heaney LG, Coyle PV and Kidney JC: Acute and latent
adenovirus in COPD. Respir Med. 101:2084–2090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ko FW, Ip M, Chan PK, Chan MC, To KW, Ng
SS, Chau SS, Tang JW and Hui DS: Viral etiology of acute
exacerbations of COPD in Hong Kong. Chest. 132:900–908. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hutchinson AF, Ghimire AK, Thompson MA,
Black JF, Brand CA, Lowe AJ, Smallwood DM, Vlahos R, Bozinovski S,
Brown GV, et al: A community-based, time-matched, case-control
study of respiratory viruses and exacerbations of COPD. Respir Med.
101:2472–2481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rohde G, Wiethege A, Borg I, Kauth M,
Bauer TT, Gillissen A, Bufe A and Schultze-Werninghaus G:
Respiratory viruses in exacerbations of chronic obstructive
pulmonary disease requiring hospitalisation: A case-control study.
Thorax. 58:37–42. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Falsey AR, Formica MA, Hennessey PA,
Criddle MM, Sullender WM and Walsh EE: Detection of respiratory
syncytial virus in adults with chronic obstructive pulmonary
disease. Am J Respir Crit Care Med. 173:639–643. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Camargo CA Jr, Ginde AA, Clark S,
Cartwright CP, Falsey AR and Niewoehner DE: Viral pathogens in
acute exacerbations of chronic obstructive pulmonary disease.
Intern Emerg Med. 3:355–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsuse T, Hayashi S, Kuwano K, Keunecke
H, Jefferies WA and Hogg JC: Latent adenoviral infection in the
pathogenesis of chronic airways obstruction. Am Rev Respir Dis.
146:177–184. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilkinson TM, Donaldson GC, Johnston SL,
Openshaw PJ and Wedzicha JA: Respiratory syncytial virus, airway
inflammation, and FEV1 decline in patients with chronic obstructive
pulmonary disease. Am J Respir Crit Care Med. 173:871–876. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wedzicha JA, Seemungal TA, MacCallum PK,
Paul EA, Donaldson GC, Bhowmik A, Jeffries DJ and Meade TW: Acute
exacerbations of chronic obstructive pulmonary disease are
accompanied by elevations of plasma fibrinogen and serum IL-6
levels. Thromb Haemost. 84:210–215. 2000.PubMed/NCBI
|
|
28
|
Sikkel MB, Quint JK, Mallia P, Wedzicha JA
and Johnston SL: Respiratory syncytial virus persistence in chronic
obstructive pulmonary disease. Pediatr Infect Dis J. 27(Suppl 10):
S63–S70. 2008. View Article : Google Scholar : PubMed/NCBI
|