Introduction
Gastric cancer, although decreasing in incidence
within the past few years, continues to be one of the serious
threats to human health (1).
Worldwide, gastric cancer is an aggressive tumor, leading to the
fourth most common type of human malignancy and the second cause of
death (2,3). Although surgical resection and
adjuvant chemotherapy have progressed, and certain types of gastric
cancer can be cured at an early stage (4), the majority of patients present at an
advanced stage at diagnosis, and effective treatment methods are
unavailable (5). Therefore,
identifying an effective biomarker for early diagnosis and
improving treatment strategies are required.
The novel candidate oncogene, tumor necrosis factor
α-induced protein 8 (TNFAIP8), has received increasing attention.
TNFAIP8, also termed NDED, GG2-1, SCCS2, SCC-S2 and MDC-3.13, is
located in 5q23.1 and is involved in the malignancies of numerous
types of tumor. Previously, the role of TNFAIP8 in the formation of
a series of tumors has been determined (6–11).
These results demonstrated that TNFAIP8 is involved in tumor
progression and in the regulation of cell proliferation, invasion,
migration, apoptosis and drug resistance among different types of
tumor.
However, the expression of TNFAIP8 and its clinical
significance in gastric cancer remain to be fully elucidated. In
the present study, the expression of TNFAIP8 in gastric cancer was
detected using immunofluorescence to confirm its cytoplasmic
localization. Immunohistochemical, western blotting and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analyses were used to evaluate the expression of TNFAIP8 in gastric
cancer tissues and cell lines, compared with adjacent normal
tissues and GES-1 cells, and its relationship with
clinicopathological characteristics and its prognostic roles were
evaluated in 86 patients on long-term follow-up. Subsequent
investigation involving knockdown of the TNFAIP8 gene was
performedd to detect its potential regulatory mechanism and
association with cell proliferation, invasion and migration.
Patients and methods
Patients and samples
Fresh samples of gastric cancer and adjacent normal
tissues (four specimens) were collected for detection of the
protein expression levels of TNFAIP8 via western blotting. A
further 86 samples (46 males and 40 females; median age, 52; age
range, 23–77) of gastric cancer tissues and adjacent normal tissues
were obtained, between January 2007 and July 2008, from the
Department of Pathology of Shandong Provincial Hospital (Shandong,
China), which had undergone radical surgical therapy, according to
the National Comprehensive Cancer Network Practice Guidelines
(12). None of the samples had
received preoperative treatment in the form of chemotherapy or
radiotherapy. The tissues were collected from patients and healthy
controls at the Provincial Hospital Affiliated to Shandong
University, following the obtaining of informed consent from the
patient’s family. The study was approved by the ethics committee of
Shandong Provincial Hospital affiliated to Shandong University
(Jinan, China). All patients had a confirmed diagnosis of gastric
carcinoma by pathological examination following resection.
Immunohistochemical (IHC) staining of
tissues
For IHC staining, the tumor specimens were embedded
in paraffin (Chemact (Liaoning) Petrochemicals Ltd., Liaoning,
China) and 4-µm thick sections were produced by the
Department of Pathology of Shandong Provincial Hospital. Briefly,
the sections were dewaxed according to the
streptavidin-biotin-peroxidase complex (Zhong Shan Golden Bridge
Biological Technology, Inc., Beijing, China) manufacturer’s
instructions. Hydrogen peroxide (0.3%) and blocking serum (goat
serum; Zhong Shan Golden Bridge Biological Technology, Inc.) were
used (one blocking step; 30 min; 37°C) to inhibit endogenous
non-specific substances. Following each blocking step, the tissues
were washed with phosphate-buffered saline (PBS; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) for 5 min three times.
The tissues were then incubated at 4°C overnight with TNFAIP8
rabbit anti-human polyclonal antibody (1:100; cat. no. 64988;
Abcam, Cambridge, MA, USA). Following washing with PBS, the tissues
were incubated with secondary goat anti-rabbit monoclonal antibody
(1:1,000; cat. no. M080825; Zhong Shan Golden Bridge Biological
Technology, Inc.) for 30 min at 37°C. Then tissues were stained
with 3,3′-diaminobenzidine (Zhong Shan Golden Bridge Biological
Technology, Inc.) and hematoxylin (Wuhan Boster Biological
Technology, Ltd.) for 30 min at 37°C. The experiment was repeated
three times.
Immunofluorescence
Immunofluorescence was also performed to confirm the
location of TNFAIP8 protein, using the following technique. The
cell climbing piece was placed into a 24-well plate with 2,000
cells for 12 h. The cells were fixed with methanol for 5 min and
then the climbing piece was washed with PBS for 5 min three times.
The cells were then blocked using 3% bovine serum albumin (BSA; 300
µl 10% BSA, 700 µl PBS and 10 µl Triton X-100)
purchased from Wuhan Boster Biological Technology, Ltd. for 30 min.
Cells were washed with PBS for 5 min three times, then were
incubated at 37°C for 3 h with the TNFAIP8 rabbit anti-human
polyclonal antibody (1:50). Subsequent to washing with PBS, the
tissues were then incubated with the monkey anti-rabbit monoclonal
secondary antibody (1:500; cat. no. A24221; Zhong Shan Golden
Bridge Biological Technology, Inc.) for 1 h at 37°C. The liquid was
then discarded, cells were stained with DAPI (Wuhan Boster
Biological Technology, Ltd.). Subsequent to mounting, images were
captured using an inverted fluorescence microscope (DP72; Olympus,
Tokyo, Japan).
Evaluation of IHC staining
The stained tissues were evaluated by two
pathologists in a blinded-manner. Scores were assigned, dependent
on the staining intensity and proportion, as previously described
(13). Based on the intensity, the
degrees were categorized as follows: 0, negative; 1, weak; 2,
moderate; and 3, strong. The proportion of TNFAIP8 staining was
scored using the following scale: 0, absent; 1, <25%; 2, 26–50%;
3, 51–75%; and 4, >75%. The final result was the sum of the
intensity and proportion scores. IHC scores of <4 were
considered to indicate low levels of expression, and those >4
were considered indicative of high levels of expression.
Cell culture
The GES-1, MKN-28, SGC-7901 and MGC-803 cells were
maintained in liquid nitrogen (Shanghai Jiayu Chemical Co., Ltd.
Shanghai, China) in the Central laboratory of Shandong Provincial
Hospital affiliated to Shandong University. The NCI-N87 human
gastric cancer cell line was provided by the Institution of
Digestive Surgery of Ruijin Hospital Affiliated to Shanghai
Jiaotong University (Shanghai, China). The cells were cultured in
RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented
with 10% heat-inactivated fetal bovine serum (FBS; GE Healthcare
Life Sciences, Beijing, China), penicillin (100 U/ml) and
streptomycin (100 mg/l) (Shanghai FMGBio Co., Ltd., Shanghai,
China), in a humidified atmosphere containing 5% CO2 at
37°C.
RT-qPCR
Total cellular RNA was isolated from the cells using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
according to the manufacturer’s instructions. The RNA was reverse
transcribed and the resulting cDNA samples were amplified by qPCR
using a LightCycler 480 Real-Time PCR system (Roche Diagnostics,
Shanghai, China) using gene-specific primers (Takara Bio, Inc.,
Dalian, China). In brief, 20 µl PrimeScript™ RT reagent kit
(DRR037A; Takara Bio, Inc., Shiga, Japan) containing 4 µl 5X
PrimeScript Buffer 2, 1 µl PrimeScriptRT Enzyme Mix I, 1
µl RT Primer Mix, 4 µl RNase Free dH2O and
10 µl RNA template. The RT-qPCR program comprised 37°C for
15 min followed by 85°C for 5 sec and 4°C for 30 min. A total of 2
µl RT product (cDNA) was amplified by RT-qPCR using SYBR
Green (DRR041A; Takara Bio, Inc.). The amplification conditions
were as follows: 95°C for 30 sec, then 40 cycles at 95°C for 5 sec
and 65°C for 30 sec. The sequences of the primer pairs were as
follows: TNFAIP8, forward 5′-TTC CAT CAG GTG GAT TAT ACC TTTG-3′
and reverse 5′-AGG TGG CGC TGA ATG ATT TG-3′. The mRNA levels were
normalized to that of GAPDH.
Sequence design and vector
construction
For small interfence (si)RNA treatment, siRNA to
TNFAIP8 was synthesized by Shanghai Genechem Co., Ltd. The DNA
target sequence for siRNA-TNFAIP8 (5′-CCA CCT TAA TAG ACG ACA
CAA-3′) was designed, based on the core sequence of human TNFAIP8
cDNA. The cells, were transfected with the siRNA, according to the
manufacturer’s instructions, and were observed under a microscope
(DP72).
Lentivirus transfection
For transfection, the SGC-7901 and MKN-28 gastric
cancer cells were pre-cultured for 30 min at 37°C in 96-well plates
at a density of 5×103 cells per well. The cells were
infected with the lentiviral vectors for 30 min at 37°C at
different multiplicities of infection (10, 20, 50, 70 and 100; 50
selected as the optimal), when they were ~50–60% confluent. The
transfection efficiency was observed using an inverted fluorescence
microscope (DP72) after 72 h.
Western blotting
The tissues and cells were washed in PBS, lysed
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) and harvested by centrifugation at 7,500 × g for 25 min. The
protein concentration in the resulting lysate was evaluated using a
Bicinchoninic Acid Protein Assay kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Appropriate quantities of protein were then
separated by electrophoresis on 12% Tris-glycine polyacrylamide
gels (Zhong Shan Golden Bridge Biological Technology, Inc.) and
transferred onto nitrocellulose membranes (Zhong Shan Golden Bridge
Biological Technology, Inc.). The membranes were blocked with 10%
skimmed milk powder and then incubated overnight at 4°C with the
rabbit anti-human TNFAIP8 primary antibody (1:100; cat. no. 64988,
Abcam). On the following day, the membranes were washed three times
with Tris-buffered saline with 1% Tween 20 (Wuhan Boster Biological
Technology, Ltd.) for 10 min and incubated with the corresponding
goat anti-rabbit monoclonal secondary antibody (1:2,000) at 37°C
for 1 h. Following washing three times for 10 min, the bound
secondary antibody was detected using an enhanced chemiluminescence
system (Pierce Biotechnology Inc.). The protein levels were
normalized against β-actin.
Colony formation assay
The gastric cancer cells were digested into single
suspension cells and 1×103 cells were plated in 60 mm
plates with 4 ml complete culture medium (5% FBS and
1%penicillin/streptomycin). The plates were cultured at 37°C in 5%
CO2 for 10 days. Colonies comprising at least 50 cells
were considered to be statistically significant. The results are
presented as the mean ± standard deviation from five randomly
selected fields.
Cell counting kit (CCK)-8 assay
The gastric cancer cells (8×102 cells)
were incubated in 96-well plates with 100µl 10% FBS, and
were continuously incubated for 24, 48, 72, 96 and 120 h at 37°C in
5% CO2. The number of cells were estimated using a CCK-8
(cat. no. C0038; Dojindo Molecular Technologies, Kumamoto, Japan).
Briefly, 10 µl CCK-8 was added to each well and, following
incubation for 1 h, the absorbance at 450 nm was measured to
calculate the number of cells. The analysis of each cell type were
repeated six times independently.
Invasion and migration assay
For invasion assays, 3×105 cells were
plated into 200 µl RPMI-1640 medium in the upper chamber of
a Transwell, which was separated from the lower chamber by a 50
µl Matrigel-coated membrane (24-well insert; 8-µm
pore size; Corning Costar, Corning, NY, USA). For the migration
assay, the membranes were not coated with Matrigel, although the
culture conditions were the same as in the invasion assay (37°C, 5%
CO2, 24 h for invasion assay, 36 h for migration).
Finally, for the two assays, the membranes were removed and stained
with hematoxylin, and images were captured using an SMZ171
microscope (Olympus).
Statistical analysis
Statistical analysis was determined using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). The protein and mRNA
expression levels of TNFAIP8 in human gastric cancer cell lines and
tissues were expressed as the mean ± standard deviation of at least
three independent experiments. The χ2 and Fisher’s exact
tests were used for the analysis of categorical variables.
P<0.05 was considered to indicate a statistically significant
difference.
Results
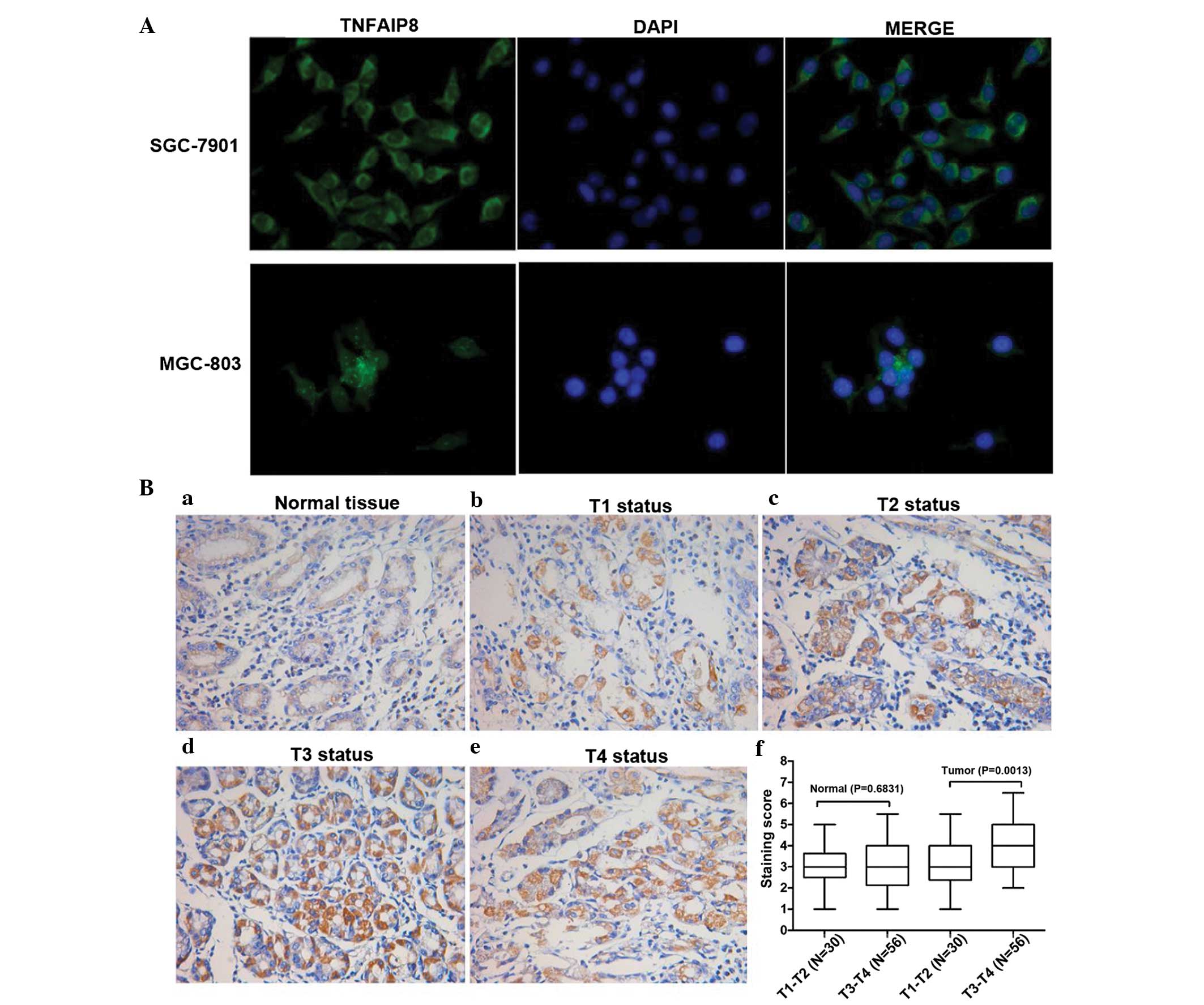
TNFAIP8 is upregulated in gastric cancer
tissues and cell lines
Although TNFAIP8 has been detected, whether its
expression is associated with tumor invasion remains to be fully
elucidated (14). Correlations
between the expression of TNFAIP8 and various clinicopathological
features are listed in Table I. In
the present study, the protein levels of TNFAIP8 were examined
using IHC staining in normal tissues and tumor tissues, varying in
depth of invasion. The tissues from 56 patients with gastric cancer
of T3+T4 status were compared with 30 patients with gastric cancer
patients of T1+T2 status. Within all the tissues, the intensity of
TNFAIP8 staining was observed in the following order:
T3+T4>T1+T2 (Fig. 1Ba–Bf). The
expression levels of TNFAIP8 were significantly associated with
tumor status (P<0.05; Table I;
Fig. 1B; n=86). Furthermore, the
expression of TNFAIP8 in the tumor tissues was higher than in the
adjacent normal tissues. In addition, the staining points of the
normal tissue indicated no significant difference in the levels of
TNFAIP8 between the T3+T4 and T1+T2 groups, however TNFAIP8 was
upregulated in the tumor tissues from T3+T4 patients, compared with
those from T1+T2 patients (Fig.
1Bf). Despite the requirement for further investigations to
confirm and develop this IHC data, these results revealed that
TNFAIP8 was commonly enhanced in gastric cancer tissues, compared
with normal tissues or deeper invasion tissues. This suggested that
TNFAIP8 can be used as a sole prognostic value for metastasis and
is expected to become a potential target for the treatment of
gastric cancer.
 | Table IComparison between the expression of
TNFAIP8 in gastric cancer tissues and clinicopathological
characteristics. |
Table I
Comparison between the expression of
TNFAIP8 in gastric cancer tissues and clinicopathological
characteristics.
| Characteristic | Number of patients
(n) | TNFAIP8 expression
level
| P-value |
|---|
| High, n (%) | Low, n (%) |
|---|
| Tumor status | | | | 0.033 |
| T1–T2 | 30 | 12 (40.00) | 18 (60.00) | |
| T3–T4 | 56 | 34 (60.71) | 22 (39.29) | |
| Lymph node
metastasis | | | | 0.030 |
| Negative | 62 | 33 (53.22) | 29 (46.77) | |
| Positive | 24 | 19 (79.17) | 5 (20.83) | |
The overexpression of TNFAIP8 protein in gastric
carcinoma tissues compared with normal tissues was demonstrated,
which was in accordance with the results of a previous study
(14). Furthermore, the four
gastric cancer cell lines exhibited higher expression levels of
TNFAIP8 than the GES-1 cells, and the SGC-7901 and MKN-28 cells
exhibited relatively high levels of expression in the four cancer
cell lines (Fig. 2).
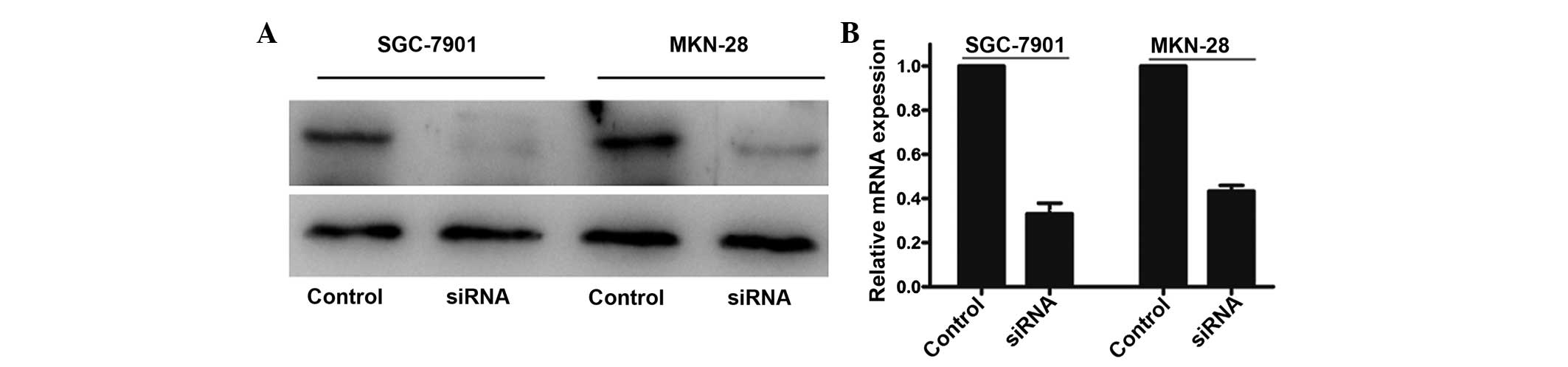
TNFAIP8 knockdown inhibited gastric
cancer cell proliferation, invasion and migration
In order to examine the biological role of TNFAIP8
in gastric cancer cells, the present study knocked down the TNFAIP8
gene in SGC-7901 and MKN-28 cell lines, which exhibited higher
protein expression levels of TNFAIP8, and performed qRT-PCR and
western blot analyses to confirm the knockdown efficiency. The
results demonstrated that treatment with siRNA markedly decreased
the protein and mRNA expression levels of TNFAIP8 (Fig. 3).
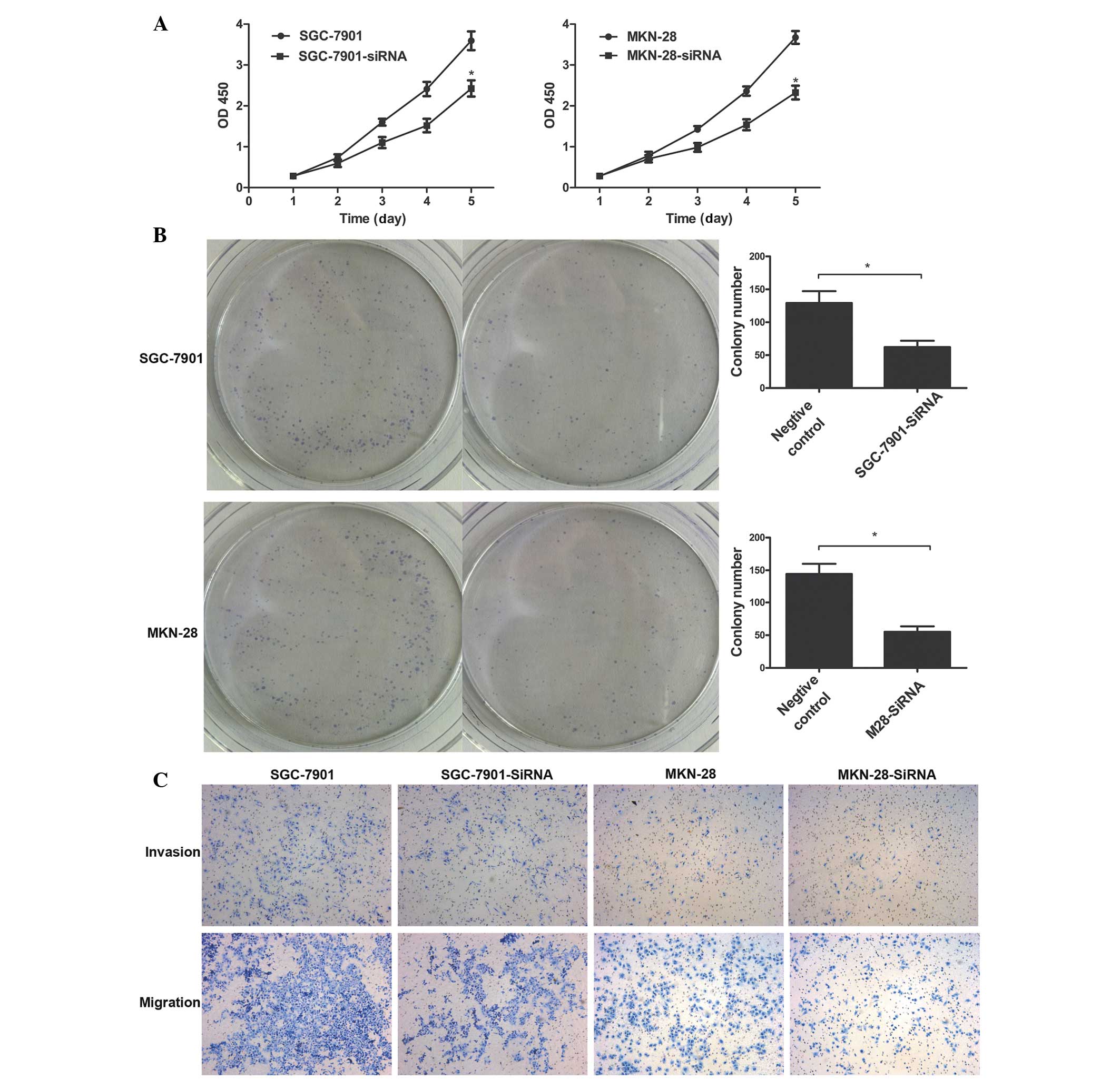
Tumorigenicity was significantly suppressed in the
TNFAIP8-transfected cells. The role of TNFAIP8 in tumor aggression
was apparent in the negative control groups, compared with the
TNFAIP8-siRNA treatment group (Fig. 4A
and B; P<0.01). In addition, decreased expression levels of
TNFAIP8 in the SGC-7901 and MKN-28 cells significantly inhibited
the invasion and migration of these cells, compared with the
invalid siRNA-treated cells (P<0.05; Fig. 4C). Taken together, these data
suggested that TNFAIP8 was involved in promoting the progression of
gastric cancer.
Discussion
Gastric cancer, a serious threat to human health, is
a complex disease affected by numerous factors. In the majority of
cases, the point at which gastric cancer is diagnosed is at a
stage, which is incurable, and the overall prognosis is poor
(15). Numerous studies have
investigated the pathogenesis and prognosis of gastric cancer
(16–18). The examination of levels of
epidermal growth factor, cyclin E, p27, E-cadherin, CD44v6, matrix
metalloproteinase (MMP-1 and tissue inhibitor of
metalloproteinase-1, human epidermal growth factor receptor-2 and
vascular endothelial growth factor (VEGF) (19) may be of important significance for
determining the prognosis and individualized treatment of patients
with gastric cancer. However, the mechanism of gastric cancer
remains to be fully elucidated, and no single index can
successfully predict or prolong the survival rate of patients with
gastric cancer. Therefore, further investigations of the mechanism
of gastric carcinogenesis, effective predictors and treatment are
required.
The expression of TNFAIP8 has been identified in
various types of human cancer (6–11).
However, prior to the present study, the expression of TNFAIP8 and
its functions associated with gastric cancer remained to be
elucidated. To investigate this, the present study firstly
corroborated the protein levels of TNFAIP8 in gastric cancer
tissues compared with corresponding adjacent normal tissues using
immunohistochemistry, western blotting and qRT-PCR. The results
demonstrated that the protein levels of TNFAIP8 in the gastric
cancer tissues were significantly higher than those of the matched
adjacent normal tissues, and there was a close correlation between
TNFAIP8 positivity, tumor, necrosis, metastasis stage, and lymph
node metastasis. These results were in agreement with previous
studies and indicated that TNFAIP8 may be involved in the
progression of gastric cancer (14,19).
The immunohistochemcal staining in the gastric cancer tissues and
corresponding normal tissues were also compared, which indicated
higher staining scores in the T3–T4 cancer group compared with the
T1–T2 cancer group. These data indicated that TNFAIP8 is of
predictive value for invasion and metastasis in gastric cancer and
provide a novel insight for the estimation of the progression of
gastric cancer. Collectively, these findings indicated that TNFAIP8
was associated with gastric cancer and can be considered as an
oncogene.
However, it is difficult to determine whether
downregulation of TNFAIP8 also affects gastric cancer cell
tumorigenesis. To further examine the expression of TNFAIP8 in
gastric cancer and the correlation of TNFAIP8 with the biological
behaviors of gastric cancer cells, the present study analyzed the
expression levels of TNFAIP8 in normal gastric mucosa epithelial
cells and in four differential gastric cancer cell lines using
western blotting. The expression levels of TNFAIP8 in the gastric
carcinoma cells were significantly higher compared with those in
the GES-1 cells. In order to investigate the functions of TNFAIP8
in tumor formation, the present study inhibited its function via
siRNA treatment in the MKN-28 and SGC-7901 cell lines, which
exhibit higher expression levels of TNFAIP8. The results revealed
that knockdown of TNFAIP8 caused a significant inhibition of cell
proliferation and colony formation ability, which were consistent
with the immunohistochemical data and with the results of previous
studies (8,14,20).
Cell migration promotes several biological processes
(21) and enhanced activation of
cell migration results in tumor metastasis, which is the
predominant factor affecting survival rates (22). In the present study, the cell
invasion and migration assay demonstrated that TNFAIP8 knockdown
inhibited cell invasion and migration in the two types of gastric
cancer cells. These data provided evidence to indicate that TNFAIP8
is important in cell invasion and metastasis. In support of this
findings, Zhang et al (11)
revealed that downregulation in the expression of TNFAIP8 decreased
lung metastasis by inhibiting MMP1 and MMP2 metastasis-associated
molecules in tumor cells, and VEGFR receptor 2 in endothelial
cells.
In conclusion, the results of the present study
indicated that the protein and mRNA expression levels of TNFAIP8 in
gastric carcinoma tissues and cells were significantly higher
compared with those in normal tissues and cells. Furthermore,
TNFAIP8 was identified as a novel independent risk factor for
predicting the prognosis of patients. Accordingly, TNFAIP8 IHC
scores may be used as a novel diagnostic biomarker for determining
the risk of metastasis and prognosis. The results of the present
study demonstrated that TNFAIP8 acted as a functional oncogene
protein in gastric tumorigenesis and was involved in promoting
lymph node metastasis. The results provide further evidence that,
in addition to enhancing tumorigenesis in other types of tumor,
TNFAIP8 may be involved in promoting cell proliferation, invasion
and migration. However, the TNFAIP8-mediated mechanisms of cell
proliferation, invasion and migration remain to be elucidated. As
TNFAIP8 is induced by the activation of the nuclear factor (NF)-kB
transcription factor, TNFAIP8 may regulate cell function via the
NF-kB signal transduction pathway. Additional investigations are
currently being performed to attempt to establish TNFAIP8 as a
potential therapeutic target.
Acknowledgments
This study was supported by a grant from the
National Youthful Science Foundation of China (grant no. 1101858)
and part research project from the Natural Science Foundation of
Shandong Province of China (grant nos. ZR2011HM041 and
ZR2011HM076).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Liang S, He L, Zhao X, et al: MicroRNA
let-7f inhibits tumor invasion and metastasis by targeting MYH9 in
human gastric cancer. PLoS One. 6:e184092011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun J, Jiang T, Qiu Z, et al: Short-term
and medium-term clinical outcomes of laparoscopic-assisted and open
surgery for gastric cancer: a single center retrospective
case-control study. BMC Gastroenterol. 11:852011. View Article : Google Scholar
|
|
5
|
Popa F, Bratucu M and Radu P: Present and
future tense in operable rectal cancer. Chirurgia (Bucur).
106:11–16. 2011.
|
|
6
|
Shi TY, Cheng X, Yu KD, et al: Functional
variants in TNFAIP8 associated with cervical cancer susceptibility
and clinical outcomes. Carcinogenesis. 34:770–778. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang C, Kallakury BV, Ross JS, et al: The
significance of TNFAIP8 in prostate cancer response to radiation
and docetaxel and disease recurrence. Int J Cancer. 133:31–42.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miao Z, Zhao T, Wang Z, et al: SCC-S2 is
overexpressed in colon cancers and regulates cell proliferation.
Tumour Biol. 33:2099–2106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu K, Qin CK, Wang ZY, Liu SY, Cui XP and
Zhang DY: Expression of tumor necrosis factor-alpha-induced protein
8 in pancreas tissues and its correlation with epithelial growth
factor receptor levels. Asian Pac J Cancer Prev. 13:847–850. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong QZ, Zhao Y, Liu Y, et al:
Overexpression of SCC-S2 correlates with lymph node metastasis and
poor prognosis in patients with non-small-cell lung cancer. Cancer
Sci. 101:1562–1569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang C, Chakravarty D, Sakabe I, et al:
Role of SCC-S2 in experimental metastasis and modulation of
VEGFR-2, MMP-1, and MMP-9 expression. Mol Ther. 13:947–955. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carlson RW, Larsen JK, McClure J,
Fitzgerald CL, et al: International adaptations of NCCN Clinical
Practice Guidelines in Oncology. J Natl Compr Canc Netw.
12:643–648. 2014.PubMed/NCBI
|
|
13
|
Liu T, Gao H, Chen X, et al: TNFAIP8 as a
predictor of metastasis and a novel prognostic biomarker in
patients with epithelial ovarian cancer. Br J Cancer.
109:1685–1692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang M, Zhao Q, Wang X, et al: TNFAIP8
overexpression is associated with lymph node metastasis and poor
prognosis in intestinal-type gastric adenocarcinoma.
Histopathology. 65:517–526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ang TL, Khor CJ and Gotoda T: Diagnosis
and endoscopic resection of early gastric cancer. Singapore Med J.
51:93–100. 2010.PubMed/NCBI
|
|
16
|
Jin Z, Jiang W and Wang L: Biomarkers for
gastric cancer: Progression in early diagnosis and prognosis
(Review). Oncol Lett. 9:1502–1508. 2015.PubMed/NCBI
|
|
17
|
Xu MD, Qi P, Weng WW, Shen XH, et al: Long
non-coding RNA LSINCT5 predicts negative prognosis and exhibits
oncogenic activity in gastric cancer. Medicine (Baltimore).
93:e3032014. View Article : Google Scholar
|
|
18
|
Ema A, Yamashita K, Ushiku H, et al:
Immunohistochemical analysis of RTKs expression identified HER3 as
a prognostic indicator of gastric cancer. Cancer Sci.
105:1591–1600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yasui W, Oue N, Aung PP, Matsumura S,
Shutoh M and Nakayama H: Molecular-pathological prognostic factors
of gastric cancer: a review. Gastric Cancer. 8:86–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar D, Gokhale P, Broustas C,
Chakravarty D, Ahmad I and Kasid U: Expression of SCC-S2, an
antiapoptotic molecule, correlates with enhanced proliferation and
tumorigenicity of MDA-MB 435 cells. Oncogene. 23:612–616. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vicente-Manzanares M, Sancho D, Yáñez-Mó M
and Sánchez-Madrid F: The leukocyte cytoskeleton in cell migration
and immune interactions. Int Rev Cytol. 216:233–289.
2002.PubMed/NCBI
|
|
22
|
Eccles SA and Welch DR: Metastasis: recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007. View Article : Google Scholar : PubMed/NCBI
|