Introduction
Pulmonary arterial hypertension (PAH) is a
progressive, fatal disorder associated with poor patient prognoses
(1,2). The progression of PAH is influenced
by numerous, complex mechanisms, including endothelial dysfunction,
the proliferation of pulmonary arterial smooth muscle cells and the
induction of inflammation (3–6).
There is evidence that vascular endothelial damage and/or
dysfunction have significant roles in the induction of pathological
vascular remodeling (7). Studies
have also indicated that inflammation may exhibit a pivotal role in
the pathogenesis of PAH (8–12).
Iptakalim, a novel compound designed and synthesized by our group,
has been confirmed to be a selective ATP-sensitive potassium
channel opener (KATPCO) by pharmacological, biochemical
and electrophysiological evaluation (13). Iptakalim has been suggested to be a
novel neuroprotective drug, which may exert its effect via the
inhibition of microglia-mediated neuroinflammation (14). Furthermore, a study indicated that
iptakalim inhibited endothelin-1 (ET-1) release and enhanced nitric
oxide release from cultured aortic endothelial cells, and that
these effects were significantly inhibited following pretreatment
with the KATP channel blocker glibenclamide, in
vitro (15). A study indicated
that iptakalim provided endothelial protection against the
progression of cardiac hypertrophy to heart failure in a rat model
of abdominal aortic banding-induced pressure-overloading. In
addition, iptakalim was able to normalize the balance between the
NO and endothelin signaling systems (16). Based on these results, it was
hypothesized that iptakalim may attenuate the inflammation and
endothelial cell injury induced in PAH.
Materials and methods
Animals and experimental design
Ninety-six male 7-week-old Sprague-Dawley rats
weighing 200–200 g (Experimental Animal Center of Nanjing Medical
University, Nanjing, China) were randomly divided into three groups
(control, hypoxia and iptakalim groups; n=32 per group; 8 rats were
sacrificed for detection of inflammatory cytokines every week). In
the hypoxia and iptakalim groups, rats were placed into normobaric
hypoxia chambers (room temperature, 12 h/12 h light/dark cycle;
CYES-II; Shanghai Anting Scientific Instrument Factory, Shanghai,
China) with 10±1% oxygen, <3% CO2 and normal
atmospheric pressure for 8 h/day, 6 days/week for 4 weeks. The
hypoxia chamber regulated the fractional O2
concentration of inspired gas by solenoid-controlled infusion of
N2, balanced against an inward leak of air through holes
in the chamber. The concentrations of O2 and
CO2 were monitored every 10–15 min. Food and water were
provided ad libitum. The control rats were kept in the same
conditions, except hypoxia was not induced. Iptakalim (Institute of
Pharmacology and Toxicology, Chinese Academy of Military Medical
Sciences, Beijing, China), at a dose of 1.5 mg/kg/day, was
administered orally by gavage tube to rats in the iptakalim group
once daily prior to hypoxia, for four weeks. The control group were
administered saline. The study was conducted in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The animal
use protocol was reviewed and approved by the Institutional Animal
Care and Use Committee of Nanjing Medical University (Nanjing,
China).
Hemodynamic measurements
On the 28th day, rats were weighed and anesthetized
with 1.0 g/kg urethane (Sigma-Aldrich, St. Louis, MO, USA),
administered intraperitoneally. The protocol was performed as
previously described (17).
Briefly, a polyethylene catheter (PE10, 427400; BD Biosciences,
Franklin Lakes, NJ, USA) and heparinsaline (125 U/ml; Changzhou
Yinsheng Pharmaceutical Co., Ltd., Changzhou, China) was inserted
into the right jugular vein and advanced into the right ventricle
and pulmonary artery. The catheter was connected to an MPA
Acquisition and Analysis system (MP100; BIOPAC Systems, Inc.,
Goleta, CA, USA) by a pressure transducer (TSD104A; BIOPAC Systems
Inc.). The mean pulmonary artery pressure (mPAP) and right
ventricular systolic pressure (RVSP) were recorded using a
multiparameter monitor PM-8000 (Zhuhai Joyful Medical Equipment
Co., Ltd., Zhuhai, China. Following measurements of hemodynamic
parameters and blood sample collection, the rats were sacrificed by
cervical dislocation, and the thorax was opened. The pulmonary
artery was carefully separated, and the heart was removed and the
right ventricle (RV), left ventricle (LV) and septum (S) were
separated. The mass ratio of RV to LV plus S (RV/LV+S) was
evaluated. The lung tissues were processed for histological
evaluation or snap-frozen in liquid nitrogen for further
analysis.
Histological analysis
Following gentle perfusion with ice-cold sterile
saline via the trachea, the lung tissue was fixed with 4% buffered
paraformaldehyde solution and embedded in paraffin. The tissues
were subsequently sectioned (4 μm) and stained with
hematoxylin and eosin. Sections were examined under a light
microscope and photomicrograph images were captured. The external
diameter and medial wall thickness of pulmonary arteries with
diameters ranging between 25 and 200 μm per lung section
were measured. A minimum of six vessels per rat were analyzed. The
medial wall thickness and medial wall area was calculated for each
pulmonary artery and expressed as follows: % wall thickness =
[(external diameter − internal diameter)/external diameter] × 100
and % wall area = [(total area − internal area)/total area] × 100.
All vessels were measured by a blinded observer with perceptible
media using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville,
MD, USA) (18,19).
Enzyme-linked immunosorbent assay
(ELISA)
The snap-frozen lungs were thawed, weighed and
transferred to tubes on ice containing 1 ml phosphate-buffered
saline with Tween-20 at 4°C for homogenization, and were
subsequently centrifuged at 6,000 × g for 15 min at 4°C. Total
protein concentrations of the homogenates were determined using a
bicinchoninic acid protein assay kit (Pierce Biotechnology, Inc.,
Thermo Fisher Scientific, Rockford, IL, USA). The concentrations of
interleukin (IL)-1β and IL-10 in the plasma and lung tissue
homogenates were evaluated using commercial ELISA kits (Fuzhou
Maixin Biotechnology Development Co., Ltd., Fuzhou, China)
according to the manufacturer’s instructions. Absorbance at 450 nm
was read on a microplate reader (Bio Tek Instruments, Inc.,
Winooski, VT, USA) and concentrations were calculated according to
the standard curve.
Immunohistochemical analysis
Immunohistochemical staining for the rat
monocyte/macrophage/microglia marker ED1 was performed on the lung
sections. The processed sections were incubated with primary
monoclonal mouse anti-rat ED1 antibodies (1:500; cat. no. 550958;
Fuzhou Maixin Biotechnology Development Co., Ltd.). The sections
were then analyzed using the avidin-biotin complex method, using an
Elite Mouse ABC kit (Vector Laboratories, Inc., Burlingame, CA,
USA). The immunoperoxidase reaction was visualized with
diaminobenzidine used as the chromogen (Vector Laboratories, Inc.).
Subsequently, the sections were washed thoroughly, mounted on
gelatin-coated slides and counterstained with hematoxylin, prior to
dehydration and clearing. Finally, the ED1 immunostained sections
were cover slipped with Permount (Shanghai Sangon Biological
Engineering Co., Ltd., Shanghai, China) and the number of
ED1+ cells was counted in ten randomly selected fields
using a Q550CW image acquistion and analysis system (Leica
Microsystems, Berlin, Germany).
Western blot analysis of platelet
endothelial cell adhesion molecule-1 (PECAM-1) and endothelial
nitric oxide synthase (eNOS) expression
RIPA lysate (200 μl; Fuzhou Maixin
Biotechnology Development Co., Ltd., Fuzhou, China) was added to
the lung tissues for 30 min lysis on ice, followed by
centrifugation (4°C, 2,862 × g) for 30 min. The supernatant was
obtained and the protein concentration was determined by
bicinchoninic acid assay (kit provided by Fuzhou Maixin
Biotechnology Development Co., Ltd.). Total protein (30
μg/lane) was separated on 12% SDS-polyacrylamide gel and
transferred to polyvinylidene fluoride membranes (Advantec MFS,
Inc., Dublin, CA, USA). The membranes were blocked in 5% non-fat
dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBST)
and incubated with polyclonal rabbit anti-mouse eNOS antibody
(dilution l:200; cat. no. 387643), monoclonal mouse anti-rat
PECAM-1 antibody (dilution l:300; cat. no. 428366) and β-actin
antibody (dilution l:200; cat. no. 653988-1; all provided by Fuzhou
Maixin Biotechnology Development Co., Ltd.) at 4°C overnight. The
membranes were subsequently washed twice with TBST and incubated
with the secondary antibody [polyclonal goat anti-mouse IgG (H+L)
antibody; dilution l:200; cat. no. 3208911-3; Fuzhou Maixin
Biotechnology Development Co., Ltd.] for 1 h. The blots were
detected using Super-Signal West Pico chemiluminescent substrate
(Pierce Biotechnology, Inc.), according to the manufacturer’s
instructions. The resulting images were analyzed with Quantity One
image analysis software (version 4.6.5; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Electron microscope analysis of
morphology
According to our previous method (20), specimens from the hilum of the lung
(1 mm3) were harvested and fixed in 2.5% glutaraldehyde.
Alterations in the ultrastructure of the pulmonary arteriole
endothelial cells were identified with a transmission electron
microscope (JEM-1010; JEOL Ltd, Tokyo, Japan).
Statistical analysis
Values are expressed as the mean ± standard
deviation. All data were statistically analyzed using SPSS version
11.5 (SPSS, Inc. Chicago, IL, USA). Statistical comparisons were
performed using one-way analysis of variance and Student
Newman-Keul’s post hoc test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
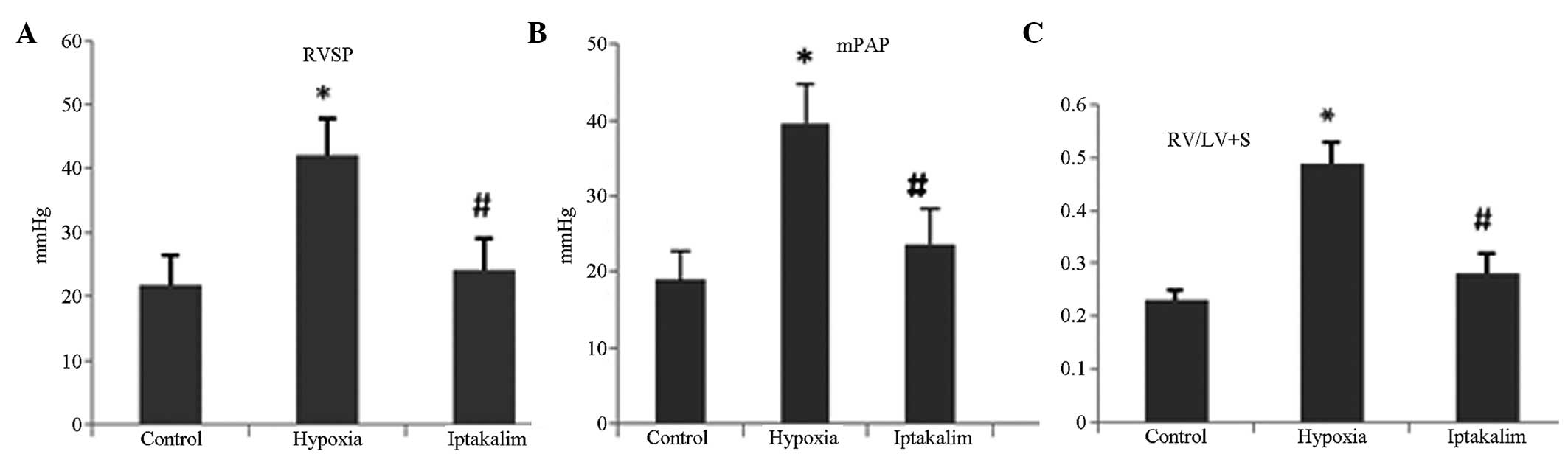
Iptakalim treatment improves hemodynamics
in hypoxia-induced PAH
On the 28th day of hypoxia, RVSP, mPAP and mass
ratio of RV to LV plus S (RV/LV+S), a reflex of PAH, were markedly
elevated in the hypoxia group compared with those in the control
group. However, iptakalim administration attenuated the
hypoxia-induced increase in RVSP, mPAP and RV/LV+S (Fig. 1; P<0.01).
Iptakalim treatment attenuates
hypoxia-induced pulmonary vascular remodeling
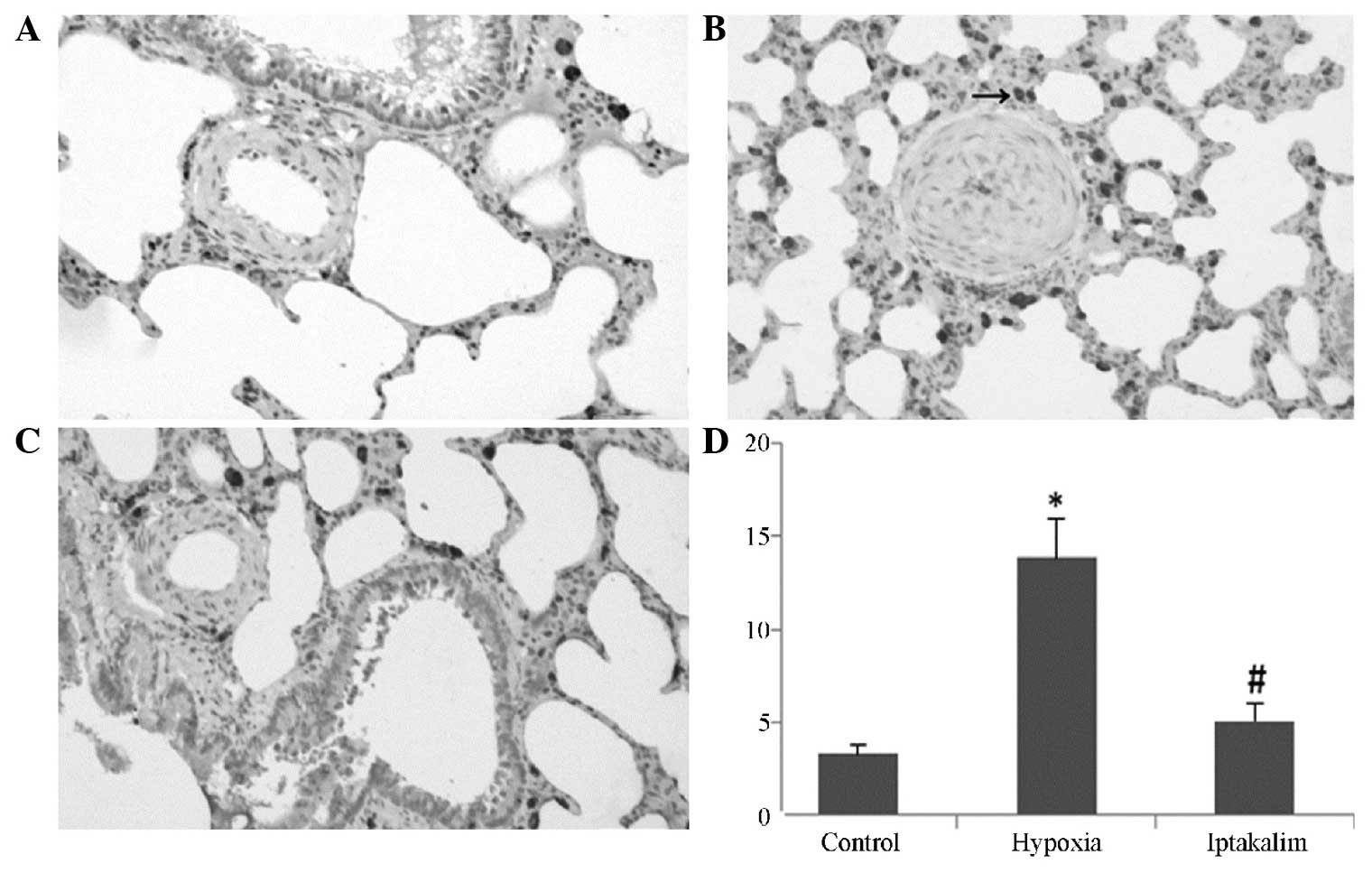
Morphological analysis was performed on small
pulmonary arteries, and relative pulmonary arterial wall thickness
(PAWT) was measured. Hypoxia induced significant thickening of the
pulmonary arterial walls, PAWT in the hypoxia group was
significantly increased compared with that of the control group.
However, iptakalim administration attenuated the hypoxia-induced
increase in PAWT observed (Fig. 2;
P<0.05).
Iptakalim treatment influences IL-1β and
IL-10 expression levels in the plasma and lung tissues in
hypoxia-induced PAH
On day 28, ELISA analysis indicated that the IL-1β
levels in lung tissues were markedly increased in the hypoxia group
compared with those of the control group. Notably, iptakalim
treatment induced a significant decrease in IL-1β expression
levels. Hypoxia also induced a significant decrease in IL-10 levels
in lung tissues, an effect that was suppressed by iptakalim
treatment (Fig. 3; P<0.05).
In the hypoxia group, IL-1β levels in the plasma
were found to gradually increase from day one to 21, and
subsequently decline between days 21 and 28. Iptakalim
administration significantly attenuated this increase in IL-1β
expression (Fig. 4).
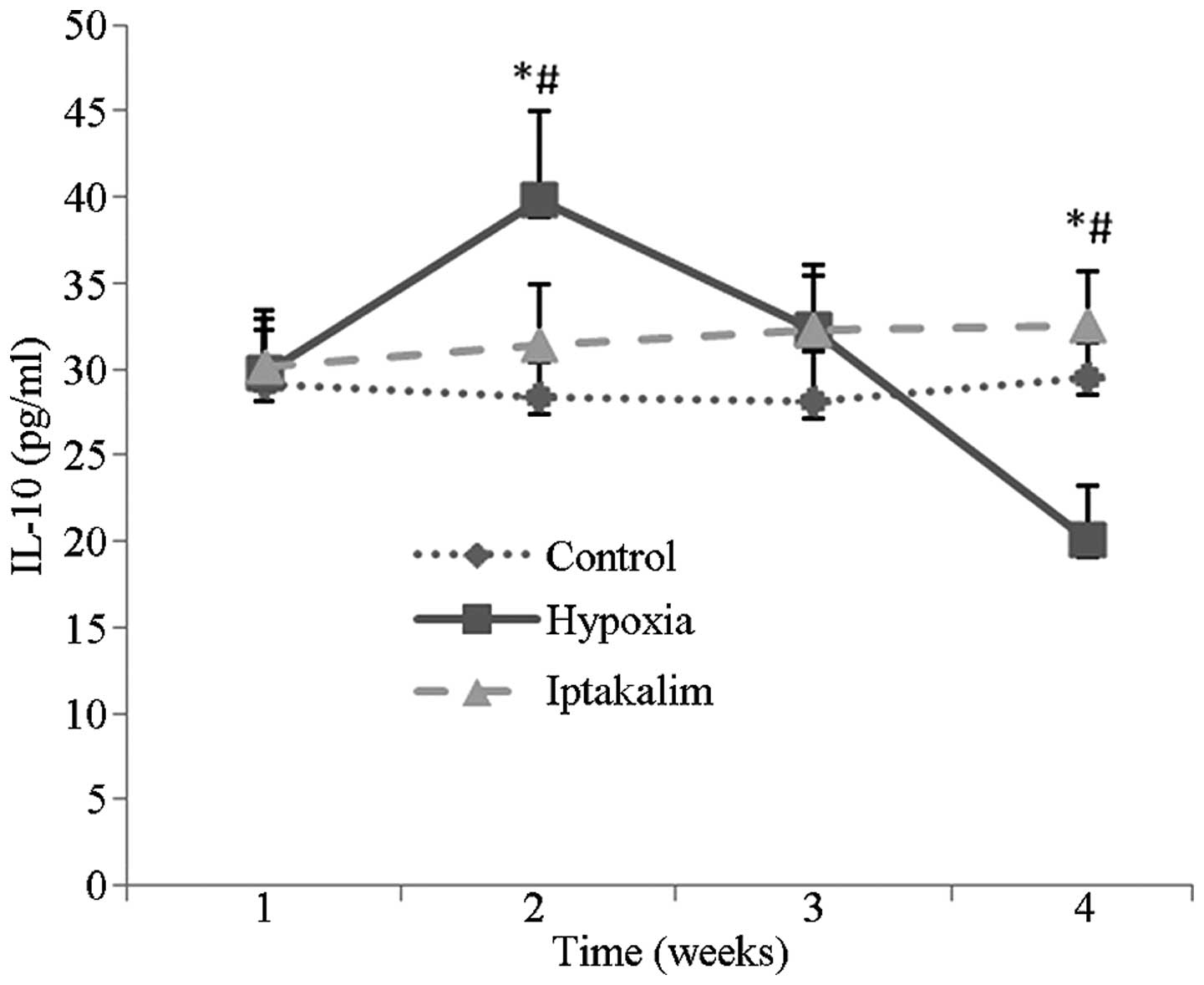
The IL-10 levels in plasma were demonstrated to
gradually increase in the hypoxia group from day seven to 14, and
then steadily decline from day 14 to 28. These fluctuations were
abrogated following iptakalim administration (Fig. 5).
Iptakalim treatment attenuates leukocyte
infiltration in the lungs of rats with hypoxia-induced PAH
In the lungs of rats in the hypoxia group, the
number of ED1+ leukocytes was significantly increased
compared with that in the control group. Notably, ED1 expression,
which signals for leukocyte invasion into lung tissue, was markedly
attenuated following iptakalim treatment (Fig. 6; P<0.01).
Iptakalim upregulates PECAM-1 and eNOS
expression
Western blot analysis revealed that the expression
levels of eNOS and PECAM-1 in the lungs were significantly
downregulated in the hypoxia group. Notably, the expression of eNOS
and PECAM-1 was upregulated following iptakalim treatment (Fig. 7).
Iptakalim abrogates hypoxia-induced
vascular endothelial damage
Electron microscopic analysis revealed that the
endothelial cells of the small pulmonary arterioles of the control
group were flat and exhibited normal morphology and mitochondrial
structures (Fig. 8A). However, the
endothelial cells of the hypoxia group exhibited damage. The
majority of the endothelial cells appeared swollen, denuded and
desquamated. Similar to the characteristic changes observed in
early apoptosis, further ultrastructural alterations identified
included cytoplasmic and nuclear condensation, a decreased number
of organelles, granules and mitochondrial swelling (Fig. 8B–E). By contrast, the destruction
of the endothelial cell ultrastructure was significantly attenuated
following treatment with iptakalim (Fig. 8F–I).
Discussion
Prolonged hypoxia results in sustained pulmonary
hypertension, inducing structural and functional changes to the
pulmonary arterial beds. These changes to the pulmonary arterial
walls include increased proliferation of smooth muscle cells and
abnormal extracellular matrix protein deposition, therefore
resulting in restriction of the vessel lumen and a reduction in the
arterial wall elasticity (21).
Studies have indicated that inflammation contributes
to the development of PAH (22,23).
Studies have suggested that pro-inflammatory cytokines and
mediators, including IL-1 have important roles in the
pathophysiology of PAH (24).
Furthermore, hypoxia induces IL-1β mRNA upregulation in the
pulmonary artery (25). IL-10 is a
multifunctional anti-inflammatory cytokine, which has been
demonstrated to exert a vasculoprotective effect (26). Throughout the progression of
inflammation, IL-10 is generated by type-2 helper T lymphocytes,
inhibiting the production of certain pro-inflammatory cytokines by
macrophages and type-1 helper T lymphocytes (27). Exogenous IL-10 administration
attenuates proliferative vasculopathy in vivo via the
inhibition of smooth muscle cell proliferation and inflammatory
cell infiltration (28,29). IL-10 has been demonstrated to
significantly reduce mPAP, RV/LV+S and percent medial thickness of
the pulmonary artery compared with control specimens (30). IL-10 was also shown to
significantly reduce the levels of macrophage infiltration and
vascular cell proliferation in the remodeled pulmonary artery in
vivo. Elevation of IL-10 expression was demonstrated to prevent
the development of PAH (13). To
the best of our knowledge, to date, IL-1β and IL-10 expression
level changes in peripheral blood and pulmonary tissue of PAH rats
have not previously been reported. In order to evaluate the
anti-inflammatory effects of iptakalim in the progression of PAH,
the IL-1β levels in serum on days 21 and 28 were dynamically
monitored, and the results indicated that expression was greatest
in the hypoxia group. The results of lung tissue evaluation on the
28th day were concurrent with those observed in the serum.
Simultaneously, the IL-10 levels in the hypoxia group were found to
initially increase, followed by a subsequent decrease. On day 28,
the IL-10 expression levels in the hypoxia group were lower than
those of the control group. However, when iptakalim was
administered prior to hypoxia, the fluctuations in IL-1β levels
were abrogated. These results indicated that iptakalim suppressed
the development of PAH by decreasing IL-1β levels and that
inhibiting the fluctuation of IL-10, as an anti-inflammatory
factor, may increase the inflammatory response to a certain extent.
The extent of inflammatory cell infiltration into the
perivasculature was determined by immunohistochemical analysis of
the ED1 antigen, which is expressed by the majority of rat tissue
macrophages (31). The results of
the present study identified a substantial increase in the number
of ED1+ cells in the lungs of rats in the hypoxia group,
which was associated with a significant upregulation in IL-1β
expression levels in plasma and lung tissue homogenates. It has
previously been generally accepted that inflammatory cytokines are
released from activated macrophages and/or neutrophils, and amplify
the inflammatory response, inducing pulmonary vasoconstriction and
promoting the proliferation of vascular cells (32). In the present study, it was
demonstrated that iptakalim treatment in PAH rats significantly
decreased the number of infiltrating ED1+ cells in the
lung and downregulated the IL-1β level in plasma and lung tissue,
thereby confirming the anti-inflammatory effect of iptakalim in the
attenuation of PAH in rats.
PAH occurs as a result of excessive vasoconstriction
and arterial remodeling, induced by endothelial dysfunction.
Endothelial cells mediate smooth muscle cell activity via the
production of vasodilators, including prostacyclin and NO, and
vasoconstrictors, for example thromboxane A2 and ET-1. Endothelial
dysfunction occurs when the physiological balance between
vasodilation and vasoconstriction is shifted towards the latter
(33,34), a state which has been identified in
PAH (35). PECAM-1 (also termed
CD31) is a major component of the intercellular junctions between
endothelial cells in confluent vascular beds (36). A previous study demonstrated that
the loss of PECAM-1 inhibits endothelial cell function (37), and a significant function of
PECAM-1 is in the prevention of excessive levels of circulating
pro-inflammatory cytokines (38).
Furthermore, PECAM-1 has been implicated in a diverse range of
inflammatory and vascular responses, including angiogenesis,
leukocyte transmigration and motility, thrombosis, vascular
permeability and multiple immune functions (39). PECAM-1 decreases plasma levels of
inflammatory cytokines, including TNF-α and MCP-1 (40). Studies have indicated that PECAM-1
is decreased in the lungs of rats following monocrotaline
administration (41). Considering
the functions of PECAM-1, it was suggested that it may be
associated with the pathogenesis of PAH. Vascular endothelial cells
expressing eNOS produce NO, which has multiple significant
physiological functions in the microvasculature (42). The rate of NO production by the
endothelium is a key determinant of NO distribution throughout the
vascular wall, and is associated with PAH progression (42). In order to evaluate the effects of
endothelial injury on microvascular function, eNOS and PECAM-1
expression levels in the lung tissue were analyzed. In the present
study, it was revealed that PECAM-1 expression in the hypoxia group
was significantly decreased compared with that of the control group
on day 28, and that iptakalim treatment significantly upregulated
the expression of eNOS and PECAM-1 in the lung tissue of PAH rats,
verifying the protective effects of iptakalim on pulmonary artery
endothelium cells. The ultrastructure was observed and pulmonary
arteriole intima hyperplasia and fibrosis were identified, as well
as pulmonary artery endothelial cell apoptosis and desquamation,
intercellular gap broadening and barrier function injury with
prolonged hypoxia under light and electron microscopic observation.
The injury in iptakalim group was markedly alleviated, as compared
with the hypoxia group.
In conclusion, the results of the present study
indicated that iptakalim alleviated PAH via suppression of the
inflammatory response and prevention of endothelial injury induced
by the upregulation of eNOS and PECAM-1. For these reasons
iptakalim may represent a potential therapeutic strategy for the
treatment of PAH.
Acknowledgments
The present study was supported by the Funds of the
National Natural Science Foundation of China (WPX) under contract
nos. 81001427 and 8127357, the Funds of the Project of Jiangsu
Pulmonology Clinical Research Center Foundation of China (grant no.
BL2012012) and the Jiangsu Province Major Scientific and
Technological Special Project (grant no. BM2011017). Furthermore,
the study was supported by grants from the National Major
Scientific and Technological Special Project for ‘Significant New
Drugs Development’ (grant no. 2011ZX09302-003-02). Finally, it is a
project funded by the Priority Academic Program Development of
Jiangsu Higher Education Institutions.
References
|
1
|
Archer S and Rich S: Primary pulmonary
hypertension: a vascular biology and translational research ‘Work
in progress’. Circulation. 102:2781–2791. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Humbert M, Sitbon O and Simonneau G:
Treatment of pulmonary arterial hypertension. N Engl J Med.
351:1425–1436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Böhm F and Pernow J: The importance of
endothelin-1 for vascular dysfunction in cardiovascular disease.
Cardiovasc Res. 76:8–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Csiszar A, Labinskyy N, Olson S, et al:
Resveratrol prevents monocrotaline-induced pulmonary hypertension
in rats. Hypertension. 54:668–675. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hironaka E, Hongo M, Sakai A, et al:
Serotonin receptor antagonist inhibits monocrotaline-induced
pulmonary hypertension and prolongs survival in rats. Cardiovasc
Res. 60:692–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Firth AL, Mandel J and Yuan JX: Idiopathic
pulmonary arterial hypertension. Dis Model Mech. 3:268–273. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Voelkel NF and Cool C: Pathology of
pulmonary hypertension. Cardiol Clin. 22:343–351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang B, Luo Y, Liu ML, et al: Macrophage
migration inhibitory factor contributes to hypoxic pulmonary
vasoconstriction in rats. Microvasc Res. 83:205–212. 2012.
View Article : Google Scholar
|
|
9
|
McNicholas WT: Chronic obstructive
pulmonary disease and obstructive sleep apnea: overlaps in
pathophysiology, systemic inflammation, and cardiovascular disease.
Am J Respir Crit Care Med. 180:692–700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Savale L, Tu L, Rideau D, Izziki M, Maitre
B, Adnot S and Eddahibi S: Impact of interleukin-6 on
hypoxia-induced pulmonary hypertension and lung inflammation in
mice. Respir Res. 10:62009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamaji-Kegan K, Su Q, Angelini DJ, Myers
AC, Cheadle C and Johns RA: Hypoxia-induced mitogenic factor
(HIMF/FIZZ1/RELMalpha) increases lung inflammation and activates
pulmonary microvascular endothelial cells via an IL-4-dependent
mechanism. J Immunol. 185:5539–5548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vergadi E, Chang MS, Lee C, et al: Early
macrophage recruitment and alternative activation are critical for
the later development of hypoxia-induced pulmonary hypertension.
Circulation. 123:1986–1995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H: Pharmacological characteristics of
the novel antihypertensive drug iptakalim hydrochloride and its
molecular mechanisms. Drug Development Research. 58:65–68. 2003.
View Article : Google Scholar
|
|
14
|
Zhou F, Wu JY, Sun XL, Yao HH, Ding JH and
Hu G: Iptakalim alleviates rotenone-induced degeneration of
dopaminergic neurons through inhibiting microglia-mediated
neuroinflammation. Neuropsychopharmacology. 32:2570–2580. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Long C, Duan Z, Shi C, Jia G and
Zhang Y: A new ATP-sensitive potassium channel opener protects
endothelial function in cultured aortic endothelial cells.
Cardiovasc Res. 73:497–503. 2007. View Article : Google Scholar
|
|
16
|
Gao S, Long CL, Wang RH and Wang H:
K(ATP) activation prevents progression of cardiac
hypertrophy to failure induced by pressure overload via protecting
endothelial function. Cardiovasc Res. 83:444–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie W, Wang H, Wang H and Hu G: Effects of
iptakalim hydrochloride, a novel KATP channel opener, on
pulmonary vascular remodeling in hypoxic rats. Life Sciences.
75:2065–2076. 2004. View Article : Google Scholar
|
|
18
|
Everett AD, Le Cras TD, Xue C and Johns
RA: eNOS expression is not altered in pulmonary vascular remodeling
due to increased pulmonary blood flow. Am J Physiol.
274:L1058–L1065. 1998.PubMed/NCBI
|
|
19
|
Megalou AJ, Glava C, Oikonomidis DL, et
al: Transforming growth factor-β inhibition attenuates pulmonary
arterial hypertension in rats. J Clin Exp Med. 3:332–340. 2010.
|
|
20
|
Qi X, Xie WP, Wang H and Hu G: Changes of
pulmonary arterial endothelial cells in chronic hypoxic rats and
intervention of iptakalim hydrochloride. Zhongguo Linchuang Kangfu
Zazhi. 9:94–96. 2005.In Chinese.
|
|
21
|
Rabinovitch M, Gamble W, Nadas AS,
Miettinen OS and Reid L: Rat pulmonary circulation after chronic
hypoxia: hemodynamic and structural features. Am J Physiol.
236:H818–H827. 1979.PubMed/NCBI
|
|
22
|
Dorfmüller P, Perros F, Balabanian K and
Humbert M: Inflammation in pulmonary arterial hypertension. Eur
Respir J. 22:358–363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kherbeck N, Tamby MC, Bussone G, Dib H,
Perros F, Humbert M and Mouthon L: The role of inflammation and
autoimmunity in the pathophysiology of pulmonary arterial
hypertension. Clin Rev Allergy Immunol. 44:31–38. 2013. View Article : Google Scholar
|
|
24
|
Humbert M, Monti G, Brenot F, et al:
Increased interleukin-1 and interleukin-6 serum concentrations in
severe primary pulmonary hypertension. Am J Respir Crit Care Med.
151:1628–1631. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsai BM, Wang M, Pitcher JM, Kher A,
Crisostomo P and Meldrum DR: Zaprinast attenuates hypoxic pulmonary
artery injury and causes less aortic relaxation than milrinone.
Shock. 24:417–420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nonaka-Sarukawa M, Okada T, Ito T, et al:
Adeno-associated virus vector-mediated systemic interleukin-10
expression ameliorates hypertensive organ damage in Dahl
salt-sensitive rats. J Gene Med. 10:368–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ito T and Ikeda U: Inflammatory cytokines
and cardiovascular disease. Curr Drug Targets Inflamm Allergy.
2:257–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen S, Kapturczak MH, Wasserfall C, et
al: Interleukin 10 attenuates neointimal proliferation and
inflammation in aortic allografts by a heme oxygenase-dependent
pathway. Proc Natl Acad Sci USA. 102:7251–7256. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mazighi M, Pellé A, Gonzalez W, et al:
IL-10 inhibits vascular smooth muscle cell activation in vitro and
in vivo. Am J Physiol Heart Circ Physiol. 287:H866–H871. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ito T, Okada T, Miyashita H, et al:
Interleukin-10 expression mediated by an adeno-associated virus
vector prevents mono-crotaline-induced pulmonary arterial
hypertension in rats. Circ Res. 101:734–741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rocha R, Martin-Berger CL, Yang P,
Scherrer R, Delyani J and McMahon E: Selective aldosterone blockade
prevents angiotensin II/salt-induced vascular inflammation in the
rat heart. Endocrinology. 143:4828–4836. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sprague AH and Khalil RA: Inflammatory
cytokines in vascular dysfunction and vascular disease. Biochem
Pharmacol. 78:539–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alan B and Nalbantgil S: Genetic, cellular
and molecular mechanisms of pulmonary arterial hypertension.
Anadolu Kardiyol Derg. 1:9–13. 2010. View Article : Google Scholar
|
|
34
|
Bauersachs J and Widder JD: Endothelial
dysfunction in heart failure. Pharmacol Rep. 60:119–126.
2008.PubMed/NCBI
|
|
35
|
Janigro D, West GA, Gordon EL, et al:
ATP-sensitive K+ channels in rat aorta and brain
microvascular endothelial cells. Am J Physiol. 265:C812–C821.
1993.PubMed/NCBI
|
|
36
|
Newman PJ: The biology of PECAM-1. J Clin
Invest. 100:S25–S29. 1997.PubMed/NCBI
|
|
37
|
DeLisser HM, Helmke BP, Cao G, et al: Loss
of PECAM-1 function impairs alveolarization. J Biol Chem.
281:8724–8731. 2006. View Article : Google Scholar
|
|
38
|
Goel R, Boylan B, Gruman L, North PE and
Newman DK: The proinflammatory phenotype of PECAM-1-deficient mice
results in atherogenic diet-induced steatohepatitis. Am J Physiol
Gastrointest Liver Physiol. 293:G1205–G1214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Woodfin A, Viosin MB and Nourshargh S:
PECAM-1: a multi-functional molecule in inflammation and vascular
biology. Arterioscler Thromb Vasc Biol. 27:G2514–G2523. 2007.
View Article : Google Scholar
|
|
40
|
Goel R, Boylan B, Gruman L, Newman PJ,
North PE and Newman DK: The proinflammatory phenotype of
PECAM-1-deficient mice results in atherogenic diet-induced
steatohepatitis. Am J Physiol Gastrointest Liver Physiol.
293:1205–1214. 2007. View Article : Google Scholar
|
|
41
|
Ikarashi N, Toba K, Kato K, et al:
Erythropoietin, but not asialoerythropoietin or
carbamyl-erythropoietin, attenuates monocrotaline-induced pulmonary
hypertension in rats. Clin Exp Hypertens. 34:575–581. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen K and Popel AS: Theoretical analysis
of biochemical pathways of nitric oxide release from vascular
endothelial cells. Free Radic Biol Med. 41:668–680. 2006.
View Article : Google Scholar : PubMed/NCBI
|