Introduction
Obesity is a major risk factor of metabolic
disorders, including type 2 diabetes, hypertension, hyperlipidemia
and arteriosclerosis. The development of obesity is characterized
by an increase in adipose tissue cell number (hyperplasia) and cell
size (hypertrophy) (1). Genetic,
metabolic and nutritional factors are crucial in the development of
obesity (2). Therefore,
understanding the nutrients that affect adipocyte differentiation
may assist in reducing the healthcare burden associated with
obesity (3).
Adipogenesis is the process by which fibroblastic
preadipocytes are converted into fat laden adipocytes. The 3T3-L1
cell line is considered an optimal in vitro model to
investigate adipogenesis, as it exhibits a high potential to
differentiate from a preadipocyte to an adipocyte and also exhibits
morphological and biochemical properties similar to the development
of obesity in humans (4).
Adipogenesis involves the stimulation of a series of
transcriptional factors, including peroxisome
proliferator-activated receptor-γ (PPARγ), CAAT/enhancer binding
protein-α (C/EBPα) and sterol regulatory element binding proteins
(SREBPs) (5). Among these, PPARγ,
which is also activated by SREBP, is considered a key transcription
factor for adipogenesis, as it stimulates the expression levels of
several genes, which are crucial for adipogenic processes,
including fatty acid synthase (FAS), fatty acid binding proteins
(aP2), hormone-sensitive lipase (HSL) and lipoprotein lipase (LPL)
(1,6,7). It
has been revealed that the insulin-induced gene (insig) protein
family of endoplasmic reticulum, insig-1 and insig-2, are important
in cholesterol metabolism (8) and
it has been reported that rats fed a high fat-diet exhibit
increased expression of insig-1 in white adipose tissue (7). Insig-1 binds to SREBP cleavage
activating protein and prevents the proteolytic action of SREBP to
activate transcription factors and, therefore, prevents its effects
on gene transcription (7), which
eventually inhibits preadipocyte differentiation and lipogenesis in
the adipocytes (8). In addition,
insig-2 suppresses the proteolytic action of SREBP, inhibiting the
development of adipogenesis (9).
Although several anti-obesity drugs are commercially
available, their safety and efficacy is questioned. Therefore, the
identification of naturally occurring compounds, which modulate
obesity, may offer beneficial therapeutic efficiency (10). Triticum aestivum sprouts
(TA), commonly termed wheat, is one of the major crops grown
worldwide. During germination/sprouting, a number of useful
phenolic compounds develop in the seeds of TA (11). The germinated wheat leaves are
consumed as a rich source of soluble fiber, vitamins, antioxidants
and minerals (11). Our previous
study demonstrated that extract from TA exhibited
antihyperlipidemic and antidiabetic effects in
streptozotocin-induced diabetic mice (12,13).
TA extract has also been observed to suppress lipid accumulation in
3T3-L1 cells (14) and TA inhibits
liver lipid accumulation in high fat-fed mice (15). Our previous study demonstrated
that, in leptin deficient ob/ob mice, administration of TA extract
reduced the levels of blood glucose and cholesterol (16). These anti-obesity-associated
effects of the TA extract prompted the purification of the
compounds present in the extract. As a result, the leuteolin,
isoscoparin and isoorientin flavonoids were present in the extract.
Although the flavonoids such as luteolin, isoscoparin and
isoorientin are present in TA (17,18),
their effects in the regulation of adipogenesis remain to be
elucidated.
The present study was performed to compare the
anti-adipogenic effects of the luteolin, isoscoparin and
isoorientin flavonoids, purified from TA, in 3T3-L1 cells.
Furthermore, the study aimed to elucidate the underlying mechanisms
associated with their anti-adipogeneic effects.
Materials and methods
Materials and reagents
Dexamethasone (Dex), insulin,
3-isobutyl-1-methylxanthine (IBMX), RNase A and Oil-Red-O (ORO)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s
modified Eagle’s medium (DMEM) and fetal calf serum (FCS) were
purchased from HyClone, (Logan, UT, USA). TRIzol reagent and the
SuperScript III kit were obtained from Invitrogen Life Technologies
(Carlsbad, CA, USA). The Cell Counting Kit-8 (CCK-8) was purchased
from Dojindo Molecular Technologies (Rockville, MD, USA). Rabbit
anti-PPARγ (cat. no. C26H12) and rabbit anti-FAS (cat. no. C20G5)
monoclonal antibodies were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Rabbit polyclonal anti-CEBPα
antibody (cat. no. sc-61), the protein assay kit, including
radioimunoprecipitation (RIPA) buffer, goat anti-rabbit (cat. no.
sc-2030) and goat anti-mouse (cat. no. sc-2005) horseradish
peroxidase (HRP)-conjugated immunoglobulin G secondary antibodies,
and mouse monoclonal anti-β-actin antibody (cat. no. sc-47778),
were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA).
Extraction and purification of the
flavonoids from TA
TA was obtained from the National Institute of Crop
Science (Jeonbuk, Korea) and was subsequently dried in a freezer
dryer. Isoscoparin and isoorientin were purified from TA as
reported previously (17).
Briefly, dried TA was extracted with MeOH (DC Chemical Co., Ltd.,
Seoul, Korea). The MeOH extracts were then partitioned in turn
using four solvents, n-hexane (DC Chemical Co., Ltd.),
CH2CL2, EtOAc and n-BuOH (Junsei Chemical
Co., Ltd., Tokyo, Japan). The EtOAc fraction of the plant was
subjected to column chromatography using silica gel (70–230,
230–400 mesh; Merck, Whitehouse Station, NJ, USA) with
CH2CL2-MeOH (5:1) as a solvent to yield
concentrated fractions. In secondary column chromatography using
YMC RP-18 resins (75 μm, Fuji Silysia Chemical Ltd.,
Kasugai, Japan) as absorbent, isoscoparin was purified with MeOH
H2O (2:3) as eluent, and then isoorientin was eluted
with acetone (DC Chemical Co., Ltd.) H2O (1:3). Luteolin
was obtained from Sigma-Aldrich.
Cell culture and adipocyte
differentiation assay
The 3T3-L1 preadipocytes were obtained from American
Type Culture Collection (Rockville, VA, USA) and maintained in
DMEM, supplemented with 10% FCS and 1% 100 U/ml penicillin and 100
U/ml streptomycin (Welgene, Daegu, Korea). incubated at 37°C in a
5% CO2 humidified incubator. The preadipocytes were
induced to differentiate 2 days post-confluence (day 0) by adding
0.5 mM IBMX, 1 μM Dex and 10 μg/ml insulin (MDI) for
2 days at 37°C in a 5% CO2 humidified incubator
(19). The culture medium was
subsequently changed to DMEM/10% FCS containing insulin (10
μg/ml). Following incubation for 2 days, the medium was
replaced with DMEM/10% FCS and incubated for another 2 days at 37°C
in a 5% CO2 humidified incubator. The individual
flavonoids (0, 1, 5, or 10 μM) were added on day 0 during
differentiation, until the cells were harvested for the subsequent
experiments.
Cytotoxicity assay
The cells were treated with MDI and various
concentrations of the flavonoids for 2, 4 or 8 days at 37°C in a 5%
CO2 humidified incubator, and cell viability was
measured using a CCK-8 kit, according to the manufacturer’s
instructions. The absorbance was measured at 450 nm on a microplate
reader (Biochrom Anthos Zenyth 200; Biochrom Ltd., Cambridge,
UK).
Oil-red staining
The cells were differentiated, as described above.
On day 8, the cells were stained with Oil-Red-O (ORO), according to
the manufacturer’s instructions to visualize the lipid accumulation
in the cells. The intracellular lipid content was measured by
extracting ORO using 100% isopropanol (Merck KGaA, Darmstadt,
Germany) and the absorbance at 520 nm was recorded using a
spectrophotometer (Optizen 2120 UV; Mecasys Co., Ltd., Daejeon,
Korea).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted using TRIzol reagent,
according to the manufacturer’s instructions. RNA (2 μg) was
used for cDNA synthesis using the Super Script™ III kit. The mRNA
expression levels were quantitatively determined using an ABI
Real-Time PCR system (Applied Biosystems, Inc., Foster City, CA,
USA) and a SYBR Green PCR Master mix (Life Technologies, Carlsbad,
CA, USA). GAPDH was used as the internal control. The specific
primers were obtained from Genotech (Daejeon, Korea), and are
listed in Table I. qPCR was
performed using the following cycle conditions: 95°C for 10 min,
followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min. Every
experiment was carried out in triplicate. The results are expressed
as the relative expression levels compared with the control.
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Primer | Accession no. | Sequence (5′-3′) | Base pairs |
|---|
| GAPDH | NM_001289726 | Forward
CATGGCCTTCCGTGTTC
Reverse CCTGGTCCTCAGTGTAGC | 152 |
|
PPAR-γ2 | XM_006505744.1 | Forward
GATGGAAGACCACTCGCATT
Reverse AACCATTGGGTCAGCTCTTG | 115 |
| C/EBPα | NM_001287514 | Forward
TTGTTTGGATTTATCTCGGC
Reverse CCAAGAAGTCGGTGGACAAG | 95 |
| FAS | NM_007988.3 | Forward
TGCTCCCAGCTGCAGGC
Reverse GCCCGGTAGCTCTGGGTGTA | 107 |
| aP2 | XM_006530048 | Forward
AGCCTTTCTCACCTGGAAGA
Reverse TTGTGGCAAAGCCCACTC | 136 |
| LPL | XM_006509563 | Forward
GGACGGTAACGGGAATGTATGA
Reverse TGACATTGGAGTCAGGTTCTCTGT | 81 |
| HSL | XM_006539572 | Forward
GGAGCACTACAAACGCAACGA
Reverse TCGGCCACCGGTAAAGAG | 67 |
| SREBP-1c | XM_006532713 | Forward
GGTTTTGAACGACATCGAAGA
Reverse CGGGAAGTCACTGTCTTGGT | 61 |
| Insig-1 | NM_153526 | Forward
TGTGGTTCTCCCAGGTGACT
Reverse TAGCCACCATCTTCTCCTCC | 109 |
| Insig-2 | NM_133748 | Forward
TGAAGCAGACCAATGTTTCAA
Reverse GGTGAACTGGGGGTCTCC | 90 |
Western blot analysis
Western blot analysis was performed, as previously
described (20). The cells were
lysed in ice-cold RIPA buffer for 40 min and centrifuged (12,000 ×
g) for 20 min at 4°C. The protein concentration was measured using
a bicinchoninic acid assay (Sigma-Aldrich). The lysates (30
μg) were separated on 8% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gels and transferred
onto polyvinylidene difluoride membranes (Amersham Pharmacia
Biotech Co., Ltd., Piscataway, NJ, USA). The membranes were
subsequently blocked with 5% non-fat milk in tris-buffered saline
(Amresco LLC, Solon, OH, USA), containing 0.1% Tween-20
(Sigma-Aldrich) (TBST) for 1 h at room temperature, and the
membranes were probed with the indicated primary antibodies
(1:1,000 dilutions) at 4°C overnight. The membranes were washed
with TBST four times and were subsequently incubated with
HRP-conjugated secondary antibodies (1:5,000 dilutions) for 45 min
at room temperature. The membranes were washed with TBST three
times, and the proteins were visualized using an enhanced
chemiluminescence detection kit (Millipore, Billerica, MA,
USA).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Statistical significance was determined using
Student’s t-test. Statistical analysis was performed using
Microsoft Excel version 2007 (Microscoft Korea, Seoul, Korea).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Flavonoids from TA inhibit lipid
accumulation without significant cytotoxicity in 3T3-L1 cells
The 3T3-L1 cells were treated with various
concentrations (0, 1, 5 and 10 μM) of the purified
flavonoids for 8 days. On the 2nd, 4th and
8th days of treatment, the cell viability was determined
using a CCK-8 assay. The results demonstrated that the flavonoids
caused no significant cytotoxicity towards the differentiating
preadipocytes (1–10 μM; Fig.
1A–C). This result ruled out the possibility that the
anti-adipogenic effects of the flavonoids resulted from
cytotoxicity to the cells. ORO staining positively correlates with
the amount of lipid stored inside the cells, and has been widely
utilized to demonstrate the potential anti-obesity effects of
natural products. Therefore, to visualize the lipid accumulation in
the cytoplasm during adipogenesis, the cells were treated with the
indicated concentrations of the flavonoids during differentiation
and were analyzed using ORO staining. As shown in Fig. 1D, lipid accumulation was inhibited
dose-dependently by all three flavonoids. At 10 μM, the
inhibitory rates were 75, 64 and 65% following treatment with
luteolin, isoscoparin and isoorientin, respectively (Fig. 1E).
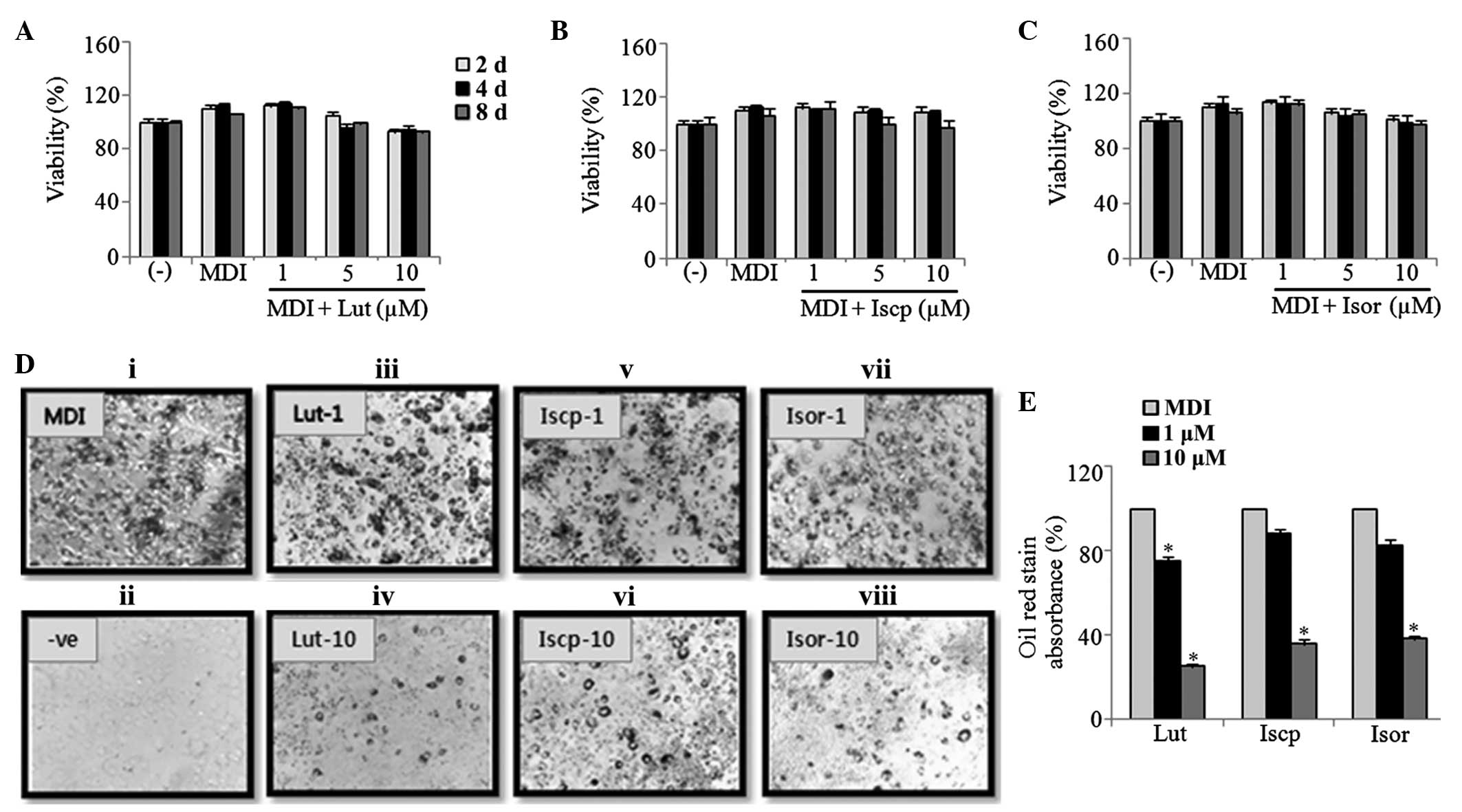 | Figure 1Effect of flavonoids from TA on the
cell viability and lipid accumulation in 3T3-L1 cells. The
viability of the 3T3-L1 cells treated with (A) Lut (B) Iscp and (C)
Isor was determined on the 2nd, 4th and
8th day of differentiation using a CCK-8 assay. (D)
Images of the microscopic visualization of the ORO staining in the
cells on 8th day. (i) MDI-induced adipocytes, (ii)
non-induced, MDI-induced and treatment with Lut at (iii) 1
μM and (iv) 10 μM; Iscp at (v) 1 μM and (vi)
10 μM; Isor at (vii) 1 μM and (viii) 10 μM
(magnification, ×40). (E) ORO-stained lipids were extracted using
isopropanol, and the absorbance was measured using a
spectrophotometer at 520 nm. The data are expressed as the mean ±
standard error of the mean (n=3; *P<0.05, compared
with the control). TA, Triticum aestivum; Lut, luteolin;
Iscp, isoscoparin; Isor, isoorienin; CCK, cell counting kit; MDI,
insulin; ORO, oil red-O. |
Effects of the flavonoids from TA on the
expression of adipogenic transcription factors in the 3T3-L1
cells
To examine the effect of the flavonoids on the
expression levels of the predominant adipogenic transcription
factors in the 3T3-L1 cells during adipogenesis, the cells were
treated with or without the flavonoids (0, 1 and 10 μM).
After 2 days, the mRNA expression levels of PPARγ and C/EBPα were
measured and the inhibition of lipid accumulation was found to be
associated with the downregulation of the transcription factors in
a dose-dependent manner, compared with the untreated control cells
(Fig. 2A and B). Furthermore, the
protein expression levels of these factors were determined on the
4th day following treatment with the flavonoids. At 10
μM, the protein expression levels of PPARγ were suppressed
47, 50 and 46%, and those of C/EBPα were suppressed 66, 58 and 76%,
by luteolin, isoscoparin and isoorientin, respectively, compared
with the controls (Fig. 2C–F).
 | Figure 2Effects of flavanoids from TA on the
expression levels of adipogenic transcriptional factors in 3T3-L1
adipocytes. The 3T3-L1 cells were incubated with or without the
Lut, Iscp or Isor flavonoids, in the presence or absence of MDI, at
the indicated doses. The analysis of the mRNA expression levels was
performed by reverse transcription-quantitative polymerase chain
reaction following 2 days treatment. The protein expression levels
were analyzed following 4 days treatment. The effect of the
flavanoids from TA on the (A) mRNA and (B) protein expression
levels of PPARγ and the (C) percentage ratio (PPARγ/actin) of the
band intensity were determined. The (D) mRNA and (E) protein
expression levels of C/EBPα, and the (F) ratio percentage
(C/EBPα/actin) of the band intensity were determined. The data are
expressed as the mean ± standard error of the mean (n=3;
*P<0.05, compared with the control). TA, Triticum
aestivum; Lut, luteolin; Iscp, isoscoparin; Isor, isoorienin;
MDI, insulin; PPAR, peroxisome proliferator-activated receptor;
C/EBP, CAAT/enhancer binding protein; Rel, relative. |
Effects of the flavonoids from TA on the
expression levels of adipogenesis-associated genes in the 3T3-L1
cells
Since the adipogenic transcription factors were
downregulated by the flavonoids, the expression levels of
adipocyte-specific genes involved in fatty acid synthesis, FAS and
aP2, and lipogenic genes, LPL and HSL, were also quantified.
Significant inhibition of the mRNA and protein expression levels of
FAS was demonstrated following treatment with the flavonoids. At 10
μM, the protein expression of FAS was suppressed by 42, 68
and 33%, by luteolin, isoscoparin and isoorientin, respectively
compared with the control (Fig.
3A–C). Other adipocyte-specific genes, including aP2, HSL and
LPL, were also inhibited in a dose-dependent manner compared with
the control (Fig. 3D–F).
 | Figure 3Effects of the flavanoids from TA on
the expression levels of adipocyte differentiation markers in
3T3-L1 adipocytes. The 3T3-L1 cells were treated with
differentiating medium in the presence or absence of flavonoids for
2 or 4 days for determining the mRNA and protein expression levels,
respectively. The effects of the flavonoids from TA on the (A) mRNA
and (B) protein expression levels of FAS, the (C) percentage ratio
(FAS/actin), and the mRNA expression levels of (D) aP2, (E) HSL and
(F) LPL were assessed. The data are expressed as the mean ±
standard error of the mean (n=3; *P<0.05, compared
with the control). TA, Triticum aestivum; Lut, luteolin;
Iscp, isoscoparin; Isor, isoorienin; MDI, insulin; FAS, fatty acid
synthase; aP2, fatty acid binding protein; HSL, hormone-sensitive
lipase; LPL, lipoprotein lipase; Rel, relative. |
Effects of the flavonoids from TA on the
expression of insig-1 and insig-2 in the 3T3-L1 cells
As insig-1 and 2 are known to inhibit the activation
of SREBP-1c and inhibit adipogenesis (7), the present study aimed to examine
whether the flavonoids from TA modulate the expression of these
genes. For this, RT-qPCR was performed to assess their levels of
expression in the cells treated with, or without, each flavonoid.
The results indicated that the flavonoids inhibited the expression
of SREBP1c (Fig. 4A). Furthermore,
the expression levels of insig-1 and 2 were upregulated by the
flavonoids in the 3T3-E1 cells compared with the control (Fig. 4B–C).
 | Figure 4Effects of the flavanoids from TA on
the expression levels of the genes involved in the insig pathway in
3T3-L1 cells. The 3T3-L1 cells were treated with differentiating
medium in the presence or absence of the flavonoids for 2 days. The
mRNA expression levels of (A) SREBP-1c, (B) insig-1 and (C) insig-2
were determined. The data are expressed as the mean ± standard
error of the mean (n=3; *P<0.05, compared with the
control). TA, Triticum aestivum; Lut, luteolin; Iscp,
isoscoparin; Isor, isoorienin; MDI, insulin; SREBP, sterol
regulatory element binding protein; Insig, insulin-induced gene;
Rel, relative. |
Discussion
Obesity is a major public health concern, which
arises due to an imbalance in energy intake and energy expenditure.
Consequently, this imbalance leads to the pathological growth of
adipocytes. Although there are medications to induce weight loss,
they are associated with negative side effects. For example,
orlistat is known to cause fecal fat loss and gastrointestinal
symptoms (19). Therefore, it is
necessary to focus investigations on healthy foods and novel drugs,
which are safe and effective, for the prevention and treatment of
obesity (10,21).
Adiposity is associated with an increase in the
number of adipocytes and the lipid content of adipocytes. The
results from the present study demonstrated that the flavonoids
(1–10 μM) purified from TA had no effect on the viability of
differentiating 3T3-L1 cells. This result suggested that
flavonoids-induced cytotoxicity did not account for the inhibition
of adipogenesis. Other experiments demonstrated that the flavonoids
significantly reduced lipid accumulation in the 3T3-L1 cells. The
regulation of differentiation of preadipocytes into adipocytes is
mediated by several transcription factors. Among these, PPARγ,
C/EBPs and SREBP1c are known to be critical during the
differentiation of the cells (7).
During the early adipocyte differentiation stages, activation of
C/EBPβ and δ is known to activate C/EBPα and PPARγ, which control
adipogenesis and insulin sensitivity in adipocytes (22). Therefore, the present study
analyzed the expression profiles of adipocyte genes that are
involved in the inhibition of adipogenesis, following treatment
with the flavonoids. Treatment with the flavonoids during the early
stages of differentiation caused a significant inhibition of the
expression levels of C/EBPα and PPARγ. Their activation is known to
regulate the adipocyte differentiation markers and genes associated
with lipid metabolism, including aP2, FAS, LPL, and HSL (21,23).
FAS is a lipogenic enzyme, which facilitates the
synthesis and cytoplasmic storage of large triglycerides (24). It is associated with lipid
accumulation during adipogenesis (25). aP2 is the terminal differentiation
marker of adipocyte differentiation, which causes the uptake of
cellular long-chain fatty acids during fatty acid metabolism and
obesity (26). Therefore, the
present study examined the effect of the flavonoids on the
regulation of the expression levels of FAS and aP2 in 3T3-L1 cells
during adipogenesis. These genes were significantly suppressed in a
dose-dependent manner, suggesting that the downregulation of FAS
and aP2 may have resulted from the decreased utilization of fatty
acid transport in the 3T3-L1 cells. HSL is a key lipolytic response
gene associated with lipid catabolism in adipocytes, as it
hydrolyzes triacylglycerol to monoacylglycerol and free fatty acid
(22). LPL is a marker of early
adipocyte differentiation, and its overexpression initiates lipid
accumulation during adipogenesis (27,28).
In the present study, the expression levels of HSL and LPL were
inhibited by the flavonoids in a dose-dependent manner, which
suggested that the anti-adipogenic effects of the flavonoids were
mediated via the downregulation of the adipogenic process.
Consistent with these results, several previous studies have
reported anti-adipogenic effects of compounds derived from
medicinal plants by inhibiting the expression levels of C/EBPα,
PPARγ and the genes involved in the adipogenic process. For
example, ursolic acid and resveratrol-amplified grape skin extracts
inhibit the adipogenesis of 3T3-L1 cells by downregulating the
expression levels of the PPARγ and CEBP isoforms (28,29).
Furthermore, the present study aimed to identify a
signaling pathway, by which the flavonoids modulate adipogenesis in
3T3-L1 cells. It was hypothesized that the flavonoids may induce a
regulator, which suppresses adipogenic gene expression. A previous
study demonstrated that the insig proteins are important for
downregulation of the SREBP pathway, which regulates cholesterol
and fatty acid synthesis (8).
Another study demonstrated that insig-1 inhibits lipogenesis and
adipocyte differentiation (30).
However, insig-2 is found to be highly expressed in adipose tissue
and has been suggested to be a crucial factor in adipose metabolism
(7). Notably, during adipocyte
differentiation, the changes in the expression of insig-2 is higher
than that of insig-1 (7).
Consistent with these studies, the data from the present study
demonstrated that the expression of insig-2 was higher than insig-1
in the flavonoid-treated cells compared with the control. This
suggested that treatment with the flavonoids increased the
expression levels of the insig proteins, which may have inhibited
the activation of SREBP1c and reduced the expression levels of
adipogenic genes in the 3T3-L1 cells.
Taken together, the results of the present study
demonstrated that the flavonoids derived from TA were natural
anti-adipogenic molecules, which inhibited adipogenic transcription
factors and, consequently, the expression levels of genes involved
in adipocyte differentiation and lipid metabolism. This potentially
occurred through the upregulation of insig-1 and 2. Additional
in vivo investigations are required to confirm the
anti-obesity effects of the purified flavonoids assessed in the
present study.
Acknowledgments
This study was supported by grants from Wonkwang
University (2014).
References
|
1
|
Kanda K, Nishil K, Kadota A, Nishimoto S,
Liu MC and Sagahara T: Nobiletin suppresses adipocyte
differentiation of 3T3-L1 cells by an insulin and IBMX mixture
induction. Biochim Biophys Acta. 1820:461–468. 2012. View Article : Google Scholar
|
|
2
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000.PubMed/NCBI
|
|
3
|
Farmer SR: Regulation of PPARγ activity
during adipogenesis. Int J Obes. 29:13–16. 2005. View Article : Google Scholar
|
|
4
|
Kwon JY, Seo SG, Heo YS, Yue S, Cheng JX,
Lee KW and Kim KH: Piceatannol, natural polyphenolic stilbene,
inhibits adipogenesis via modulation of mitotic clonal expansion
and insulin receptor-dependent insulin signaling in early phase of
differentiation. J Biol Chem. 287:11566–11578. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
White UA and Stephens JM: Transcriptional
factors that promote formation of white adipose tissue. Mol Cell
Endocrinol. 318:10–14. 2010. View Article : Google Scholar
|
|
6
|
Park HS, Kim SH, Kim YS, et al: Luteolin
inhibits adipogenic differentiation by regulating PPARgamma
activation. Biofactors. 35:373–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ka SO, Kim KA, Kwon KB, Park JW and Park
BH: Silibinin attenutates adipogenesis in 3T3-L1 preadipocytes
through potential upregulation of the insig pathway. Int J Mol Med.
23:633–637. 2009.PubMed/NCBI
|
|
8
|
Yang T, Espenshade PJ, Wright ME, et al:
Crucial step in cholesterol homeostasis: sterols promote binding of
SCAP to INSIG-1, a membrane protein that facilitates retention of
SREBPs in ER. Cell. 110:489–500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldstein JL, DeBose-Boyd RA and Brown MS:
Protein sensors for membrane sterols. Cell. 124:35–46. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Montagut G, Blade C, Blay M, et al:
Effects of a grapeseed procy-anidin extract (GSPE) on insulin
resistance. J Nutr Biochem. 21:961–967. 2010. View Article : Google Scholar
|
|
11
|
Lee SH, Lim SW, Lee YM, Lee HS and Kim DK:
Polysaccharide isolated from Triticum aestivum stimulates insulin
release from pancreatic cells via the ATP-sensitive K+ channel. Int
J Mol Med. 29:913–919. 2012.PubMed/NCBI
|
|
12
|
Lee SH, Lee YM, Lee HS and Kim DK:
Anti-oxidative and anti-hyperglycemia effects of Triticum
aestivumwheat sprout water extracts on the streptozotocin-induced
diabetic mice. Korean J Pharmacogn. 40:408–414. 2009.
|
|
13
|
Lee SH, Lim SW, Lee YM, Hur JM, Lee HS and
Kim DK: Anti-diabetic effects of Triticum aestivum L. water
extracts in db/db mice as an animal model of diabetes mellitus type
II. Korean J Pharmacogn. 41:282–288. 2010.
|
|
14
|
Lee SH, Xin M, Luyen BTT, et al:
Inhibitory effect of Triticum aestivum ethanol extract on lipid
accumulation in 3T3-L1 preadi-pocytes. Yakhak Hoeji. 55:478–484.
2011.
|
|
15
|
Lee SH, Lim SW, Lee YM, Lee SH and Kim DK:
Inhibitory effects of Triticum aestivum L. extracts on liver lipid
accumulation in high fat-fed mice. Korean J Pharmacogn. 42:309–316.
2011.
|
|
16
|
Lee SH, Lim SW, Mihn NV, et al:
Administration of Triticum aestivum water extracts reduce the level
of blood glucose and cholesterol in leptin deficient ob/ob mice. J
Korean Soc Food Sci Nutr. 40:401–408. 2011. View Article : Google Scholar
|
|
17
|
Luyen BTT, Tai BH, Thao NP, Cha JY, Lee YM
and Kim YH: A new phenolic component from Triticum aestivum sprouts
and its effects on LPS-stimulated production of nitric oxide and
TNF-alpha in RAW 264.7 cells. Phytother Res. 28:1064–1070. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wojakowska A, Perkowski J, Goral T and
Stobiecki M: Structural characterization of flavanoid glycosides
from leaves of wheat (Triticum aextivum L.) using LC/MS/MS
profiling of the target compounds. J Mass Spectrom. 48:329–339.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Poudel B, Lim SW, Ki HH, Nepali S, Lee YM
and Kim DK: Dioscin inhibits adipogenesis through the AMPK/MAPK
pathway in 3T3-L1 cells and modulates fat accumulation in obese
mice. Int J Mol Med. 34:1401–1408. 2014.PubMed/NCBI
|
|
20
|
Poudel B, Yoon DS, Lee JH, Lee YM and Kim
DK: Collagen I enhances functional activities of human
monocyte-derived dendritic cells via discoidin domain receptor 2.
Cell Immunol. 278:95–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morimoto C, Kameda K, Tsujita T and Okuda
H: Relationships between lipolysis I induced by various lipolytic
agents and hormone-sensitive lipase in rat fat cells. J Lipid Res.
42:120–127. 2001.PubMed/NCBI
|
|
22
|
Bennett MK, Lopez JM, Sanchez HB and
Osborne TF: Sterol regulation of fatty acid synthase promoter.
Coordinate feedback regulation of two major lipid pathways. J Biol
Chem. 270:25578–25583. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Claycombe KJ, Jones BH, Standridge MK, Guo
Y, Chun JT, Taylor JW and Moustaïd-Moussa N: Insulin increases
fatty acid synthase gene transcription in human adipocytes. Am J
Physiol. 274:1253–1259. 1998.
|
|
24
|
Schmid B, Rippmann JF, Tadayyon M and
Hamilton BS: Inhibition of fatty acid synthase prevents
preadipocyte differentiation. Biochem Biophys Res Commun.
328:1073–1082. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haunerland NH and Spener F: Fatty
acid-binding proteins-insights from genetic manipulations. Prog
Lipid Res. 43:328–349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chirala SS, Jayakumar A, Gu ZW and Wakil
SJ: Human fatty acid synthase: role of interdomain in the formation
of cata-lytically active synthase dimer. Proc Natl Acad Sci USA.
98:3104–3108. 2001. View Article : Google Scholar
|
|
27
|
Gonzales AM and Orlando RA: Role of
adipocyte-derived lipo-protein lipase in adipocyte hypertrophy.
Nutr Metab (Lond). 4:222007. View Article : Google Scholar
|
|
28
|
He Y, Li Y, Zhao T, Wang Y and Sun C:
Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through
LKB1/AMPK pathway. PLoS One. 8:e701352013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang XH, Huang B, Choi SK and See JS:
Anti-obesity effects of resveratrol-amplified grape skin extracts
on 3T3-L1 adipocytes differentiation. Nutr Res Pract. 6:286–293.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li J, Takaishi K, Cook W, McCorkle SK and
Unger RH: Insig-1 ‘brakes’ lipogenesis in adipocytes and inhibits
differentiation of preadipocytes. Proc Natl Acad Sci USA.
100:9476–9481. 2003. View Article : Google Scholar
|


















