Introduction
Detection and monitoring of hepatic injury after
warm ischaemia reperfusion (IR) is an important but difficult task
in the clinic. Measurement of serum activities of aspartate
aminotransferase (AST), alanine aminotransferase (ALT) and lactate
dehydrogenase (LDH) is the gold standard to evaluate hepatocellular
damage. However, interpretation of elevated serum activities of
AST, ALT and LDH may be difficult due to their occurrence in other
tissues, such as skeletal or heart muscle (1).
Characteristics of an ideal biomarker for clinical
use to detect hepatocellular injury involve organ specificity, fast
and extensive release into the serum, fast degradation in
vivo and high stability ex vivo to ensure early
detection of organ injury, fast recognition of therapy success and
unimpaired extended storage.
The potential role of circulating microRNAs (miRNAs)
as specific biomarkers of pathological conditions was first
described in several types of cancer, such as prostate cancer
(2) and B-cell lymphoma (3). Elevated levels of circulating miR-21,
miR-122 and miR-223 have been detected in patients with
hepatocellular carcinoma (HCC) (4,5) and
may aid in discriminating between patients with HCC and patients
with chronic hepatitis B or cirrhosis (6). Laterza et al (7) showed that miR-122 is exclusively
expressed in large quantities in hepatocytes. After application of
different toxicants, expression of serum miR-122 specifically
indicates liver cell injury with higher sensitivity compared with
histology and ALT activity without any interference with muscle
cell damage. A clinical study reported higher sensitivity of
circulating miR-122 in comparison to ALT activity as a biomarker of
toxic liver injury (8).
Furthermore, the increase in miR-122 after exposure to paraquat and
cholestatic liver injury correlated with the activity of ALT
(9,10).
Only a few studies have analysed alterations of
circulating miRNAs after hepatocellular injury by IR thus far. In a
porcine model of cardiogenic shock (11) hypothermia was shown to decrease
miR-122 serum expression, but not serum activity of ALT. In
patients with a liver transplant, serum miR-122 correlated with the
serum activity of AST and ALT (12). In a case of rejection of the
transplanted liver, serum expression of miR-122 increased earlier
than serum activity of AST and ALT and decreased faster after
resolved rejection (12). However,
the effect of exclusive warm IR on the levels of circulating
miR-122 and the degree of correlation with common markers of liver
injury, such as AST, ALT and LDH remains unknown.
Elevated levels of miR-21 are associated with
non-alcoholic fatty liver disease and biliary tract cancer
(13,14). Hepatocellular expression of miR-223
correlates with elevated serum levels of AST and AST after IR in
mice (15). However, whether this
correlates with an increase in circulating miR-223, has not been
investigated.
In blood samples, miRNAs are extremely stable and
are resistant to digestion, DNase treatment, freeze/thaw cycles,
boiling, low or high pH and extended storage (16,17).
This could possibly be explained by the finding, that serum miRNAs
are bound to high-density lipoprotein and thus, are protected
against digestion (18). In
addition, Turchinovic et al (19) stated, that a high quantity of
extracellular miRNAs is bound to Argonaute-proteins, which
stabilizes them for up to several months.
It was hypothesised that circulating miRNAs
correlate with the liver enzymes AST, ALT and LDH and may serve as
novel and improved biomarkers for liver injury after warm hepatic
IR. Therefore, the present study analysed the relative expression
of miR-122, miR-21 and miR-223 after hepatic IR in serum and liver
tissue and tested whether the serum miRNA expression was correlated
with the serum activity of liver enzymes. Furthermore, it was
analysed whether increased levels of circulating miRNAs are
associated with increased miRNA expression in liver tissue.
Materials and methods
Animal models
Animal maintenance and treatment were conducted in
accordance with the National Institute of Health Guide for Animal
Welfare. The local animal care and use committee approved this
study (State Agency for Nature, Environment and Consumer Protection
of North Rhine-Westphalia, Germany; reference number: 8.87–50.
10.34.09.017; approved by Dr. A. Blankenhorn on May 10, 2010). Male
Wistar rats (animals were obtained from breedings of the Central
Facility for Animal research and welfare, University Hospital,
Duesseldorf, Germany; 300±20 g body weight) were housed in a
controlled environment (temperature 25±1°C, humidity 50±10%, 12 h
light/12 h dark cycle) with access to food, until 16 h prior the
experiment, and water ad libitum. After induction of
anaesthesia with sodium pentobarbital (60 mg/kg intraperitoneally;
Rhone-Merieux, Laupheim, Germany), the trachea was intubated and
the rats were mechanically ventilated with a mixture of nitrogen
(70%) and oxygen (30%; Linde gas, Höllrieglskreuth, Germany). Vena
jugularis and a tail vein were cannulated for continuous
administration of pentobarbital (40 mg/kg/h) and saline infusion
(20 ml/kg/h; Jonosteril, Fresenius, Bad Homburg, Germany). Blood
gases were analysed via the arterial line of the left carotid
artery (Radiometer Medical ApS, Brønshsøj, Denmark) and ventilation
was adjusted to obtain normocapnia (temperature corrected
pCO2: 40 mmHg). The adequacy of anaesthesia was assessed
by arterial blood pressure and heart rate. The body temperature was
monitored using a rectal temperature probe (GHM Messtechnik GmbH,
Regenstauf, Germany) and was maintained between 36.5 and 37.5°C by
a heating plate (customized; connected with a heat exchanger; Dr R
Lauda, Wobser GmbH & Co., KG, Lauda-Königshofen, Germany)
After midline laparotomy, the portal triad including
the hepatic artery, the portal vein and the bile duct supplying the
middle and left hepatic lobes was prepared, as described previously
(20,21). Sham-operated animals received no
further treatment (n=5). In the IR group, partial hepatic ischaemia
(70%) was induced by clamping of the portal triad with an
atraumatic vascular clip (Aesculap, Tuttlingen, Germany). After 45
min of ischaemia, a 240 min reperfusion period was initiated by
removal of the clip (n=7).
At the end of the experiment, animals were
euthanized by exsanguination after an overdose of pentobarbital.
Liver tissue samples were harvested immediately and snap frozen in
liquid nitrogen and stored at −80°C for subsequent analysis. Blood
samples were incubated at room temperature for 30 min and
centrifuged twice at 13,000 × g for 5 min. Serum was stored at
−80°C until use.
Liver enzymes
Serum ALT, AST and LDH levels were measured by a
standard automated procedure using the method of activation of
pyridoxal-phosphate with subsequent photometric analysis (MODULAR
analyser, Roche Diagnostics, Mannheim, Germany). Values are
expressed as U/l.
Isolation of microRNAs from serum and
liver tissue
Circulating miRNAs were isolated with the miRNeasy
mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s
instructions. C. elegans miR-54 (5 μl) from a 5
fmol/μl stock were added to each sample (Applied Biosystems,
Carlsbad, CA, USA). RNA from liver tissue was isolated using
TRIzol® (Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s instructions. RNA purity and concentration
were assessed by spectophotometry at 260 and 280 nm (NanoDrop
Products, Wilmington, DE, USA).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) analysis
Reverse transcription of the total RNA into cDNA was
performed using the High Capacity RNA-to-cDNA transcription kit
(Applied Biosystems), according to the manufacturer’s instructions.
The RT-qPCR assays for rno-miR-122 (Assay ID: 002245), rno-miR-21
(Assay ID: 000397), rno-miR-223 (Assay ID: 000526), cel-mir-54
(Assay ID: 001361) and U6 (Assay ID: 001973) were purchased from
Applied Biosystems and were performed, according to the
manufacturer’s instructions. RT-qPCR was performed according to the
manufacturer’s instructions on a 7300 Real-Time RT-qPCR system
(Applied Biosystems). The RT-qPCR conditions were as follows: 50°C
for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 sec and
60°C for 60 sec.
The relative expression level of serum miRNAs was
normalised to the relative expression level of miR-54 C.
elegans, expression of liver miRNAs was normalised to U6 (Assay
ID: 001973). The relative expression of miRNAs was calculated using
the 2−ΔΔCt equation (22).
Statistical analysis
Statistical calculations were performed using
GraphPad Prism 5 software (GraphPad Software Inc, La Jolla, CA,
USA). To compare liver enzymes of sham and IR, Student’s t-test was
performed. Comparison of the relative expression of miR-122, -21
and -223 in serum and liver (sham vs. IR) was performed by the
Relative Expression Software Tool (REST, version 2.0.13). To
calculate the degree of correlation between the liver enzymes AST,
ALT, LDH and the relative expression of miRNAs after IR, values
were tested by linear regression analysis after logarithmic
transformation (x=lnx; y=lny). P<0.05 was considered to indicate
a statistically significant difference. Statistical analysis was
reviewed and approved by an independent statistician (Dr Pablo E.
Verde, Coordination Centre for Clinical Studies, University
Hospital Düsseldorf).
Results
Serum activity of liver enzymes after
IR
To determine the severity of hepatocellular injury
after IR, serum activity of AST, ALT and LDH were quantified. Serum
activity of AST (IR: 995.4±523.7 vs. sham: 105.8±23. U/l;
P<0.05) and ALT (IR: 840.9±339.1 vs. sham: 30±3 U/l, P<0.05)
were significantly elevated, whereas the increase of LDH serum
activity did not reach a level of significance (1836±2091 vs.
741.4±437.3 U/l; P=0.28) (Fig.
1).
Relative expression of miR-122, -21 and
-223 in serum and liver
The levels of circulating miR-122 (93.65±139-fold,
P=0.001) and miR-21 (6.72±8.8-fold, P=0.01) were increased after
IR, while the level of miR-223 (0.99-fold±0.45, P=0.97) remained
unchanged (Fig. 2A). Relative
expression of miR-122 (0.69±0.38-fold, P=0.35) and miR-21
(1.62±0.63-fold, P=0.11) in liver tissue remained unchanged after
IR, whereas IR induced an increase of miR-223 in liver tissue after
IR compared with the sham group (1.99±0.83-fold, P=0.03) (Fig. 2B).
Correlation of relative expression of
circulating and hepatic miR-122, -21 and -223 with liver enzymes
AST, ALT and LDH
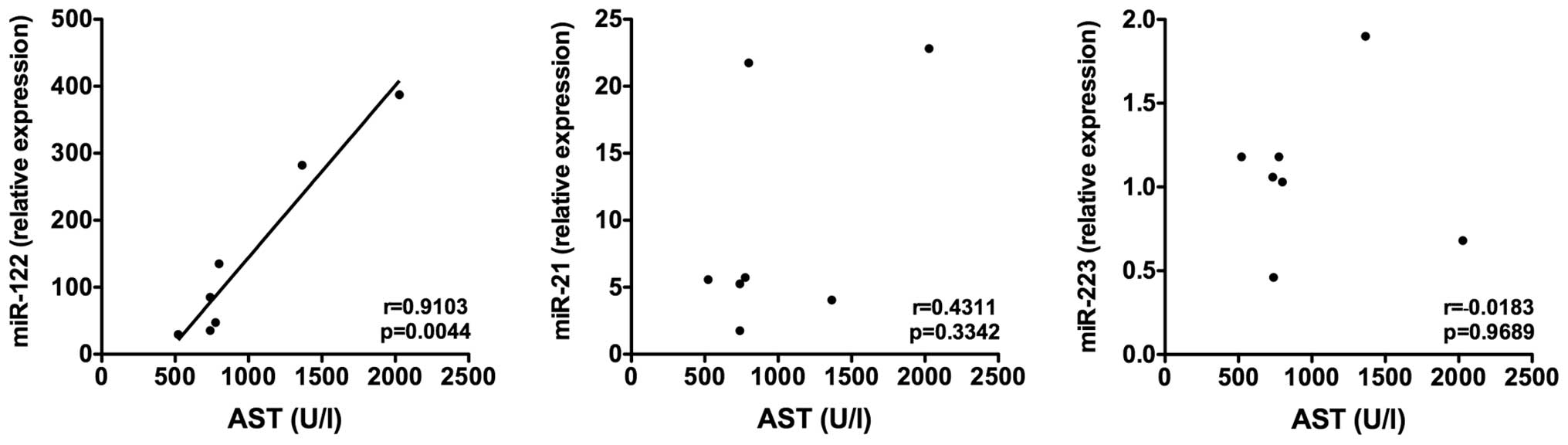
The serum expression of miR-122 strongly correlated
with serum activity of AST, ALT and LDH (AST, r=0.9103, P=0.0044;
ALT, r=0.8323, P=0.02; LDH, r=0.8772, P=0.0095). No correlation
with the liver enzymes was detected for circulating miR-21 and
miR-223 (Fig. 3). Relative hepatic
expression levels of the investigated miRNAs did not correlate with
the serum activity of AST, ALT and LDH (Fig. 4).
Discussion
In the present study, it was investigated whether
circulating miR-122, -21 or -223 could serve as potential
indicators of hepatocellular IR injury. Therefore serum was
analysed and hepatocellular expression levels of these miRNAs after
partial hepatic IR and were tested to determine whether their
expression levels correlated with the activity of the established
enzyme markers of hepatocellular injury AST, ALT and LDH in serum
and liver tissue.
miR-122 represents a promising candidate in the
context of hepatic IR, as it is highly and exclusively expressed in
the liver (23). In the present
study, the relative expression of circulating miR-122 increased
after IR and was correlated with the serum activities of liver
enzymes. This result is in line with previous studies, showing
correlations between ALT and plasma miR-122 after toxic (7,24) or
viral (25,26) liver injury. The potential role of
circulating miR-122 as a biomarker of acute hepatic IR was first
investigated by Andersson et al (11). In a porcine model of cardiogenic
shock they showed a strong increase in circulating miR-122. The
relative serum expression of miR-122 and the serum activity of ALT
correlated with hemodynamic parameters in shock and no correlation
between the relative expression of circulating miR-122 and the
serum activity of ALT was observed. Therefore, the present results
provide the first evidence for a correlation between circulating
miR-122 and serum activity of ALT, AST and LDH after warm hepatic
IR. However, elevated levels of circulating miR-122 following
hepatic IR may be caused by an increased level of expression of
miR-122 in the liver. This would in general reduce the suitability
of this miRNA as a potential biomarker. Therefore, the expression
of miR-122 in liver tissue was analysed and it was found that
miR-122 expression was unchanged following IR. This is a novel
finding, as the influence of liver IR on hepatocyte expression
levels of miR-122 has not been previously determined and confirms
that elevated circulating miR-122 levels following hepatic IR are
not influenced by a higher hepatic expression of this miRNA. An
advantage of circulating miR-122 in comparison to ALT/AST could be
the faster onset of elevation. Farid et al showed that an
elevation of circulating miR-122 occurred earlier than an elevation
of AST and ALT in an acute rejection of transplanted livers
(12). Additionally, the miR-122
level dropped more quickly after the initiation of a glucocorticoid
therapy in these patients (27).
The present study aimed to identify other miRNAs
than miR-122 that could also be indicators of hepatic IR. Recent
studies have described miR-21 as a potential biomarker of
non-alcoholic fatty liver disease, hepatocellular cancer (27) and chronic type B hepatitis
(27). The present study shows
that the relative expression of miR-21 in serum increased
significantly after IR, while the expression of miR-21 was
unaffected in liver tissue compared with sham. In contrast to
miR-122, the elevated level of circulating miR-21 did not correlate
with the activity of liver enzymes. This may be due to the lower
expression level of miR-21 compared with miR-122 in liver tissue
(28), which leads to a decreased
release of miR-21 following hepatocellular damage.
Another promising miRNA for quantification of
hepatic injury after IR is miR-223. Yu et al showed in a
murine in vivo model of hepatic IR an increase in the
expression level of miR-223 in liver tissue (15). Furthermore, relative hepatic
expression of miR-223 correlated well with ALT and AST activity in
the context of IR. However, the expression of circulating miR-223
was not analysed. In the present study these findings of elevated
expression of miR-223 in the liver after IR were confirmed;
however, no correlation with the serum activity of liver enzymes
could be detected. In addition, the level of circulating miR-223
remained unchanged. The role of miR-223 in the context of hepatic
IR is unclear. Myeloid cells show a high expression of miR-223 with
granulocytes expressing the highest levels (29). Therefore, the elevated level of
miR-223 in the liver following IR most likely reflects the invasion
of inflammatory cells, particularly granulocytes (30).
In conclusion, it was demonstrated that circulating
miR-122 levels are elevated after exclusive warm hepatic IR.
Furthermore, the level of circulating miR-122 correlates with ALT,
AST and LDH levels and therefore represents a promising candidate
biomarker of liver injury following warm IR.
Acknowledgments
The authors would like to thank Dr Carmen Barthuber, Mrs. Yvonne Grueber and Mrs. Antje Nebert for helpful assistance with the study. They would also like to acknowledge the statistical review and approvement of Dr Pablo E. Verde, Coordination Centre for Clinical Studies, University Hospital Duesseldorf. The work was funded by a starter grant of the Research Committee of the Medical Faculty of the Heinrich-Heine-University Düsseldorf.
References
|
1
|
Ozer J, Ratner M, Shaw M, Bailey W and
Schomaker S: The current state of serum biomarkers of
hepatotoxicity. Toxicology. 245:194–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitchell PS, Parkin RK, Kroh EM, et al:
Circulating microRNAs as stable blood-based markers for cancer
detection. Proc Natl Acad Sci USA. 105:10513–10518. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lawrie CH, Gal S, Dunlop HM, et al:
Detection of elevated levels of tumour-associated microRNAs in
serum of patients with diffuse large B-cell lymphoma. Br J
Haematol. 141:672–675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu J, Wu C, Che X, et al: Circulating
microRNAs, miR-21, miR-122 and miR-223, in patients with
hepatocellular carcinoma or chronic hepatitis. Mol Carcinog.
50:136–142. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomimaru Y, Eguchi H, Nagano H, et al:
Circulating microRNA-21 as a novel biomarker for hepatocellular
carcinoma. J Hepatol. 56:167–175. 2012. View Article : Google Scholar
|
|
6
|
Zhou J, Yu L, Gao X, et al: Plasma
microRNA panel to diagnose hepatitis B virus-related hepatocellular
carcinoma. J Clin Oncol. 29:4781–4788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laterza OF, Lim L, Garrett-Engele PW, et
al: Plasma MicroRNAs as sensitive and specific biomarkers of tissue
injury. Clin Chem. 55:1977–1983. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Antoine DJ, Dear JW, Lewis PS, et al:
Mechanistic biomarkers provide early and sensitive detection of
acetaminophen-induced acute liver injury at first presentation to
hospital. Hepatology. 58:777–787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding X, Ding J, Ning J, et al: Circulating
microRNA-122 as a potential biomarker for liver injury. Mol Med
Rep. 5:1428–1432. 2012.PubMed/NCBI
|
|
10
|
Shifeng H, Danni W, Pu C, Ping Y, Ju C and
Liping Z: Circulating liver-specific miR-122 as a novel potential
biomarker for diagnosis of cholestatic liver injury. PLoS One.
8:e731332013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andersson P, Gidlöf O, Braun OO, Götberg
M, van der Pals J, Olde B and Erlinge D: Plasma levels of
liver-specific miR-122 is massively increased in a porcine
cardiogenic shock model and attenuated by hypothermia. Shock.
37:234–238. 2012. View Article : Google Scholar
|
|
12
|
Farid WR, Pan Q, van der Meer AJ, et al:
Hepatocyte-derived microRNAs as serum biomarkers of hepatic injury
and rejection after liver transplantation. Liver Transpl.
18:290–297. 2012. View
Article : Google Scholar
|
|
13
|
Yamada H, Suzuki K, Ichino N, et al:
Associations between circulating microRNAs (miR-21, miR-34a,
miR-122 and miR-451) and non-alcoholic fatty liver. Clin Chim Acta.
424:99–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kishimoto T, Eguchi H, Nagano H, et al:
Plasma miR-21 is a novel diagnostic biomarker for biliary tract
cancer. Cancer Sci. 104:1626–1631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu CH, Xu CF and Li YM: Association of
MicroRNA-223 expression with hepatic ischemia/reperfusion injury in
mice. Dig Dis Sci. 54:2362–2366. 2009. View Article : Google Scholar
|
|
16
|
Chen X, Ba Y, Ma L, et al:
Characterization of microRNAs in serum: a novel class of biomarkers
for diagnosis of cancer and other diseases. Cell Res. 18:997–1006.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Jiang Z, Xu L, Yao H, Guo J and Ding
X: Stability analysis of liver cancer-related microRNAs. Acta
Biochim Biophys Sin (Shanghai). 43:69–78. 2011. View Article : Google Scholar
|
|
18
|
Vickers KC, Palmisano BT, Shoucri BM,
Shamburek RD and Remaley AT: MicroRNAs are transported in plasma
and delivered to recipient cells by high-density lipoproteins. Nat
Cell Biol. 13:423–433. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Turchinovich A, Weiz L, Langheinz A and
Burwinkel B: Characterization of extracellular circulating
microRNA. Nucleic Acids Res. 39:7223–7233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmidt R, Tritschler E, Hoetzel A, et al:
Heme oxygenase-1 induction by the clinically used anesthetic
isoflurane protects rat livers from ischemia/reperfusion injury.
Ann Surg. 245:931–942. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Braun S, Plitzko G, Bicknell L, et al:
Pretreatment with helium does not attenuate liver injury after warm
ischemia reperfusion. Shock. 41:413–419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific microRNAs from mouse. Curr Biol. 12:735–739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang K, Zhang S, Marzolf B, et al:
Circulating microRNAs, potential biomarkers for drug-induced liver
injury. Proc Natl Acad Sci USA. 106:4402–4407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Waidmann O, Bihrer V, Pleli T, et al:
Serum microRNA-122 levels in different groups of patients with
chronic hepatitis B virus infection. J Viral Hepat. 19:e58–e65.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cermelli S, Ruggieri A, Marrero JA,
Ioannou GN and Beretta L: Circulating microRNAs in patients with
chronic hepatitis C and non-alcoholic fatty liver disease. PLoS
One. 6:e239372011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu J, Wu C, Che X, et al: Circulating
MicroRNAs, miR-21, miR-122 and miR-223, in patients with
hepatocellular carcinoma or chronic hepatitis. Mol Carcinog.
50:136–142. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Landgraf P, Rusu M, Sheridan R, et al: A
mammalian microRNA expression atlas based on small RNA library
sequencing. Cell. 129:1401–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramkissoon SH, Mainwaring LA, Ogasawara Y,
et al: Hematopoietic-specific microRNA expression in human cells.
Leuk Res. 30:643–647. 2006. View Article : Google Scholar
|
|
30
|
Honda M, Takeichi T, Asonuma K, Tanaka K,
Kusunoki M and Inomata Y: Intravital imaging of neutrophil
recruitment in hepatic ischemia-reperfusion injury in mice.
Transplantation. 95:551–558. 2013. View Article : Google Scholar : PubMed/NCBI
|