Introduction
Parkinson’s disease (PD) is one of the most common
degenerative diseases of the nervous system. The major pathological
change observed in PD is degeneration and necrosis of the
dopaminergic neurons of the substantia nigra, which results in a
decrease in dopamine synthesis (1). The low dopamine level disrupts the
balance between the dopaminergic and cholinergic activity that is
required for normal movement, which accounts for a series of
clinical symptoms, including tremor, muscle stiffness and slowness
of movement. Genetic and environmental factors contribute to this
disease. While the majority of cases of PD are sporadic, certain
cases occur as a result of genetic factors and are associated with
certain gene mutations, including parkin (2), α-synuclein (α-Syn) and leucine-rich
repeat kinase 2 (3,4). Lewy bodies (LBs) are a common feature
of hereditary and idiopathic PD. α-Syn, the main component of LBs,
is encoded by SNCA. Point mutations in SNCA, such as A30P and A53T,
may cause familial cases of PD (5,6).
α-Syn misexpression has been experimentally demonstrated to mimic
several aspects of PD in transgenic animals, including motor
dysfunction, α-Syn aggregation and neurodegeneration (7–9). At
present, several molecular mechanisms have been proposed to explain
the causes of PD, including misfolding and aggregation of α-Syn,
posttranslational modifications of α-Syn and oxidative stress
(10).
In addition to motor dysfunction, numerous patients
with PD have non-motor symptoms, including depression, sleep
disorders, dementia, autonomic dysfunction, hyposmia and
gastrointestinal symptoms (11).
Previous investigations have demonstrated that a specific
cholinergic neurotransmitter defect in patients with senile
dementia contributed significant damage to cholinergic neurons.
This observation suggests a close association between the
cholinergic system and cognitive function in the human brain. As a
critical cognitive function, memory has been used to demonstrate
that synaptic plasticity in the Drosophila melanogaster
mushroom body neurons is critical for learning and memory of
olfactory stimuli (12). The
projection neurons (PNs) in the antennal lobe of the mushroom body
are cholinergic and neurotransmission at the synapse conducts
messages in the form of electrical activity (13). Voltage-gated calcium channels (CAC)
are located at the plasma membrane of nerve terminals and are
crucial for the process of synaptic transmission and neuronal
communication. Cav2-type calcium channels are encoded by
cac and regulate action potential (AP)-independent neurotransmitter
release at cholinergic synapses in the adult Drosophila
brain (14). While certain details
have been determined concerning the molecular mechanisms of PD,
direct evidence for the association between α-Syn and cholinergic
transmission remains to be demonstrated. The aim of the present
study was to use Drosophila as a model organism to observe
whether α-Syn affects cholinergic neuronal transmission and
consequentially alters their learning and memory capabilities.
Materials and methods
Drosophila strains
Drosophila stocks were reared on standard
cornmeal agar medium supplemented with dry yeast for 24 h at 60%
relative humidity. α-Syn-expressing Drosophila were modified
by microinjection and expression of A53T α-Syn transgenes was
driven by the elav-GAL4 expression system. In brief, the α-Syn
clone was constructed using the elav-GAL4 expression system, then
this was injected into the eggs of white eye Drosophila
(control flies). After hybridization of the red eye flies, α-Syn
flies were created. Elav-GAL4 flies were crossed with UAS-A53T
flies (provided by the Biochemistry and Cell Institute of Shanghai
Life Science Research Institute of the Chinese Academy of Sciences,
Shanghai, China), to produce the A53T flies.
Western blot analysis
The cerebral proteins of α-Syn Drosophila
brains were extracted from tissue lysate with the protease
inhibitor phenylmethylsulfonyl fluoride (Sigma-Aldrich, St. Louis,
MO, USA). The solution was sonicated and ultracentrifuged for 30
min at 4695 × g at below 4°C. The resulting supernatant was boiled
for 15 min in a water bath. Adult fly heads of the appropriate
genotypes were prepared and analyzed using standard SDS-PAGE with
10% Tris-glycine gradient gels. The primary α-Syn antibody (cat.
no. 610786) was developed by BD Biosciences (Mountain View, CA,
USA) and was diluted to 1:1,000 prior to use. The goat anti-mouse
secondary antibody (cat. no. 81-6511; Thermo Fisher Scientific, San
Francisco, CA, USA) was then diluted to 1:10,000 and applied, and
an X-ray film exposure was conducted.
Climbing assays
Flies were divided into random groups of 20–30
individuals per vial and then assessed for geotaxis as described
previously (8). Briefly, groups of
10 flies were placed in a 95×27 mm empty vial. Flies were gently
taped to the bottom of the vial and the number of flies crossing an
8 cm mark was recorded after 10 sec. Researchers were blinded to
the corresponding genotype and condition of the flies.
T maze test
Hungry flies (80–100), which had been in the dark
for 30 min prior to the experiment were placed in the maze. After
90 sec, smell A was released in one side of the T maze and at the
same time, twelve 1.5 sec of the 70 volt DC electrical stimulation
was applied over 60 sec. Flies were then allowed to breathe air for
45 sec. Subsequently, smell B was released for 60 sec in the other
side of the T maze followed by 45 sec air. The flies were then
allowed to move around the maze freely for 2 min, after which the
numbers of flies at the sides of the maze with smells A and B were
counted.
Immunofluorescence
The flies brains were stripped and placed on a
slide. The slide was rinsed with 1% PBS (Sigma-Aldrich) three
times, blocked and incubated in a blocking buffer (0.1 M PBS, 0.1%
Triton X-100 and 1% BSA; Sigma-Aldrich) for 3 h at room
temperature. The α-synuclein antibody (1:250; 610786; BD
Biosciences) was then applied and washed by PBS for 5 min, three
times. Subsequently, the rabbit anti-human IgG secondary antibody
(BA1020; Boster, Wuhan, China) was applied. Following incubation,
the brain was washed three times with PBS with 5 min intervals and
fixed with a coverslip. The brains of flies were observed with an
immunofluorescence laser scanning confocal microscope (Zeiss
LSM710; Carl Zeiss, Oberkochen, Germany).
Biocytin staining and
immunohistochemistry images
In order to identify and confirm that the cells
recorded were PNs, cells recorded were stained by biocytin
(Sigma-Aldrich). While recording, cells were injected with biocytin
through a recording pipette filled with internal solution
containing biocytin in whole-cell recording mode for a minimum of
30 min. After recording, the brains were collected and fixed in 4%
formaldehyde (Sigma-Aldrich) in phosphate-buffered saline (PBS;
Sigma-Aldrich) at 4°C for 10 h. The brain was then rinsed in 1% PBS
three times, blocked and incubated in a blocking buffer (0.1 M PBS,
0.1% Triton X-100, and 1% bovine serum albumin; Sigma-Aldrich)
containing streptavidin-Cy3 (Molecular Devices Ltd., Wokingham, UK)
for 3 h at room temperature. After incubation, the brain was washed
three times with 5 min intervals in PBS. A BX51WI microscope
(Olympus, Tokyo, Japan) with a ×40 objective and confocal camera
was used to capture images of dendritic arborization of the PNs in
the antennal lobe.
Immunohistochemistry
Adult Drosophila brains were fixed in 4%
paraformaldehyde and embedded in paraffin. The paraffin-embedded
brains were then cut into sections. The α-Syn antibody (1:250; BD
Biosciences) was used to detect the expression of α-Syn.
Electrophysiological recordings from PNs
in isolated whole brain
All brains were obtained from female flies two days
prior to eclosion. The entire brain, including optic lobes, was
removed from the head and prepared for recordings in standard
external solution containing 20 U/ml papain (Sigma-Aldrich) with 1
mM l-cysteine. Subsequently, the dissected brains were mounted in
an RC-26 perfusion chamber (Sigma-Aldrich) containing the recording
solution bubbled with 95% O2 and 5% CO2 (2
ml/min) throughout the experiments, with the ventral surface of the
brain facing up. Pipettes were targeted to PNs in the dorsal neuron
cluster in the antennal lobe. For measurements of cholinergic
miniature excitatory postsynaptic currents (mEPSCs), tetrodotoxin
(TTX; 1 μM) was added to the external solution to block
voltage-gated sodium currents and γ-aminobutyric (GABA)ergic
synaptic currents, and picrotoxin (PTX; 10 μM;
Sigma-Aldrich) was added to block GABAergic synaptic currents.
CaCl2 was omitted and tetraethylammonium (TEA; 10 mM;
Sigma-Aldrich) and 4-aminopyridine (4-AP; 1 mM) were added to the
external solution to measure Ca2+ currents. For
Ca2+ current measurements, the external solution
consisted of 101 mM NaCl, 1.8 mM CaCl2, 0.8 mM
MgCl2, 5.4 mM KCl, 5 mM glucose, 1.25 mM
NaH2PO4, 20.7 mM NaHCO3, 1
μM TTX, 10 mM TEA and 1 mM 4-AP.
Statistical analysis
Statistical analysis was conducted using SPSS
software, version 11.5 (SPSS, Inc., Chicago, IL, USA) Comparison of
the magnitude of the calcium currents prior to and following
application of permethrin was made using an unpaired t-test.
Comparison of the area below the baseline of sodium currents prior
to and following application of permethrin was made using a paired
t-test. Comparisons of more than two treatments were made with
analysis of variance (ANOVA) and the Bonferroni post hoc
correction.
Results
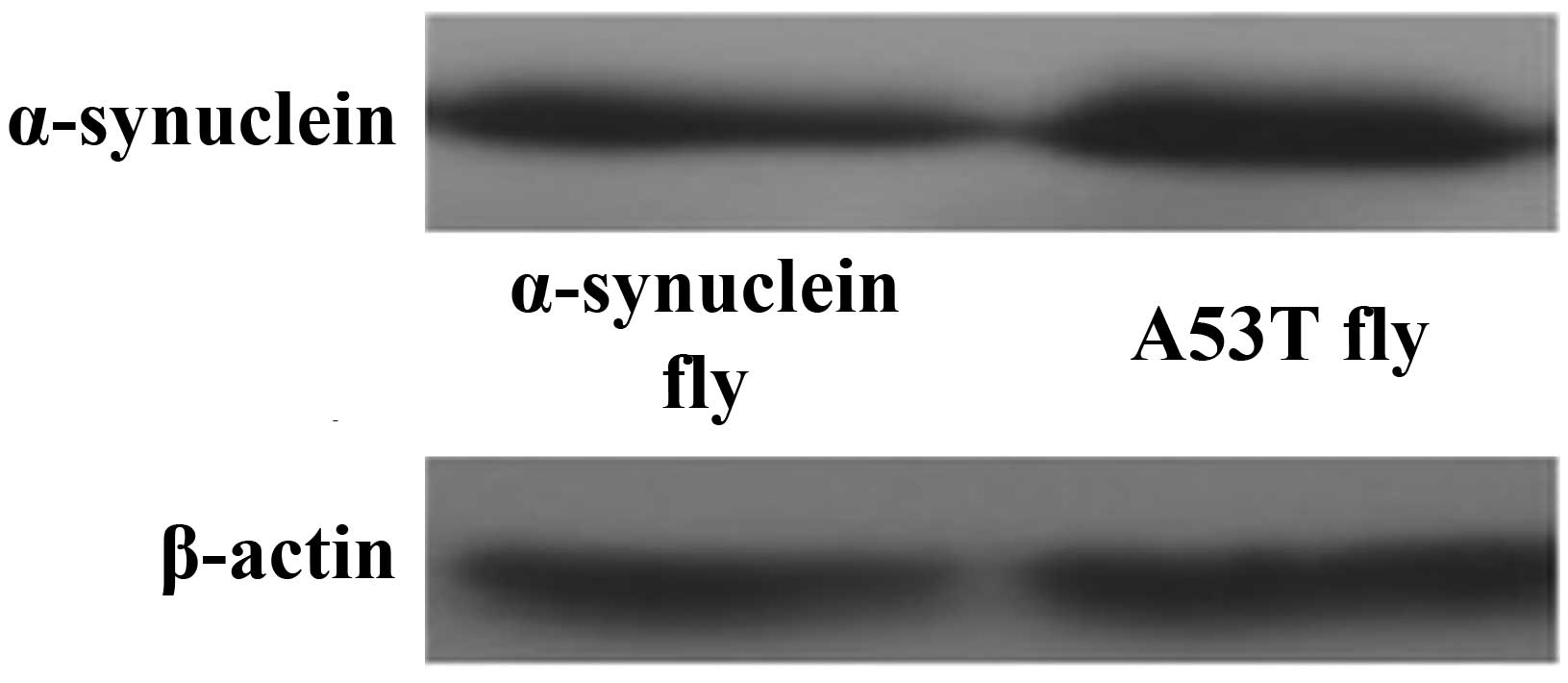
Expression of α-Syn protein
The pan-neuronal driver elav-GAL4 was used to drive
expression of normal and mutant (A53T) human α-Syn throughout the
nervous system. Western blot analysis was performed to verify the
expression of α-Syn (Fig. 1).
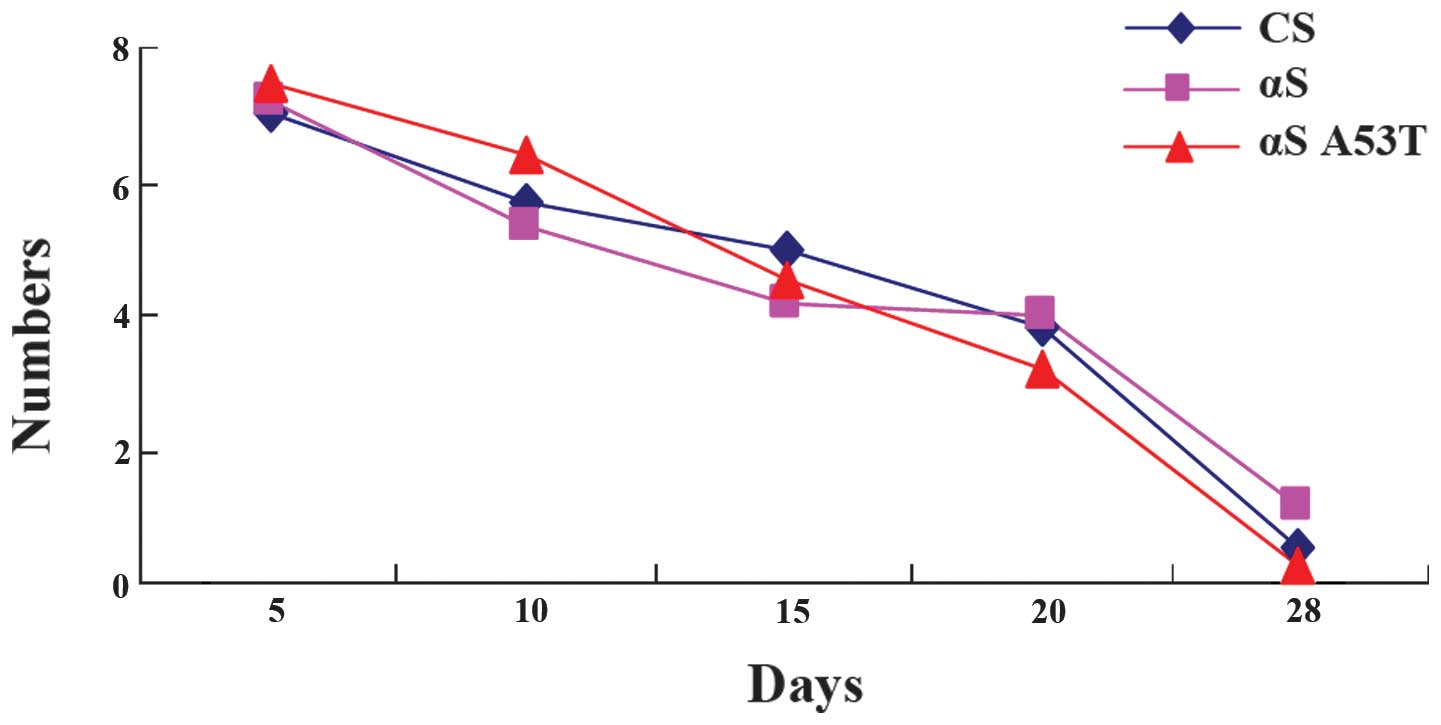
No differences are present in locomotor
ability of α-Syn Drosophila
Previous studies have demonstrated that a decrease
in the ability of flies to climb up the wall of a plastic vial is
associated with the progression of the pathology in PD (15,16).
However, in the present study, no significant decrease in climbing
ability was observed (Fig. 2).
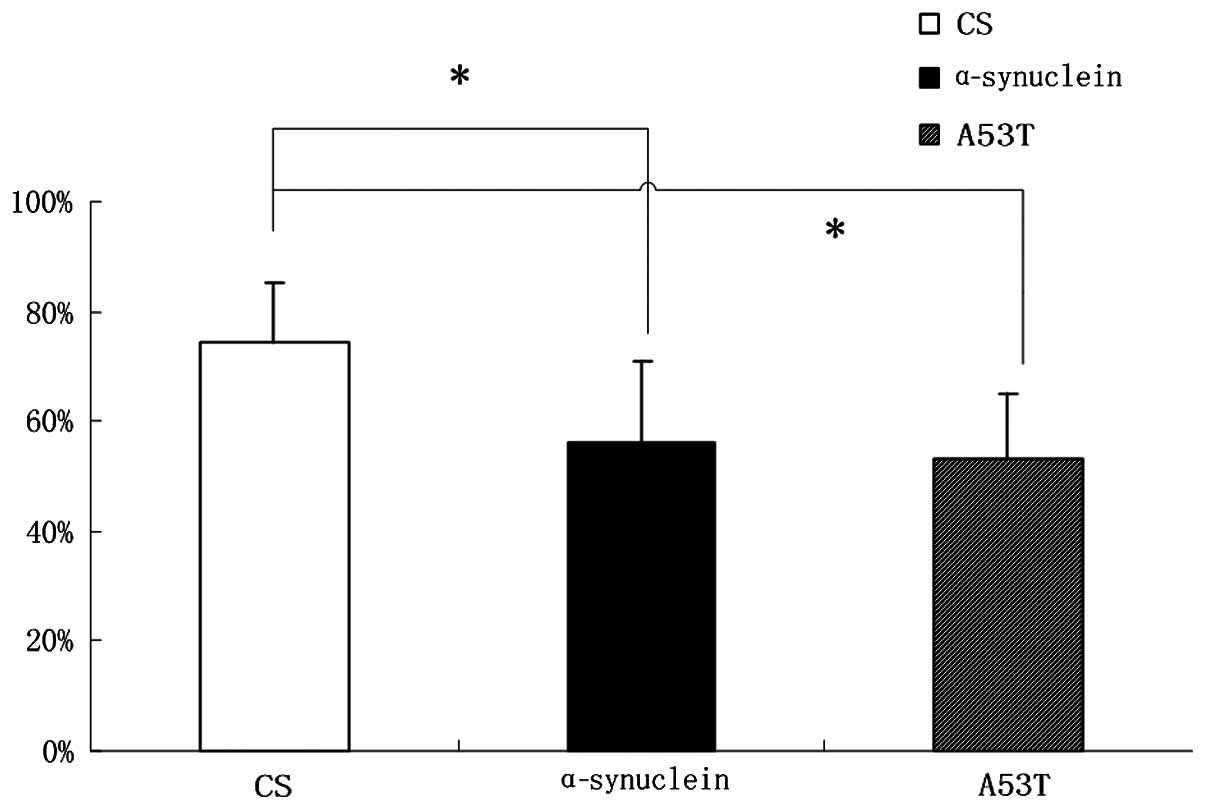
Learning and memory skills are reduced in
α-Syn Drosophila
Compared with CS flies, the learning and memory
ability of α-Syn wild-type and A53T mutation flies decreased
exhibiting a reduction of ~18% (Fig.
3) as elucidated through a T maze experiment.
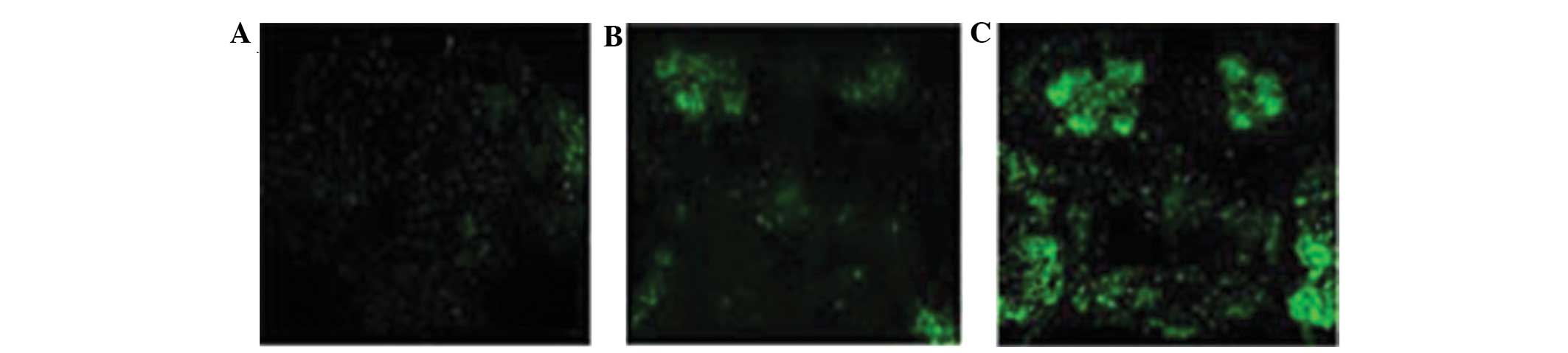
Immunofluorescence analysis of α-Syn in
Drosophila
The whole brain of the Drosophila were
observed with an immunofluorescence laser scanning confocal
microscopy, and it was identified that the levels of green
fluorescence were greater in the α-Syn Drosophila compared
with the control saline group. In addition, green fluorescence was
particularly evident in the mushroom body (Fig. 4).
mEPSC frequency is decreased in α-Syn
Drosophila
Previous studies have demonstrated that cac-encoded
voltage-gated calcium channels are important in regulating the
spontaneous release of neurotransmitter at excitatory cholinergic
synapses and mEPSC frequency in the PNs of Drosophila
(7,17,18).
Recordings were performed at room temperature for a minimum of 1
min in the presence of TTX to block sodium channels and PTX to
block GABA receptors. The dorsal antennal lobe glomeruli is the
location of the PNs, with two main branches projecting to the
lateral horn and the local mushroom body. The PNs were identified
morphologically from biocytin fills and their specific electrical
activities (Fig. 5). The PNs were
selected for analysis as they were cholinergic and cholinoceptive.
PNs firing spontaneously were recorded in α-Syn Drosophila
in the presence of TTX and PTX. The experiments indicated that the
frequency of PNs was reduced. Sodium AP-independent mEPSCs in the
antennal lobe PNs were monitored in the whole brain, which was
isolated from female fly pupae two days prior to eclosion. mEPSCs
were directly monitored by whole cell patch-clamp recordings from
PNs. The average mEPSC frequency was 1.92±0.3 Hz under normal
conditions. The average mEPSC frequency decreased significantly by
0.62±0.11 Hz in the α-Syn Drosophila. The present results
demonstrated that the mEPSC frequency in the α-Syn
Drosophila was decreased (Fig.
6).
Cholinergic mEPSCs were recorded from single PNs
following the addition of TTX and PTX to the external solution. The
mean frequency of mEPSCs was significantly reduced by α-Syn (ANOVA,
Bonferroni post hoc test; P<0.01). No significant difference was
identified between the mean mEPSC amplitude of the α-Syn and A53T
groups and that of the CS group.
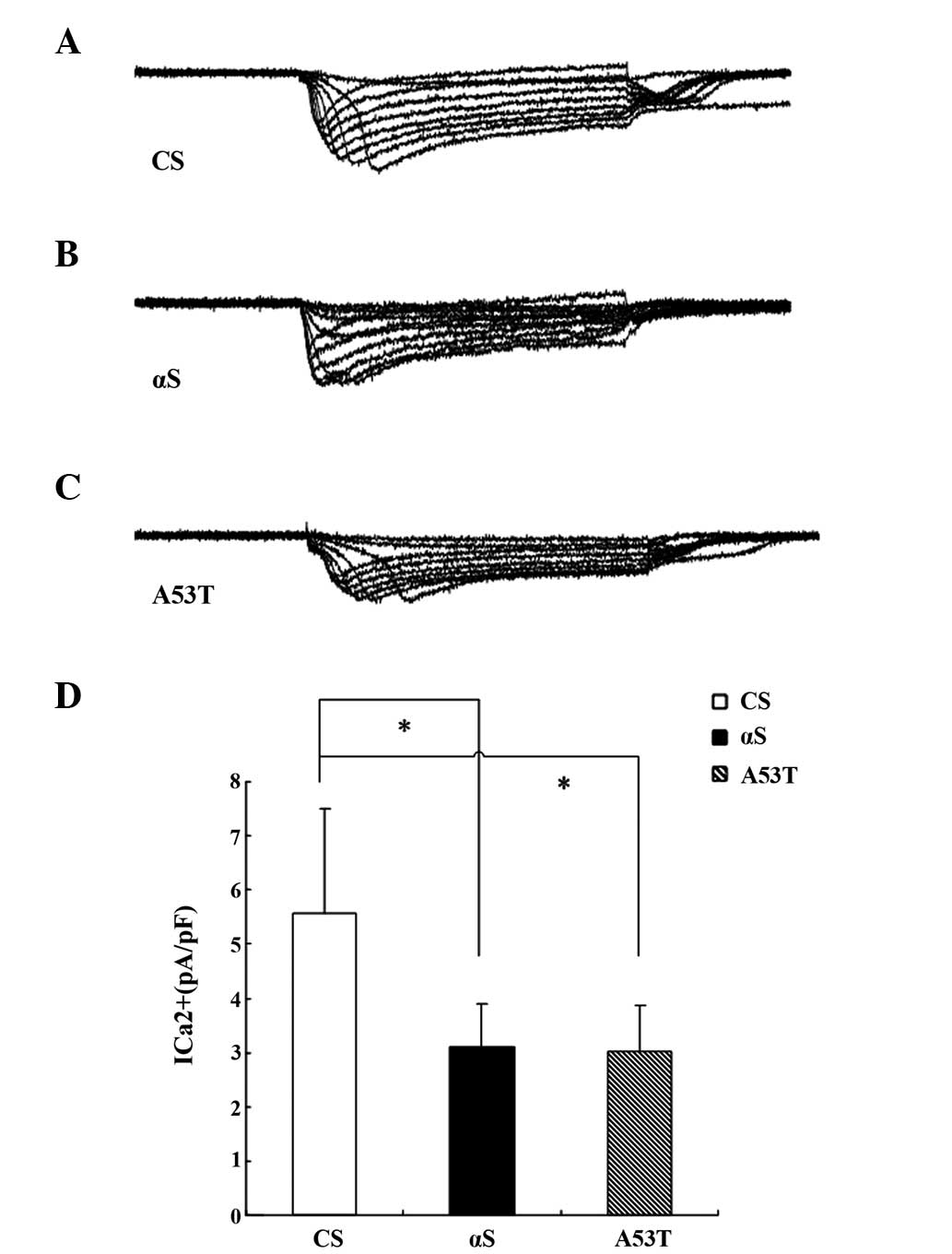
Calcium currents of PNs in brains of
α-Syn Drosophila pupae two days prior to eclosion
Calcium currents have been revealed to have broad
effects on neuronal function, particularly in presynaptic terminals
for neurotransmitter release, electrical excitability and
activity-dependent gene regulation (19,20).
Calcium currents were directly calculated in antennal lobe PNs
isolated from the whole brain of fly pupae two days prior to
eclosion. Using whole cell voltage-clamp recordings, the inward
calcium current was isolated in PNs as previously described
(21). The average calcium current
in normal PNs and α-Syn PNs were 5.57±1.94 and 3.03±0.85 pA/pF,
respectively (Fig. 7).
Discussion
Drosophila are an ideal model for
investigating the association between PD and Alzheimer’s disease
(AD) as transgenic techniques can be used to express human α-Syn in
the CNS of Drosophila. The abundant expression of
acetylcholine and GABA throughout the Drosophila CNS
suggested that these classic neurotransmitters have a major role in
mediating fast synaptic transmission (22). The PNs were selected for analysis
as they are cholinergic and cholinoceptive, receiving cholinergic
synaptic input from olfactory receptor neurons and potentially
lateral excitatory input (13).
The preparations used in the current study and described in a
previous study (23) allowed
assessment of the cholinergic synaptic transmission in PNs in an
isolated whole brain. Cholinergic currents were recorded using a
whole-cell patch clamp at room temperature following treatment with
TTX for at least 1 min to block sodium channels and PTX to block
GABA receptors. The neurons were identified as cholinergic and
mediated by nicotinic acetylcholine receptors based on the finding
that the majority of the mEPSCs recorded could be inhibited by a
competitive antagonist of the nicotinic acetylcholine receptor at
the neuromuscular junction termed curare, which is classified as a
long-duration (24),
non-depolarizing neuromuscular blocking agent. A number of studies
have revealed that the morbidity associated with senile dementia is
more prevalent in PD patients and cholinergic neurons exhibit a
critical role in AD. A number of studies had reported difficulties
in recording cholinergic currents in the mammalian nervous system
and as a result and in addition to their amenability to transgenic
studies, Drosophila became a useful model for studying of
the association between AD and PD.
Certain non-motor symptoms of PD, including
cognitive impairment and sleep disorders often appear a number of
years prior to the onset of motor symptoms. The main pathology
associated with PD involves dopaminergic degeneration, which is the
main factor contributing to the motor symptoms. The motor symptoms
observed in patients with PD are often accompanied by cognitive
impairment, depression and other symptoms associated with
degeneration of the forebrain. Mutations in α-Syn are associated
with genetic and sporadic cases of PD. Previous studies have
demonstrated that an α-Syn mutation in Drosophila, which
leads to increased oligomerization, enhances the degree of
neurotoxicity in the fruit fly (25). A previous study demonstrated that
there is a long time period between the degeneration of
dopaminergic neurons and the appearance of the typical motor
symptoms of PD (26), which
indicates that there is a compensatory mechanism in the central
nervous system. It is hypothesized that the dynamic balance between
dopamine and acetylcholine is important. Inhibiting the cholinergic
system may cause a decline in learning and memory abilities and
symptoms similar to senile amnesia (27). In addition, numerous patients with
PD eventually develop cognitive deficits and dementia (28). The present experiments demonstrate
that the non-motor symptoms in patients with PD may be associated
with the cholinergic system.
The present results also demonstrate that learning
and memory skills reduced in α-Syn wild-type and A53T mutant
Drosophila by ~18%. Previous studies have demonstrated that
the expression of human α-Syn may impair the motor capability of
Drosophila (8,29,30).
However, in the present study, no significant difference was
identified in the motor function of the α-Syn and A53T
Drosophila. It is possible that the flies in the present
study were too young and the expression of α-Syn may only effect
non-motor symptoms at this stage, without a marked impact on motor
symptoms. α-Syn may reduce the frequency of mEPSCs in PNs and
reduce the electrical signals of the calcium channel. Thus, it has
been demonstrated that α-Syn expression in Drosophila may
affect cholinergic synaptic transmission. The frequency of mEPSCs
in PNs and the calcium current were reduced, which may be
associated with the decline in the ability to learn and in memory
in Drosophila. The present study demonstrated that mutations
in α-Syn may be one of the reasons for the higher incidence of
senile dementia in patients with PD. However, the exact mechanism
requires further investigation.
References
|
1
|
Janezic S, Threlfell S, Dodson PD, et al:
Deficits in dopaminergic transmission precede neuron loss and
dysfunction in a new Parkinson model. Proc Natl Acad Sci USA.
110:E4016–E1025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kitada T, Asakawa S, Hattori N, Matsumine
H, Yamamura Y, Minoshima S, et al: Mutations in the parkin gene
cause autosomal recessive juvenile parkinsonism. Nature.
392:605–608. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paisán-Ruiz C, Jain S, Evans EW, Gilks WP,
Simòn J, van der Brug M, et al: Cloning of the gene containing
mutations that cause PARK8-linked Parkinson’s disease. Neuron.
44:595–600. 2004. View Article : Google Scholar
|
|
4
|
Zimprich A, Biskup S, Leitner P, Lichtner
P, Farrer M, Lincoln S, et al: Mutations in LRRK2 cause
autosomal-dominant parkinsonism with pleomorphic pathology. Neuron.
44:601–607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krüger R, Kuhn W, Müller T, et al:
Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s
disease. Nature Genetics. 18:106–108. 1998. View Article : Google Scholar
|
|
6
|
Polymeropoulos MH, Lavedan C, Leroy E, et
al: Mutation in the α-synuclein gene identified in families with
Parkinson’s disease. Science. 276:2045–2047. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feany MB and Bender WW: A Drosophila model
of Parkinson’s disease. Nature. 404:394–398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee MK, Stirling W, Xu Y, et al: Human
α-synuclein-harboring familial Parkinson’s disease-linked
Ala-53→Thr mutation causes neurodegenerative disease with
α-synuclein aggregation in transgenic mice. Proc Nat Acad Sci USA.
99:8968–8973. 2002. View Article : Google Scholar
|
|
9
|
Masliah E, Rockenstein E, Veinbergs I, et
al: Dopaminergic loss and inclusion body formation in α-synuclein
mice: implications for neurodegenerative disorders. Science.
287:1265–1269. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizuno H, Fujikake N, Wada K, et al:
α-Synuclein transgenic Drosophila as a model of Parkinson’s disease
and related synucleinopathies. Parkinsons Dis. 2011:2127062011.
|
|
11
|
Chaudhuri KR, Yates L and Martinez-Martin
P: The non-motor symptom complex of Parkinson’s disease: A
comprehensive assessment is essential. Curr Neurol Neurosci Rep.
5:275–283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heisenberg M: Mushroom body memoir: from
maps to models. Nat Rev Neurosci. 4:266–275. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kazama H and Wilson RI: Homeostatic
matching and nonlinear amplification at identified central
synapses. Neuron. 58:401–413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bosboom JL, Stoffers D and Wolters ECh:
Cognitive dysfunction and dementia in Parkinson’s disease. J Neural
Transm. 111:1303–1315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rieckhof GE, Yoshihara M, Guan Z and
Littleton JT: Presynaptic N-type calcium channels regulate synaptic
growth. J Biol Chem. 278:41099–41108. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pendleton RG, Parvez F, Sayed M and
Hillman R: Effects of pharmacological agents upon a transgenic
model of Parkinson’s disease in Drosophila melanogaster. J
Pharmacol Exp Ther. 300:91–96. 2002. View Article : Google Scholar
|
|
17
|
Stone E, Haario H and Lawrence JJ: A
kinetic model for the frequency dependence of cholinergic
modulation at hippocampal GABAergic synapses. Math Biosci.
258:162–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhaowei L, Yongling X, Jiajia Y and Zhuo
Y: The reduction of EPSC amplitude in CA1 pyramidal neurons by the
peroxynitrite donor SIN-1 requires Ca2+ influx via
postsynaptic non-L-type voltage gated calcium channels. Neurochem
Res. 39:361–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu H, Jiang SA, Campusano JM, Iniguez J,
Su H, Hoang AA, et al: Cav2-type calcium channels
encoded by cac regulate AP-independent neurotransmitter release at
cholinergic synapses in adult Drosophila brain. J Neurophysiol.
101:42–53. 2009. View Article : Google Scholar :
|
|
20
|
Kawasaki F, Zou B, Xu X and Ordway RW:
Active zone localization of presynaptic calcium channels encoded by
the cacophony locus of Drosophila. J Neurosci. 24:282–285. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Catterall WA: Structure and regulation of
voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol.
16:521–555. 2000. View Article : Google Scholar
|
|
22
|
Karpinar DP, Balija MBG, Kügler S, et al:
Pre-fibrillar α-synuclein variants with impaired β-structure
increase neurotoxicity in Parkinson’s disease models. EMBO J.
28:3256–3268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rascol O, Payoux P, Ory F, et al:
Limitations of current Parrkinson’s disease therapy. Ann Neuro.
53:s3–s15. 2003.discussions 12–5. View Article : Google Scholar
|
|
24
|
Restifo LL and White K: Molecular and
genetic approaches to neurotransmitter and neuromodulator systems
in Drosophila. Adv Insect Physiol. 22:115–219. 1990.
|
|
25
|
Gu H and O’Dowd DK: Cholinergic synaptic
transmission in adult Drosophila Kenyon cells in situ. J Neurosci.
26:265–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thompson MA: Muscle relaxant drugs. Br J
Hosp Med. 23:1531980.PubMed/NCBI
|
|
27
|
Rinne JO, Myllykylä T, Lönnberg P and
Marjamäki P: A postmortem study of brain nicotinic receptors in
Parkinson’s and Alzheimer’s disease. Brain Res. 547:167–170. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Foltynie T, Brayne CE, Robbins TW and
Barker RA: The cognitive ability of an incident cohort of
Parkinson’s patients in the UK. The CamPaIGN study. Brain.
127:550–560. 2004. View Article : Google Scholar
|
|
29
|
Chen AY, Wilburn P, Hao X and Tully T:
Walking deficits and centrophobism in an α-synuclein fly model of
Parkinson’s disease. Genes Brain Behav. 13:812–820. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gajula Balija MB, Griesinger C, Herzig A,
Zweckstetter M and Jäckle H: Pre-fibrillar α-synuclein mutants
cause Parkinson’s disease-like non-motor symptoms in Drosophila.
PLoS One. 6:e247012011. View Article : Google Scholar
|