Introduction
The prevalence of diabetes in Korean patients has
dramatically increased from 1.5 to 9.9% over the last 40 years
(1). It is anticipated that the
prevalence of diabetes will rise to 11.4% by 2030. This drastic
increase in diabetic patients is ultimately associated with
secondary micro- and macro-vascular complications (2). Therefore, effective approaches to
control blood glucose are required to prevent vascular
complications and improve the quality of life of diabetic patients.
Initial management often involves lifestyle interventions,
including diet and exercise, but in most cases, pharmacotherapy is
also required as the disease progresses (2). However, medications used to control
blood glucose often cause metabolic side effects, including weight
gain and organ toxicity (3,4).
Thus, development of alternative therapies is of paramount
importance, and natural products that can manage patients’ blood
glucose levels without noticeable side effects are gaining
considerable attention.
Persimmon (Diospyros kaki), a fruit tree that
is native to China and belongs to the family of Ebenaceae,
is widely distributed in eastern Asia. The fruit of the persimmon
tree is consumed as food, whereas the young leaf is mainly used for
tea. Persimmon leaf tea contains several bioactive compounds,
including flavonoids, triterpenoids, tannins and carotenoids
(5–9). A number of studies have reported the
beneficial effects of persimmon leaf extract (PLE) on hypertension
(5), stroke (10), atherosclerosis (11) and dermatitis (12). Recently, Jung et al
(13) investigated the metabolic
effects of PLE using type 2 diabetic (db/db) mice. After
oral administration of powdered persimmon leaves for five weeks,
glucose- and lipid-lowering effects were observed in the animals,
which also led to amelioration of hyperglycemia, dyslipidemia and
fatty liver.
In the present study, the anti-diabetic efficacy of
PLE in streptozotocin-induced diabetic mice and db/db mice
was investigated. Furthermore, the underlying mechanism of the
anti-diabetic effect of PLE was investigated, particularly focusing
on α-glucosidase inhibition and pancreatic β-cell-protecting
activities.
Materials and methods
Reagents
Unless otherwise stated, all reagents were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Collagenase was purchased
from Roche Diagnostics (Indianapolis, IN, USA).
Preparation of PLE
Persimmon leaves were raised and harvested in Wanju
(Jeonbuk, Korea) in June 2013 by Dongsangmyeon Saramdeul Inc.
(Jeonbuk, Korea). Persimmon leaves were dried in the shade for one
week prior to being powdered and passed through 60-mesh sieves. One
volume of persimmon leaf powder was added to 10 volumes of
distilled water and extracted at 90–100°C for 3 h. The aqueous
phase was filtered and concentrated with a vacuum evaporator
(Eyela, Japan). After lyophilization, the powder was stored at
−80°C until used. The components of PLE were analyzed by the
Development Institute of Traditional Korean Medicine (Jeonnam,
Korea) using a high-pressure liquid chromatography workstation
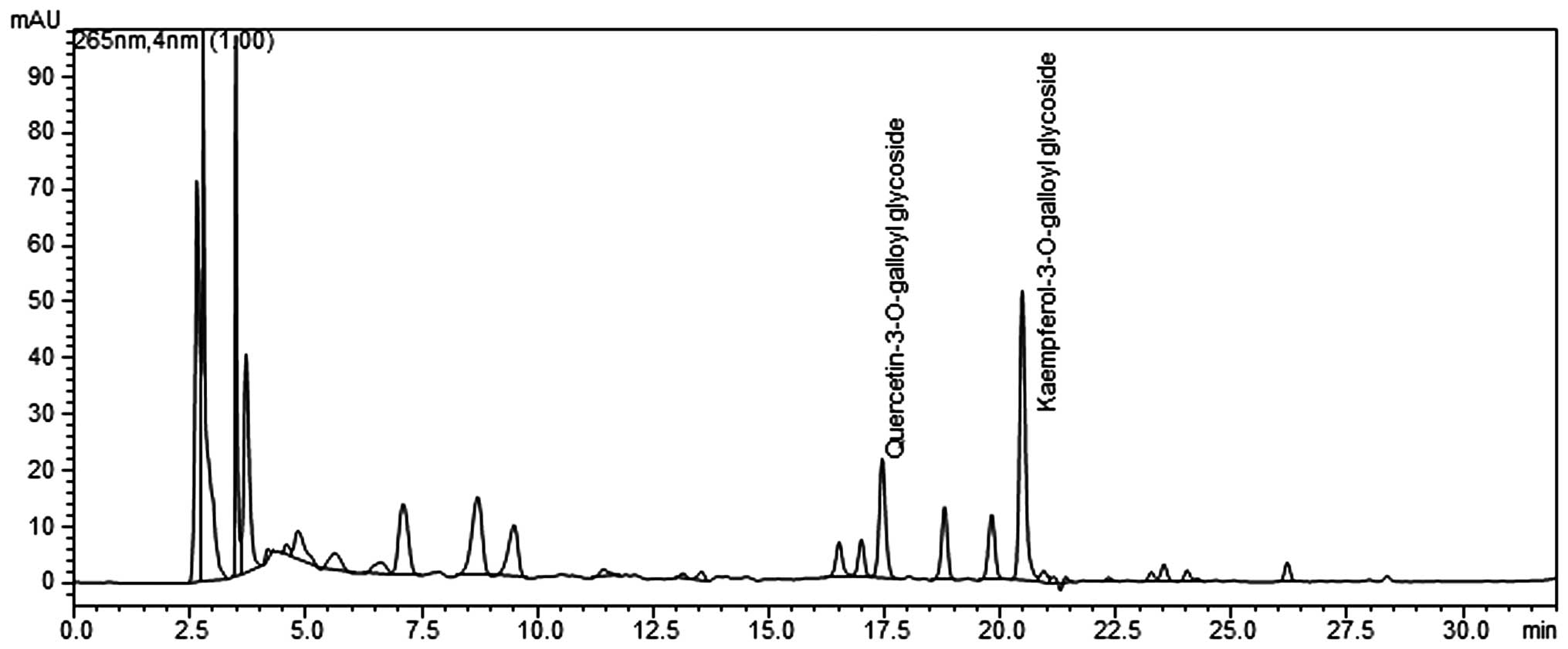
(Shimadzu, Japan) (Fig. 1).
Analyses were performed on an X-bridged C18 column with a mobile
phase gradient of A) 0.1% formic acid and B) acetonitrile over 50
min. Gradient elution was programmed at a flow rate of 0.25 ml/min
as follows: 0 min (100%), 10 min (90%), 30 min (40%), 45 min (30%)
and 50 min (90%). The injection volume was 20 μl. The column
temperature was kept constant at 25°C, and the mobile phase flow
rate was 1 ml/min with ultraviolet detection at 265 nm. PLE was
standardized to contain 4–7 mg total quercetin
3-O-2′galloylglucoside
(C24H24O19) and kaempferol
3-O-2′galloylglucoside
(C24H24O18) per 1 g of
extract.
In vitro α-glucosidase assay
Yeast α-glucosidase (0.5 U) dissolved in 0.2 M
potassium phosphate buffer (pH 6.8) was mixed with various
concentrations of PLE or acarbose. After incubation at 37°C for 15
min, 3 mM p-nitrophenyl-α-D-gluc opyranoside was added. The
reaction was further incubated at 37°C for 10 min and then stopped
by the addition of 0.1 M Na2CO3. The
absorption (Abs) of 4-nitrophenol was measured at 405 nm. Reaction
mixture without any sample was used as a control, and the mixture
without substrate was used as a blank. The percent inhibition of
α-glucosidase was calculated as
[1-(Abssample−Absblank)/Abscontrol]×100.
Measurements were performed in triplicate.
Oxygen free radical scavenging assay
The anti-oxidant activity of each sample extract was
assessed by the ability of the extract to scavenge
2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals. The extracts,
in separate test tubes, were allowed to react with DPPH. DPPH free
radical scavenging activity was monitored by measuring the decline
in absorbance at 517 nm. Butylated hydroxyanisole was used as the
standard compound.
Experimental design
Pathogen-free, male C57BL/6 mice were purchased from
Orientbio (Sungnam, Korea). The mice were housed at 20°C with 50%
relative humidity, a 12-h light/dark cycle (light from 6:00 am to
6:00 pm) and were provided free access to drinking water. To induce
diabetes, eight-week-old male C57BL/6 mice were injected via the
tail vein with 100 mg/kg body weight streptozotocin (STZ) dissolved
in 0.1 mol/l sodium citrate buffer (pH 4.0). The control mice
received citrate buffer alone. PLE (50 or 250 mg/kg body weight)
was injected daily for five days via oral gavage prior to
administration of STZ. STZ was first administered on day one. On
day six, mice were sacrificed by decapitation without anesthesia
and trunk blood was collected.
In addition, seven-week-old male
C57BL/KsJ-db/db (db/db) mice were purchased from the
Jackson Lab (Bar Harbor, ME, USA) and fed a normal chow diet.
Starting at eight weeks of age, the point at which the mice become
diabetic, the db/db mice were treated with PLE (50 or 250
mg/kg) for eight weeks via oral gavage once daily. Each group was
made up of five mice. As a positive control, acarbose (10 mg/kg)
was administered instead of PLE. Food consumption and body weight
were recorded every week. At the end of the experimental period, an
oral glucose tolerance test (OGTT; 1 g/kg body weight) was
performed. After a 14 h fast, glucose was administered by oral
gavage (2 mg/g). The blood glucose level was subsequently
determined from the tail vein at 0, 15, 30, 60 and 120 min
following the glucose administration. Animals were sacrificed by
decapitation, after which blood samples were collected, and livers
were removed and weighed. All of the animal experiments were
performed in accordance with the Guide for the Care and Use of
Laboratory Animals published by the US National Institutes of
Health (NIH Publication no. 85-23, revised 2011). The protocol of
the present study was approved by the Institutional Animal Care and
Use Committee of Chonbuk National University (permit no. CBU
2014-00048).
Oral maltose tolerance test in
streptozotocin-induced diabetic mice
Mice were classified into four groups (1–4)
containing five mice each. Groups 1 and 2 received
phosphate-buffered saline (PBS) as a negative control or acarbose
(3 mg/kg) as a positive control, respectively. Groups 3 and 4 were
treated with PLE at two doses (50 and 250 mg/kg). All samples were
administered orally to 12-h fasted mice, and 3 g/kg of maltose was
administered 5 min thereafter. Blood was collected from the tail
vein at 0, 15, 30, 60 and 120 min after loading maltose.
Biochemical analyses
Blood glucose levels were measured by Accu-Chek
Aviva glucose monitors (Roche Diagnostics, Indianapolis, IN, USA)
and plasma insulin was measured using an ELISA kit (cat. no.
EZRMI-13K; Millipore, Bedford, MA, USA). Plasma levels of total
cholesterol (TC), triglyceride (TG) and HDL-cholesterol were
measured using commercially available kits (cat. no’s. AM202-K,
AM157S-K and AM203-K, respectively; Asan Pharmaceutical, Seoul,
Korea). For liver TG quantification, liver tissues were homogenized
and extracted in chloroform, methanol and DW (2/1/1 ratio).
Histology
Tissues were removed and immediately placed in 10%
formalin solution, embedded in paraffin and cut into 5-μm
sections. Specimens were stained with hematoxylin and eosin
(H&E) to identify morphological changes. For
immunohistochemical analysis, tissue sections were subjected to a
microwave antigen retrieval procedure (1,000 watts for 5 min;
CPC-600; Cuisinart, East Windsor, NJ, USA) in 0.01 mol/l sodium
citrate buffer. After blocking endogenous peroxidase, the sections
were incubated with Protein Block Serum-Free (DAKO, Glostrup,
Denmark) to block non-specific staining and then with rabbit
anti-insulin antibody (cat. no. sc-9168; 1:100; Santa Cruz
Biotechnology, Dallas, TX, USA) for 12 h at 4°C. Peroxidase
activity was detected using 3-amino-9-ethyl carbazole. Tissue
sections were observed using a light microscope (Eclipse E600
polarizing microscope; Nikon, Tokyo, Japan).
Islet isolation and glucose-stimulated
insulin secretion (GSIS) assay
Pancreatic islets were isolated from 12-week-old
mice using the collagenase digestion method as previously described
(14). Following isolation, islets
were cultured overnight in RPMI-1640 supplemented with 2 mM
L-glutamine, 10% heat-inactivated fetal calf serum, 100 units/ml
penicillin and 100 μg/ml streptomycin in humidified air
containing 5% CO2 at 37°C. Prior to experiments, islets
were washed three times in RPMI-1640 and cultured overnight. After
the initial culture period, islets were cultured for three days in
identical RPMI-1640 containing 5.5 or 30 mmol/l glucose and
subsequently washed three times in Krebs-Ringer bicarbonate buffer
[25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 115
mmol/l NaCl, 24 mmol/l NaHCO3, 5 mmol/l KCl, 1 mmol/l
MgCl2, 2.5 mmol/l CaCl2 and 0.1% bovine serum
albumin, pH 7.4] containing 2.8 mmol/l D-glucose. Insulin secretion
assays were performed with 2.8 or 16.7 mmol/l glucose and measured
using an ELISA kit (cat. no. EZRMI-13K; Millipore, Bedford, MA,
USA).
Statistical analysis
Statistical analysis was performed using analysis of
variance and Duncan’s tests on through GraphPad Prism v5.02
(GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
PLE inhibits α-glucosidase activity
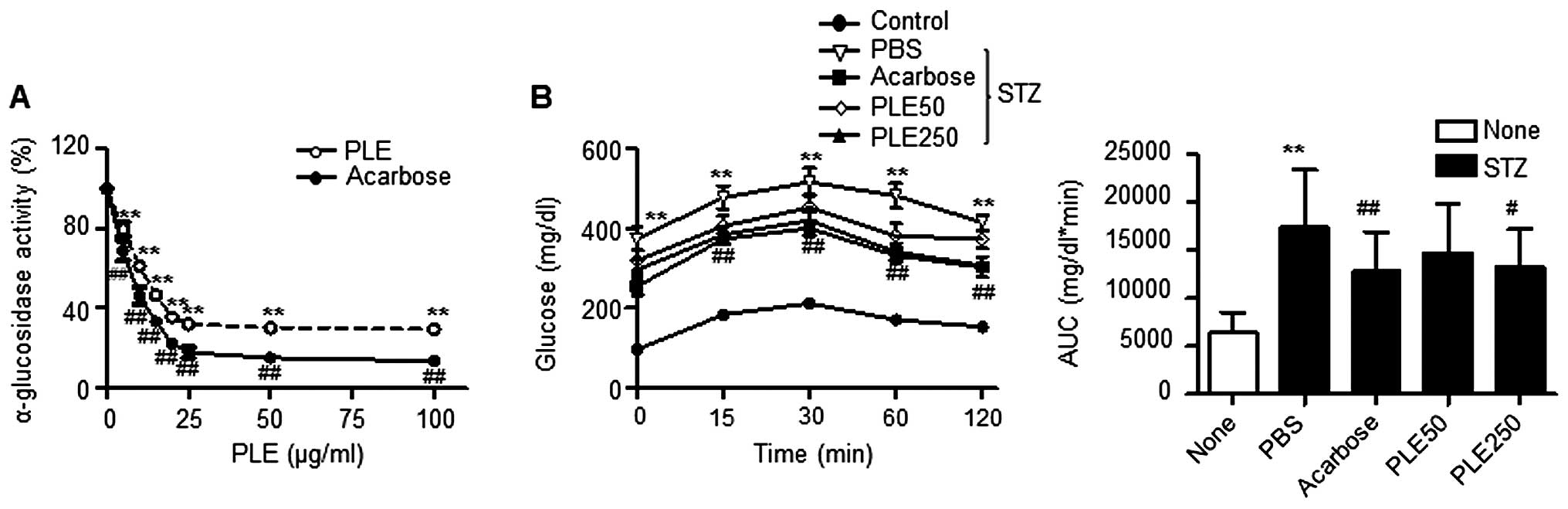
The in vitro inhibitory activity of PLE
against yeast α-glucosidase is shown in Fig. 2A. PLE inhibited α-glucosidase
activity in a dose-dependent manner and therefore should be
considered an effective α-glucosidase inhibitor: PLE at a
concentration of 100 μg/ml inhibited α-glucosidase activity
by 70.5% and was 16.0% less potent than acarbose, which was used as
a positive control. The half maximal inhibitory concentration
(IC50) value on α-glucosidase activity was 4.7
μg/ml.
 | Figure 2α-Glucosidase inhibitory activity of
PLE. (A) Effects of different concentrations of PLE on
α-glucosidase activity in vitro (**P<0.01,
##P<0.01, vs. no treatment). (B) Effects of PLE on
blood glucose levels after administration of 3 g/kg maltose in
STZ-induced diabetic mice. Values are expressed as the mean ±
standard error of the mean (n=5; **P<0.01, vs.
control mice; #P<0.05, ##P<0.01, vs.
PBS-treated STZ mice). PLE50, persimmon leaf extract (50 mg/kg);
PLE250, persimmon leaf extract (250 mg/kg); STZ, streptozotocin;
PBS, phosphate-buffered saline; AUC, area under curve. |
The results of the maltose tolerance experiment are
presented in Fig. 2B. After the
administration of maltose, the blood glucose levels in normal mice
increased after 15 min. This elevation was statistically
significant compared with the levels at time 0 for each group. At
120 min, the blood glucose levels returned to their basal values.
Oral administration of 250 mg/kg PLE inhibited the increases in
glucose levels after 30 min, which was statistically significant
compared with levels in the corresponding controls at each
time-point. The administration of acarbose also significantly
decreased the increase of postprandial blood glucose. The areas
under the curve (AUC) for glucose were reduced by 15.34% by PLE at
50 mg/kg, 23.63% by PLE at 250 mg/kg and 26.51% by acarbose.
Furthermore, the anti-oxidant properties of PLE were
determined using the cell-free DPPH assay. PLE inhibited DPPH
activity in a dose-dependent manner (Fig. 3), which confirms the findings of a
previous study (15).
PLE protects mice against STZ-induced
diabetes
Powdered persimmon leaf has previously been reported
to have glucose-and lipid-lowering effects in db/db mice
(13). First, the present study
evaluated the hypoglycemic effects of PLE at concentrations of 50
mg/kg (low-dose) and 250 mg/kg (high-dose) in STZ-induced diabetic
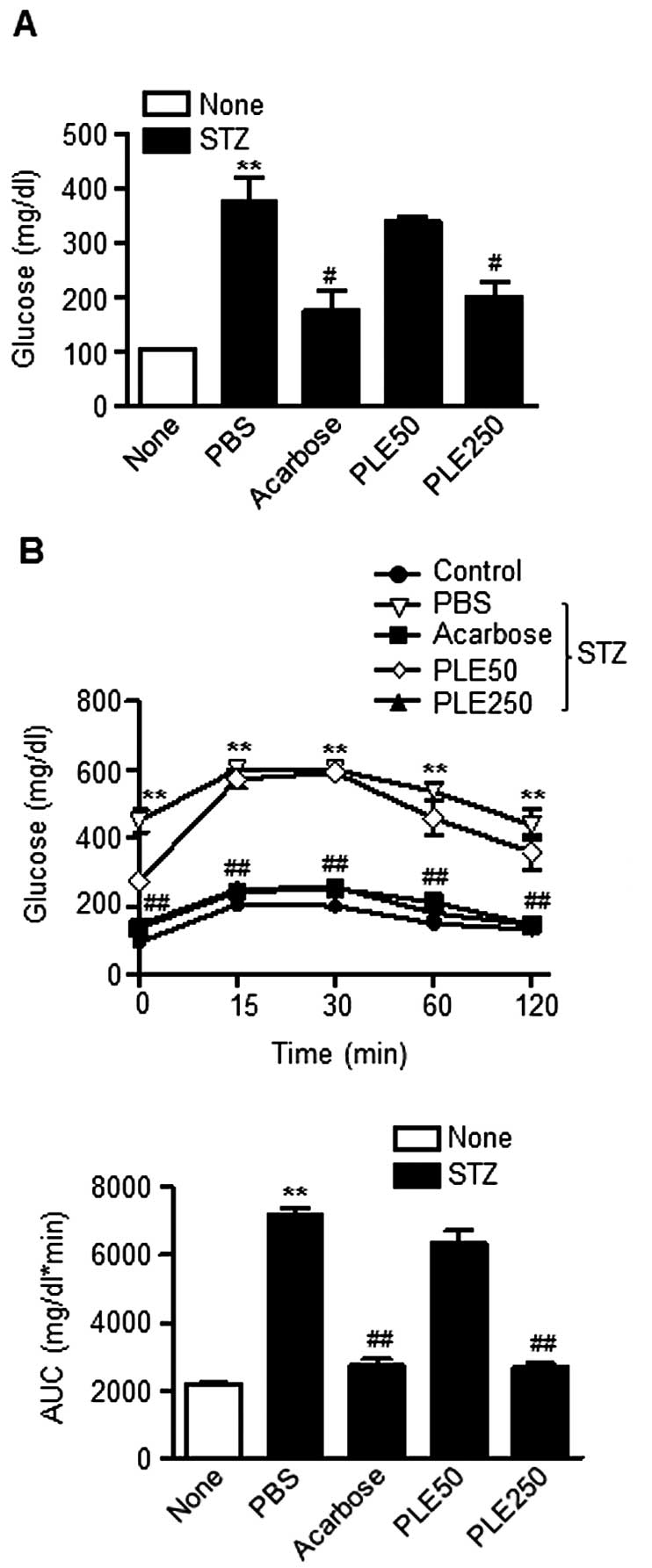
mice. As shown in Fig. 4A, a
single intravenous injection of STZ induced the increase of fasting
blood glucose levels to ~400 mg/dl. However, the fasting glucose
levels were significantly reduced to 176 mg/dl in the STZ mice
pre-treated with high-dose PLE. The group of mice treated with
acarbose (10 mg/kg) showed similar blood glucose levels. Of note,
the body weight among the groups did not change over the course of
the study (data not shown).
The effect of PLE on the post-prandial increase in
blood glucose in STZ-induced diabetic mice was determined via OGTT
Consistent with the above results, oral administration of high-dose
PLE significantly prevented an increase in plasma glucose levels
(Fig. 4B). High-dose PLE and
acarbose decreased the AUCs for the postprandial glucose responses
by 61.6 and 62.4%, respectively, compared with that in the
PBS-treated group (P<0.01). These results indicated that
treatment with PLE prevents STZ-induced β-cell damage in mice.
Long-term treatment with PLE ameliorates
hyperglycemia and dyslipidemia in db/db mice
To further evaluate the therapeutic effects of PLE
on diabetes, the long-term anti-diabetic effects of PLE in
db/db mice were evaluated. Food intake was not significantly
influenced by PLE treatment (Fig.
5A); however, the body weight was significantly decreased by
high-dose PLE treatment (Fig. 5B).
Diabetic db/db mice at 16 weeks of age that were fed
normally had hyperglycemia with postprandial blood glucose levels
of ~494 mg/dl (Fig. 5C). High-dose
PLE decreased postprandial blood glucose levels in db/db
mice as effectively as acarbose. The decrease in blood glucose
levels by PLE appeared to be dose-dependent, as low doses of PLE
(50 mg/kg) revealed no glucose lowering effects, whereas high doses
of PLE (250 mg/kg) significantly decreases glucose levels (P<
0.01).
An OGTT was performed to determine the effects of
PLE on glucose tolerance after eight weeks of PLE treatment
(Fig. 5D). The AUC for glucose
response of high-dose PLE-treated db/db mice was
significantly lower than that of the (db/db) mice. By
contrast, oral administration of low-dose PLE did not show any
improvement in glucose tolerance.
Plasma insulin levels between db/db mice that
received PBS and those that had been administered PLE were also
compared. The serum insulin levels in high-dose PLE-treated mice
tended to be higher than those in PBS-treated mice (Fig. 6A). In addition, as shown in
Fig. 6B and C, high-dose PLE
significantly lowered plasma TG (160.5±15.9 mg/dl; P<0.01) and
TC levels (209.2±74.5 mg/dl; P<0.05) compared with those in the
control mice (208.9±32.4 and 263.9±19.3 mg/dl, respectively).
Plasma HDL-cholesterol levels were not significantly different
among groups (Fig. 6D). Overall,
these results suggested that PLE has glucose- and lipid-lowering
effects in db/db mice.
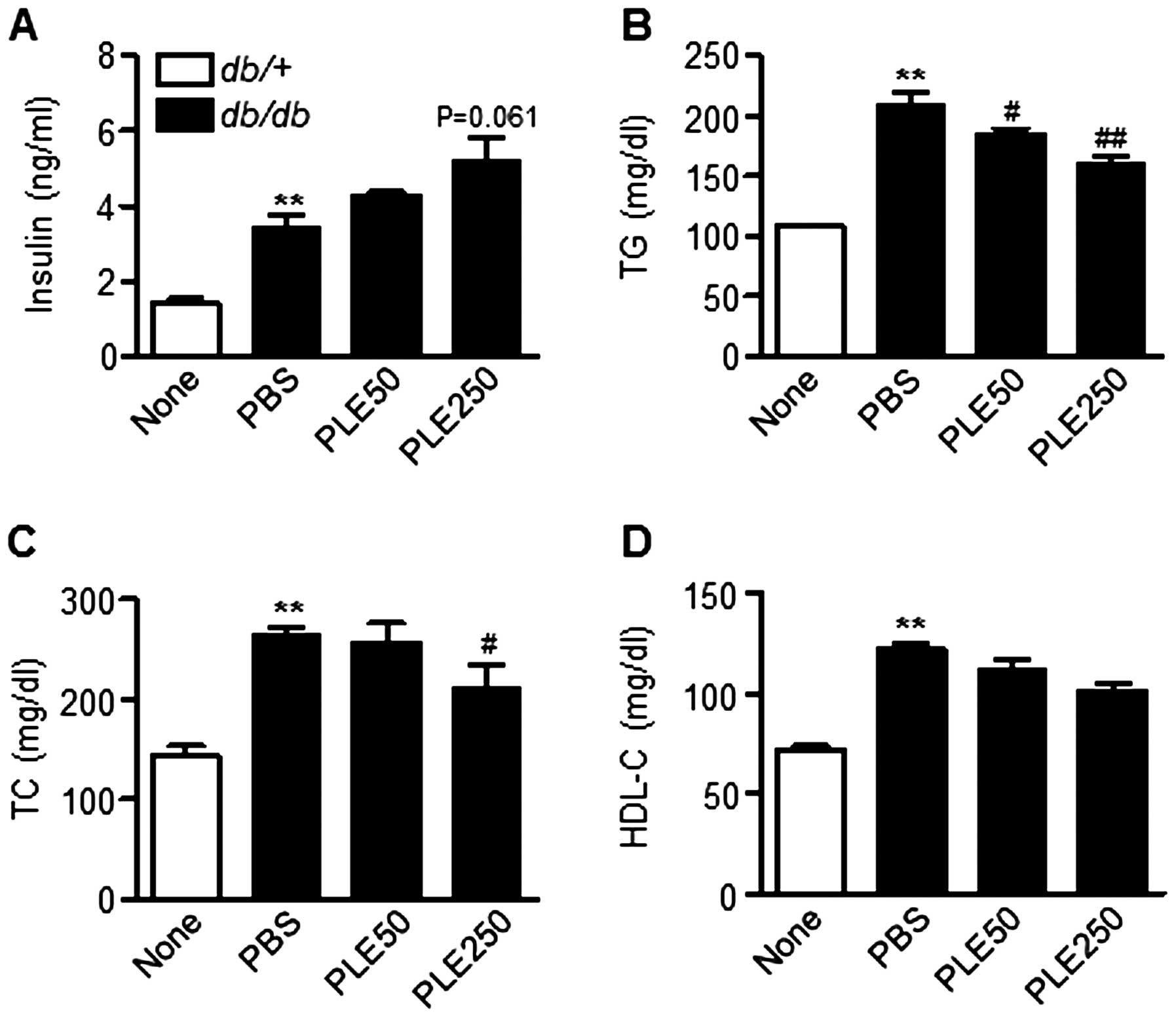 | Figure 6Biochemical parameters in db/db
mice after eight weeks of treatment with persimmon leaf extract.
Mice received daily oral supplementation with PLE50 or PLE250. At
the end of the study, plasma concentrations of (A) insulin, (B) TG,
(C) TC and (D) HDL-C were determined. Values are expressed as the
mean ± standard error of the mean (n=5). **P<0.01 vs.
control mice; #P<0.05, ##P<0.01 vs.
PBS-treated db/db mice. TG, triglyceride; TC, total
cholesterol; HDL-C, high-density lipoprotein cholesterol; PLE50,
persimmon leaf extract (50 mg/kg); PLE250, persimmon leaf extract
(250 mg/kg); PBS, phosphate-buffered saline; db/db,
diabetic. |
Long-term treatment with PLE prevents
fatty liver development in db/db mice
As diabetes can trigger hepatic steatosis, livers
were assessed for the extent of fat accumulation. The results
indicated that PLE supplementation was associated with less
steatotic livers (data not shown). Examination of H&E-stained
sections demonstrated marked macrovesicular steatosis in
db/db mice, and the degree of hepatic steatosis was
markedly alleviated by PLE (Fig.
7A). Liver TG and liver weight were concordant with the
histological findings (Fig. 7B and
C). Treatment of db/db mice with high-dose PLE resulted
in significant decreases in hepatic TG contents (85.3±12.5 mg/100
mg vs. 54.2±15.8 mg/100 mg; P<0.01) and liver weight (3.8±0.8
vs. 2.7±0.6 g; P=0.088) as compared with that in the control
db/db mice.
Long-term treatment with PLE protects
pancreatic β-cells in db/db mice
As PLE administration increased plasma insulin
levels (Fig. 6A), pancreata were
examined using H&E staining. Pancreatic islets of diabetic
db/db mice exhibited degeneration and poorly defined margins
(Fig. 8A). Immunostaining with an
insulin antibody showed weak insulin staining. By contrast,
pancreatic islets of high-dose PLE-treated mice had a round shape
and high insulin immunoreactivity, suggesting the protection of
β-cells by PLE.
To determine the protective effects of PLE on
β-cells, the present study examined whether it was effective in
protecting pancreatic islets from glucotoxicity. Islets from mice
were isolated and incubated under normal glucose (5.5 mM) or
high-glucose (30 mM) culture conditions. Basal and
glucose-stimulated insulin secretion was assessed after three days
of culture (Fig. 8B). Results
showed that the amount of glucose-stimulated insulin secretion was
6.65±0.48 ng/islet/h in normal glucose-cultured islets and
4.80±1.48 ng/islet/h in high-glucose-cultured islets. Following
pre-treatment with PLE, however, the insulin secretion under
high-glucose conditions was significantly restored to a level
closer to the control value. Basal insulin release among the groups
was similar. In conclusion, in vitro and in vivo
results of the present study suggested that PLE exhibits protective
effects on β-cells.
Discussion
In the present study, PLE was shown to improve the
biochemical parameters of glucose and lipid metabolism and
prevented fatty liver development in db/db mice after eight
weeks of oral supplementation. Similar results have been reported
by a study of five-week treatment with powdered persimmon leaves
(13), suggesting that long-term
oral supplementation with persimmon leaf can effectively exert
glycemic control in diabetic mice. In addition, five-day oral
supplementation with PLE prevented diabetes development in
STZ-treated mice. In recent meta-analysis studies, flavonoids have
been reported to have acute and chronic effects on glucose and
lipid metabolism (16,17). As PLE contains a considerable
amount of flavonoids, including quercetin and kaempferol (4), it is reasonable to expect PLE to
exhibit acute as well as chronic anti-diabetic effects. However,
even though PLE possesses hypoglycemic effects, the blood glucose
levels in PLE-supplemented db/db mice were still higher
throughout the experimental period than those of wild-type db/m
mice, suggesting that PLE causes a certain improvement in glucose
tolerance under hyperglycemic conditions.
Although the body weight decreased in db/db
mice after PLE supplementation, a similar decrease in food intake
was not observed. This finding is in contrast with results from a
study by Jung et al (13),
in which oral administration of the powder of persimmon leaves
caused significant decreases in body weight gain and food intake
compared to those in control group mice. This discrepancy may
result from a difference in persimmon leaf sources between the two
studies. Furthermore, the present study used PLE, whereas Jung
et al fed mice with 5% powder of persimmon leaves. As the
aqueous extract and powdered persimmon leaves display differences
in their chemical composition, their application is likely to have
different outcomes. In the present study, the conclusion that PLE
reduced body weight and/or improved glucose tolerance simply by
reducing food intake can be excluded.
Of note, the hypoglycemic effect of PLE in
db/db mice was observable as early as one week after PLE
supplementation. This finding led to the hypothesis that
α-glucosidase and α-amylase inhibition are possible underlying
mechanisms of the anti-diabetic effects of PLE, as these enzymes
are involved in the digestion of complex carbohydrates from food
into absorbable monosaccharides (18). Accordingly, an in vitro
study was performed to examine the effects of PLE on α -glucosidase
activity. The results revealed that PLE inhibited -glucosidase
activity in a dose-dependent manner with an IC50 value
of 4.7 μg/ml. In the following oral maltose tolerance test
in normal mice, PLE showed marked α-glucosidase inhibitory
activity. In addition, a study by Kawakami et al (9) reported α-amylase inhibitory activity
of persimmon leaves. Therefore, inhibition of α-glucosidase and/or
α-amylase by PLE may prolong overall digestion time, causing a
delay in glucose absorption, consequently reducing the rapid
increase of postprandial blood glucose.
PLE significantly suppressed the increase in the
post-prandial blood glucose levels as compared to those in
PBS-treated db/db mice. Insulin has a pivotal role in
maintaining the post-prandial glucose levels within a normal range
by enhancing glycogen synthesis and glycolysis, and by suppressing
gluconeogenesis (19). In general,
the db/db mice exhibited an initial phase of
hyperinsulinemia to compensate for insulin resistance and
progressively develop insulinopenia with age, a characteristic
commonly observed in patients during late stages of type 2 diabetes
(20). In db/db mice
supplemented with PLE, the plasma insulin levels were higher than
those in PBS-treated db/db mice. In addition, islet
architecture was relatively well preserved and the mass of
insulin-immunoreactive β-cells was increased in PLE-supplemented
mice, suggesting that PLE-supplemented db/db mice still
displayed insulin-secreting β-cell masses. This assumption was
evidenced by ex vivo experiments using isolated islets.
Assessment of insulin secretion capacities after culturing of
islets under glucotoxic conditions showed that glucose-stimulated
insulin secretion was increased in PLE-treated islets as compared
with that in untreated islets.
In conclusion, the present study provided further
evidence for the anti-diabetic efficacy of PLE in STZ-induced
diabetic mice and db/db mice, which was comparable to the
effect elicited by acarbose. Glucose tolerance during OGTT was
enhanced, lipid parameters were improved and fat accumulation in
the liver was suppressed in PLE-supplemented mice compared to those
of the control mice. These beneficial effects are at least
partially mediated via suppression of α-glucosidase activity and
preserved, functional β-cell masses. The former leads to decreased
blood glucose levels and the latter leads to increased insulin
levels. Therefore, the results of the present study implied that
supplementing pre-diabetes or diabetes patients with PLE may be a
way to maintain blood glucose levels within a normal range.
Acknowledgments
The present study was supported by a Regional
Agri-Food Lead Cluster Promotion Project from Wanju-gun, Republic
of Korea.
References
|
1
|
Kim DJ: The epidemiology of diabetes in
Korea. Diabetes Metab J. 35:303–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamagishi S and Imaizumi T: Diabetic
vascular complications: Pathophysiology, biochemical basis and
potential therapeutic strategy. Curr Pharm Des. 11:2279–2299. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Derosa G and Maffioli P:
Thiazolidinediones plus metformin association on body weight in
patients with type 2 diabetes. Diabetes Res Clin Pract. 91:265–270.
2011. View Article : Google Scholar
|
|
4
|
Mitri J and Hamdy O: Diabetes medications
and body weight. Expert Opin Drug Saf. 8:573–584. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawakami K, Shibukura Y, Kanno T, Furuki
T, Aketa S and Hirayama M: Identification of 2′-galloylated
flavonol 3-o-glycosides accumulating in developing leaves of
persimmon. Phytochem Anal. 22:403–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu L, Liu RL, Zhang J and Zhang ZQ: Study
on the PEG-based microwave-assisted extraction of flavonoid
compounds from persimmon leaves. J Sep Sci. 35:3412–3420. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen G, Lu H, Wang C, et al: Effect of
five triterpenoid compounds isolated from leaves of Diospyros kaki
on stimulus-induced superoxide generation and tyrosyl
phosphorylation in human polymorphonuclear leukocytes. Clin Chim
Acta. 320:11–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen G, Wang ZQ and Jia JM: Three minor
novel triterpenoids from the leaves of Diospyros kaki. Chem Pharm
Bull (Tokyo). 57:532–535. 2009. View Article : Google Scholar
|
|
9
|
Kawakami K, Aketa S, Nakanami M, Iizuka S
and Hirayama M: Major water-soluble polyphenols, proanthocyanidins,
in leaves of persimmon (Diospyros kaki) and their alpha-amylase
inhibitory activity. Biosci Biotechnol Biochem. 74:1380–1385. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bei W, Peng W, Zang L, Xie Z, Hu D and Xu
A: Neuroprotective effects of a standardized extract of Diospyros
kakileaves on MCAO transient focal cerebral ischemic rats and
cultured neurons injured by glutamate or hypoxia. Planta Med.
73:636–643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katsube T, Tabata H, Ohta Y, et al:
Screening for antioxidant activity in edible plant products:
comparison of low-density lipoprotein oxidation assay, DPPH radical
scavenging assay and Folin-Ciocalteu assay. J Agric Food Chem.
52:2391–2396. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kotani M, Matsumoto M, Fujita A, et al:
Persimmon leaf extract and astragalin inhibit development of
dermatitis and IgE elevation in NC/Nga mice. J Allergy Clin
Immunol. 106:159–166. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung UJ, Park YB, Kim SR and Choi MS:
Supplementation of persimmon leaf ameliorates hyperglycemia,
dyslipidemia and hepatic fat accumulation in type 2 diabetic mice.
PLoS One. 7:e 490302012. View Article : Google Scholar
|
|
14
|
Lee JH, Song MY, Song EK, et al:
Overexpression of SIRT1 protects pancreatic beta-cells against
cytokine toxicity by suppressing the nuclear factor-κB signaling
pathway. Diabetes. 58:344–351. 2009. View Article : Google Scholar :
|
|
15
|
Sun L, Zhang J, Lu X, Zhang L and Zhang Y:
Evaluation to the antioxidant activity of total flavonoids extract
from persimmon (Diospyros kaki L.) leaves. Food Chem Toxicol.
49:2689–2696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu YJ, Zhan J, Liu XL, Wang Y, Ji J and
He QQ: Dietary flavonoids intake and risk of type 2 diabetes: A
meta-analysis of prospective cohort studies. Clin Nutr. 33:59–63.
2014. View Article : Google Scholar
|
|
17
|
van Dam RM, Naidoo N and Landberg R:
Dietary flavonoids and the development of type 2 diabetes and
cardiovascular diseases: review of recent findings. Curr Opin
Lipidol. 24:25–33. 2013. View Article : Google Scholar
|
|
18
|
Kumar S, Narwal S, Kumar V and Prakash O:
α-glucosidase inhibitors from plants: A natural approach to treat
diabetes. Pharmacogn Rev. 5:19–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bouché C, Serdy S, Kahn CR and Goldfine
AB: The cellular fate of glucose and its relevance in type 2
diabetes. Endocr Rev. 25:807–830. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kodama H, Fujita M and Yamaguchi I:
Development of hyper-glycaemia and insulin resistance in conscious
genetically diabetic (C57BL/KsJ-db/db) mice. Diabetologia.
37:739–744. 1994. View Article : Google Scholar : PubMed/NCBI
|