Introduction
Tuberculosis (TB) is one of the most prevalent
infectious diseases worldwide, accounting for ~1.4 million
mortalities and 8.7 million novel cases annually, which occurs as a
results of Mycobacterium tuberculosis (Mtb) infection
(1). Due to Mtb reactivation at a
latent state in immunocompromised individuals, slow progress in
dealing with drug-resistant Mtb infection and the co-infection of
Mtb with human immunodeficiency virus, the global burden of TB
remains high, particularly in developing countries (2,3).
Effective vaccines are of key importance in ending
the global TB epidemic (1).
However, a consistently effective vaccine is not currently
available. The only available TB vaccine, attenuated
Mycobacterium bovis Bacillus Calmette Guerin (BCG) has made
a marked contribution to Mtb infection control, especially in
juvenile population and newborns (4). However, BCG does not provide
effective protection for all age groups, particularly in adults;
its protective efficacy is highly varied from different trials,
with certain studies observing negative effects associated with BCG
revaccination (0-80%) (5,6). Therefore, the development of more
effective vaccines or feasible vaccination strategies that provide
better protection from Mtb infection are urgently required.
It is widely accepted that homologous boosting with
the same vaccine is not sufficient for protecting against Mtb
(7); therefore, heterologous
prime-boost immunization strategies using BCG and a novel anti-TB
vaccine have been investigated. Such prime-boost vaccination
strategies have demonstrated the potential to elicit protective
immune responses, including cellular immune responses against Mtb
infection in animal models and in humans (8–12).
Mtb is an intracellular pathogen transmitted via a
mucosal route; mucosal and cellular immunity have thus been
suggested to have pivotal roles in protection against Mtb
infection. Therefore, a vector that is able to be delivered via a
mucosal route and elicit potent antigen-specific immune responses
may be an ideal candidate for anti-TB vaccines. Recombinant
adenoviral vectors have gained increasing attention in anti-TB
vaccine development due to their properties of type 1 immune
adjuvant activity, excellent safety record in humans and high
levels of antigen release as well as their suitability for
parenteral and intranasal mucosal delivery (13,14).
In addition, recombinant adenoviral vectors are highly effective at
eliciting robust cellular immunity in experimental animal models
(15), implicating them as
promising antigen delivery vectors for the development of an
anti-TB vaccine. In addition to a delivery vector, proper Mtb
antigens used for vaccine development are also key factor for
effectiveness of a vaccine candidate (16,17).
Previously, a number of microbial antigens of Mtb were tested as TB
vaccine candidates, including 10-kDa culture filtrate protein
(CFP10), 6-kDa early-secreted antigenic target (ESAT6), the 30–32
kDa family of three proteins [antigen 85 (Ag85)A, Ag85B and Ag85C],
the Mtb protein 64 (MPT64) and TB10.4 (a protein of 96 amino acids
with a theoretical molecular mass of 10.4 kDa) (18–23).
Among them, CFP10 and ESAT6 are immunodominant antigens encoded by
region of difference-1 (RD1) that are present in virulent strains
of Mtb and Mycobacterium bovis; however, these antigens are
absent in BCG (24–26). Loss of RD1 was hypothesized to be
the contributing factor for the attenuation of BCG (27,28);
therefore, RD1-encoded CFP10 and ESAT6 have often been selected as
potential antigen candidates in the development of novel anti-TB
vaccines (19,29–32).
In addition to CFP10 and ESAT6, Ag85A and Ag85B have also been
widely employed in anti-TB vaccine development (32–36).
In the present study, BCG and a recombinant
adenoviral vector (Ad5-CEAB) co-expressing CFP10, ESAT6, Ag85A and
Ag85B of Mtb were used in combination to investigate the effects of
this prime-boost strategy in mice.
Materials and methods
Animals
Female ICR mice (n=72, 6-8 weeks old) were purchased
from the Animal facility of Ningxia Medical University (Yinchuan,
China) and housed in a special pathogen-free environment with free
access to food and water and a constant temperature of 18°C. All
animal experiments were performed in accordance with the guidelines
of the Chinese Council on Animal Care and were approved by the
Committee for Animal Care and Use of Ningxia University.
Bacterial strains and Mtb antigens
The BCG vaccine, which was produced by Chengdu
Institute of Biological Products (Chengdu, China), was a gift from
the Centers for Disease Control and Prevention in Ningxia Province
of China (Ningxia, China) while colony-forming units (CFU) were
determined on 7H11 agar plates. For preparation of Mtb antigens of
CFP10, ESAT6, Ag85 and Ag85B, the target gene fragments were
amplified from Mtb H37Rv genomic DNA, which was extracted using
Myco DNAout Kit (Beijing Tiandz Gene Technology Company, Beijing,
China), by polymerase chain reaction (PCR), as previously described
(18). PCR fragments were codon
optimized prior to being subcloned in frame into a prokaryotic
expression plasmid pET-28a (Novagen, Madison, WI, USA) for E.
coli expression of His-tagged proteins (Novagen) (26). The His-tagged CFP10, ESAT6, Ag85A
and Ag85B proteins were purified using ÄKTA protein purification
system (GE Healthcare, Pittsburgh, PA, USA) according to the
manufacturer’s instruction. Endotoxins were removed from the
purified proteins using ToxinEraser™ Endotoxin Removal kit
(GenScript, Piscataway, NJ, USA) prior to use. The antigenic
proteins used in the present study had a purity of >85%, which
was determined as previously described (18).
Recombinant adenovirus Ad5-CEAB
preparation and immunization
The recombinant adenovirus Ad5-CEAB in which the
four genes of CFP10, ESAT6, Ag85A and Ag85B were expressed as a
mixture of proteins, rather than a fusion protein, was prepared as
described previously, and the titer of virus stock was determined
by a plaque assay (18). For
immunization, mice were randomly divided into three groups (n=8 per
group) as follows: Phosphate-buffered saline (PBS; Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) group, mice
were treated intranasally with 100 μl PBS three times
separated by two-week intervals; BCG group, mice were injected
subcutaneously with 1×106 CFU of BCG vaccine three times
separated by 2 week intervals; BCG/Ad5 group, mice received a
subcutaneous injection with 1×106 CFU of BCG and
following a 2 week interval, mice were intranasally boosted with
100 μl 1×109 plaque-forming units (PFU) of
Ad5-CEAB twice with 2 week intervals. At 2 weeks following the
final immunization, animals were euthanized under ether anesthesia
(Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China) by
exsanguination for analysis of immune responses.
Flow cytometric analysis of
splenocytes
Lymphocytes were isolated from the spleens of mice 2
weeks following the final immunization. Briefly, following
sacrifice, spleens were aseptically harvested and the mouse spleen
cells were obtained by carefully mashing the spleens with a syringe
plunger, passing the product through a cell strainer (BD
Biosciences, San Jose, CA, USA) and suspending it in preheated
(37°C) RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA). Splenocytes
from each mouse were then isolated through density gradient
centrifugation (1.092±0.001 g/ml; 500 × g, 20 min) with Mouse
Lymphocyte Separation Medium (Solarbio Science & Technology
Co., Ltd, Beijing, China). Splenocytes at a concentration of
5×106/ml were cultured in RPMI-1640 medium with 5% fetal
calf serum (FCS) (Gibco-BRL, Eggenstein, Germany) supplemented with
a mixture of purified Mtb proteins (containing 5 μg/ml of
each purified Mtb: CFP10, ESAT6, Ag85A and Ag85B; Mtb CEAB antigen
mixture) in an atmosphere of 5% CO2 at 37°C for 48 h.
The frequencies of CD4+ and CD8+ T cells were
characterized through flow cytometric analysis on a FACSCalibur
instrument (BD Biosciences). Briefly, splenocytes from each animal
were stained with a combination of allophycocyanin (APC) rat
anti-mouse CD4 (1 μg/mouse; 553051), phycoerythrin (PE) rat
anti-mouse CD8a (1 μg/mouse; 553033) and peridinin
chlorophyll (PerCP) hamster anti-mouse CD3e (1 μg/mouse;
553067) antibodies (diluted 1:500; BD Biosciences) for 30 min at
4°C. The APC rat immunoglobulin (Ig)G2aκ, PE rat IgG2aκ and PerCP
hamster IgG1κ isotype controls were included for isotype control
staining (diluted 1:500; BD Biosciences).
Antigen-specific lymphocyte proliferation
test
A total of 5×105 isolated splenocytes
from individual mice were seeded into 96-well plates and stimulated
in triplicate, with or without Mtb CEAB antigen mixture, in 5% FCS
RPMI-1640 medium at 37°C in an atmosphere of 5% CO2 for
72 h. T cell proliferation was evaluated using the CellTiter
96® Aqueous One Solution Cell Proliferation Assay
(Promega Corp., Madison, WI, USA), which is a sensitive
fluorescence based microplate assay, according to the
manufacturer’s protocol. The proliferative responses were expressed
as stimulation index (SI) that was calculated using the following
formula: SI = mean optical density (OD) value of antigen-stimulated
cells/mean OD value of control cells.
Enzyme-linked immunospot (ELISPOT) assays
for interferon (IFN)-γ
The frequency of splenic antigen-specific
IFN-γ-secreting spot forming cells (SFC) was determined by ELISPOT
using a Mouse IFN-γ ELISPOT Ready-SET-Go! Reagent set (eBioscience,
San Diego, CA, USA) according to the manufacturer’s instructions
with minor modifications. Briefly, isolated splenocytes were seeded
at a density of 1×105cells/well in a 96-well filter
plate (MSIPS4510; Millipore, Billerica, MA, USA) pre-coated with
mouse IFN-γ antibodies overnight. Cells were then incubated with or
without the stimulator (Mtb CEAB antigen mixture) for 40 h at 37°C.
Visible spots were counted under a light microscope (SZX16;
Olympus, Tokyo, Japan).
Cytokine induction and
quantification
Splenocytes at a concentration of
5×105/well were seeded into 24-well plates and cultured
in 5% FCS RPMI-1640 medium containing Mtb CEAB antigen mixture for
72 h at 37°C in an atmosphere of 5% CO2. Culture
supernatants were then harvested by centrifugation at 500 x g for
10 min and the concentrations of IFN-γ, tumor necrosis factor
(TNF)-α and interleukin (IL)-2 were determined using an
enzyme-linked immunosorbent assay (ELISA) cytokine detection system
(RayBiotech, Inc., Norcross, GA, USA) according to the
manufacture’s instructions. All experiments were performed in
triplicate.
ELISA assay for antigen-specific
secretory (s)IgA and IgG
For sIgA measurement, bronchoalveolar lavage (BAL)
samples were collected according to a method previously described
(37). SIgA in the supernatant of
BAL fluid was determined using an ELISA kit (eBioscience) according
to the manufacturer’s protocol. ELISA plates were pre-coated with
Mtb CEAB antigen mixture (5 mg/ml) at 4°C overnight.
For IgG measurement, mouse peripheral blood (~600
ml) was collected 2 weeks following the final immunization and the
concentration of serum antigen-specific IgG was ascertained using a
mouse ELISA Ready-SET-Go! kit (eBio-science) according to the
manufacturer’s instructions with minor modifications. ELISA plates
were customized by pre-coating with Mtb CEAB antigen mixture at 4°C
overnight, rather than directly coated with the capturing
antibodies provided in the kits.
Statistical analysis
Experimental data were expressed as the mean ±
standard deviation. Differences between groups were analyzed using
a one-way analysis of variance followed by Tukey’s post-hoc test
with SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA).
Results
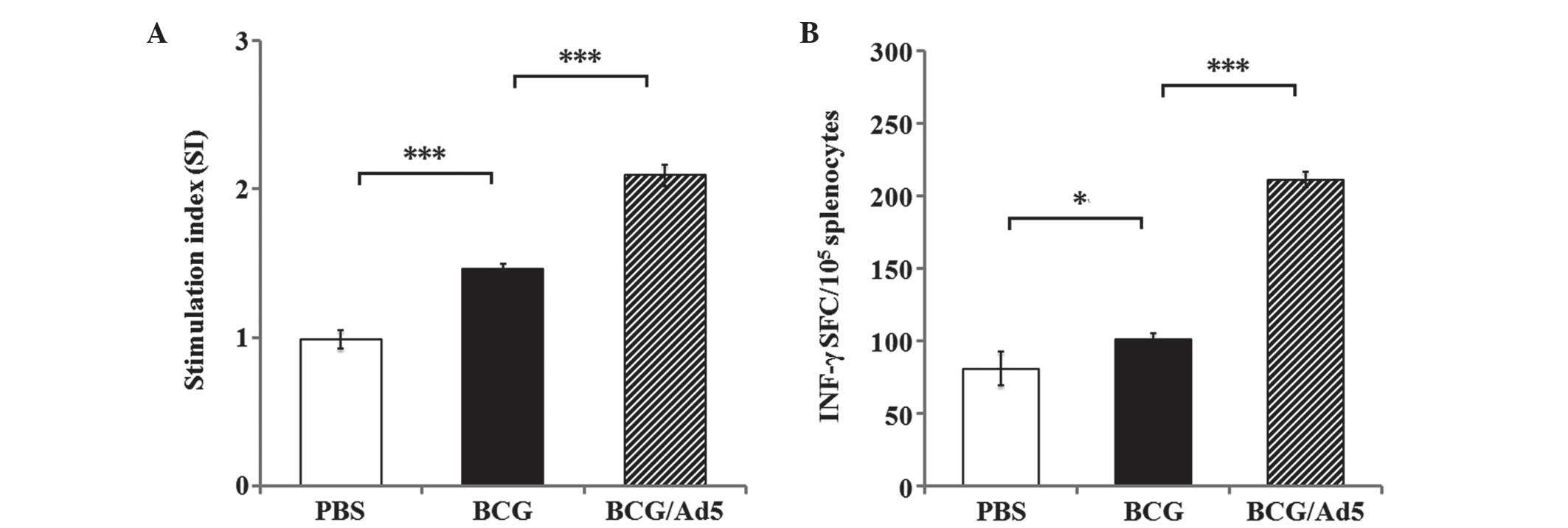
Antigen-specific T cell responses
It is widely accepted that T cell responses have
important roles in the host defense against Mtb infection (16). In the present study, the Mtb
antigen-specific T cell response was analyzed in vitro by
assessing the ability of the prime-boosted strategy to induce T
cell responses. The results of Mtb CEAB antigen-specific splenic T
cell responses revealed a significantly elevated splenic T cell
proliferation in immunized mice in the BCG and BCG/Ad5 groups
compared with the PBS-treated group (Fig. 1A). In addition, the IFN-γ ELISPOT
assay revealed an increased frequency of Mtb antigen-specific
IFN-γ-secreting splenic T cells in the mice immunized with BCG
(P<0.05) and BCG/Ad5 (P<0.001) compared with the PBS-treated
group (Fig. 1B). Furthermore,
higher frequencies of CD4+ (Fig. 2A) and CD8+ (Fig. 2B) T cell populations were observed
in mice immunized with BCG (P<0.001) and BCG/Ad5 (P<0.001)
compared with the PBS-treated group. Of note, all of the above
examined indexes of immune response in mice immunized with BCG/Ad5
were significantly increased compared with those of the BCG group.
These results clearly demonstrated that the subcutaneous BCG prime
mucosal Ad5-CEAB-boosted strategy was capable of stimulating a more
potent antigen-specific T cell response in mice compared with that
of the BCG group.
Antigen-specific cytokines responses
Cytokines were previously suggested to have
important roles in the host defense against Mtb (16). In the present study, concentrations
of cyto-kines INF-γ, TNF-α and IL-2 were detected using ELISA
analysis of the culture supernatant of lymphocytes stimulated with
Mtb antigens in vitro (Fig.
3). The results showed that the levels of all the tested
cytokines were significantly higher in the BCG
prime-Ad5-CEAB-boosted group compared with the PBS-treated group.
Of note, significantly elevated levels of antigen-induced cytokines
INF-γ (P<0.001) (Fig. 3A),
TNF-α (P<0.05) (Fig. 3B) and
IL-2 (P<0.001) (Fig. 3C) were
reported in the BCG prime-Ad5-CEAB-boosted group compared with the
BCG group. This therefore indicated that the BCG prime-Ad5-CEAB
boost strategy had a greater potency to enhance antigen-specific
immunity in mice compared with BCG alone.
Antigen-specific antibody responses
Mucosal immunity is known to have important roles
against Mtb infection and sIgA is the most abundant antibody
isotype produced in mucosal tissues (38); therefore, sIgA production was
examined in BAL fluid of mice. As shown in Fig. 4A, sIgA levels in BAL were markedly
elevated in mice immunized with the BCG/Ad5 compared with the BCG
group (P<0.01). However, no statistically significant difference
was observed between the BCG group and PBS-treated group
(P>0.05) (Fig. 4A). These data
suggested that the prime-boost strategy was able to potently
augment mucosal immune responses in vivo.
Humoral immunity has also been demonstrated to have
a protective role in mycobacterial infections (39). In order to evaluate the IgG
antibody response in immunized mice in the BCG or BCG prime
Ad5-CEAB-boosted groups, the titers of IgG in mice were examined at
2 weeks following the final immunization. As shown in Fig. 4B, mice immunized with BCG or
BCG/Ad5 elicited significantly higher titer of antigen-specific IgG
compared with the group treated with PBS. Furthermore, IgG levels
in mice immunized with BCG/Ad5 were significantly higher compared
with those of the BCG group (P<0.001), which indicated that the
prime-boosted strategy may elicit more efficient antibody
responses.
Discussion
It has been reported that repeated vaccination with
the same vaccine induces increased levels of antibody production
compared with a single vaccination. However, such homologous boosts
with the same vaccine may not be sufficient for protection against
intracellular pathogens, such as Mtb (7). Studies in humans have demonstrated
that revaccination with BCG does not confer additional protection
against TB (40,41) and certain studies in humans and
animals even reported negative effects associated with BCG
revaccination (40-43). However, heterologous prime-boost
strategies using BCG and a novel anti-TB vaccine may elicit robust
immune responses, which are more efficacious than BCG alone. Since
BCG is the only commonly used anti-TB vaccine in most developing
countries, employment of a second vaccine to boost BCG-primed
immunity may be the most practical novel strategy. In accordance
with the heterologous prime-boost strategy, the present study
investigated the safety and effi-cacy of a novel recombinant
vaccine candidate, Ad5-CEAB, using the BCG-prime-boost strategy in
mice. To the best of our knowledge, the recombinant adenovirus
Ad5-CEAB used as booster in the present study was the first attempt
for the co-expression of four Mtb antigens as a mixture of
individual proteins. The results demonstrated that the adenovirus
vector may be a promising novel vaccine platform capable of
boosting BCG-induced immunity.
Vaccines against intracellular infections are
dependent on the induction of cell-mediated immunity (44). As an intracellular pathogen, Mtb
localizes to the vacuole of the host’s macrophages and cellular
immunity has a crucial role in the immune response against Mtb
infection. The cellular immune response is primarily composed of
CD4+, CD8+ and other subsets of T cells.
CD4+ T cells were reported to contribute to the initial
resistance to Mtb via the production of IFN-γ and other cytokines
in order to induce macrophage activation (45). However, CD8+ T cells
produce IFN-γ and cytokines in addition to producing perforin and
granulysin, which act to directly kill Mtb-infected cells and
attack the invaded Mtb bacilli (46). In the present study, significantly
increased frequencies of antigen-specific CD4+,
CD8+ and INF-γ-secreting T cells were detected in the
splenocytes of mice boosted with Ad5-CEAB compared with those
primed with BCG alone. In addition to its ability to induce
antigen-specific T cell responses, the prime-boost strategy also
displayed a capacity to augment antigen-specific T helper type-1
cytokine production, including the secretion of INF-γ, TNF-α and
IL-2. Cytokines have also been demonstrated to have important roles
in host defense against Mtb infection. For example, IFN-γ was
reported to activate infected macrophages and directly inhibit
intracellular replication and growth of Mtb (47,48).
By contrast, TNF-α was demonstrated to be essential for the
initiation of the immune response against Mtb infection (49).
In addition to T cell responses, the prime-boost
strategy exhibited a capacity to evoke antibody responses in the
present study. Antibody responses have a protective role in
preventing mycobacterial infections, particularly the mucosal
antibodies. For example, sIgA, the most abundant naturally-produced
antibody isotype in mucosal tissue, has an indispensable role in
preventing primary Mtb infection at the mucosal surfaces; in
addition, sIgA was reported to prevent the adsorption of pathogens
at the mucosal epithelium (50,51).
A murine study demonstrated that sIgA may act to prevent the
entrance of mycobacterial bacilli into the lungs (52). The results of the present study
revealed that the BCG prime and mucosal Ad5-CEAB boost strategy was
able to significantly augment mucosal immune responses in
vivo.
Antigen-specific IgG antibodies are commonly used as
biomarkers to confirm the expression of Mtb antigens in animal
models; however, the role of serum antibodies in the pathogenesis
and control of TB has been controversial for a long time. Previous
studies have demonstrated that serum antibodies may have protective
effect in animal models of tuberculosis (39,53).
In addition, analysis of the isotypic distribution of
immuno-globulin may offer an insight into the possible
immunological mechanisms involved in cellular immunity (54). Together with the observation of
increased CD4+ and CD8+ T cell populations in
BCG/Ad5-CEAB-immunized mice, the results of the present study
clearly demonstrated that the BCG prime mucosal Ad5-CEAB boost
vaccination strategy effectively evoked the immune system for T
cell- and antibody-mediated antigen-specific immune responses in
mice.
The mucosal immune response is the first line of
defense against infectious agents and is crucial for the immune
response against Mtb infection. Increasing evidence has indicated
the effectiveness of vaccination at the mucosal site compared with
vaccination via other routes for inducing protection from mucosal
infectious diseases (15,55). Numerous studies have verified that
mucosal immunity may provide unique advantages for protection
against Mtb infection (34,56–58).
Therefore, any vaccines or vaccination strategies that are able to
elicit the mucosal immune response may enhance the efficacy of
protection against Mtb infection. Great efforts have been made to
improve the protective efficacy of TB vaccines and various types of
vaccine candidates or vectors have been developed, including the
recombinant BCG (rBCG), DNA vaccines, nanoparticle vaccines,
recombinant modified vaccinia virus Ankara and recombinant
adenoviral-based vaccines (12,15,32,59,60).
Among them, the adenoviral-based TB vaccines have gained increased
attention, as they were first evaluated as mucosal TB vaccine
candidates (15). An increasing
number of studies have thus focused on mucosal immunity; these
studies have suggested that intranasal/intrapulmonary vaccination
with recombinant adenoviral vaccines may induce an antigen-specific
mucosal immune response (15,61–66).
In line with these findings, the results of the present study
demonstrated that intranasal boost with the recombinant adenovirus
Ad5-CEAB was able to enhance the BCG-primed immune response. In
addition, the present study reported that levels of
antigen-specific sIgA in BAL fluid were significantly increased in
the Ad5-CEAB-boosted group, indicating that intranasal boost with
Ad5-CEAB may induce a potent antigen-specific mucosal immune
response in mice.
In conclusion, the results of the present study
demonstrated that the heterologous prime-boost strategy of
subcutaneously primed BCG-intranasal boost with recombinant
adenovirus Ad5-CEAB was able to elicit an enhanced antigen-specific
immune response in mice compared with that conferred by homologous
prime-boost immunization with BCG. These results provided evidence
for the effectiveness of TB vaccines from recombinant adenoviral
vectors and novel anti-TB vaccination strategies. The BCG prime
Ad5-CEAB boost vaccination strategy appears promising as an anti-TB
vaccination strategy, thus we aim to evaluate this in mice infected
with Mtb in future studies.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 31160515), the
National Key Basic Research Program of China (973 Program) (nos.
2012CB126301 and 2012CB518801) and the Key Technologies Research
and Development Program of China (no. 2012BAD12B07-4).
References
|
1
|
World Health Organization (WHO): Global
Tuberculosis Report 2012. WHO; Geneva, Switzerland: pp. 3062012
|
|
2
|
Churchyard GJ, Chaisson RE, Maartens G and
Getahun H: Tuberculosis preventive therapy: An underutilised
strategy to reduce individual risk of TB and contribute to TB
control. S Afr Med J. 104:339–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muwonge A, Malama S, Johansen TB, et al:
Molecular epidemiology, drug susceptibility and economic aspects of
tuberculosis in Mubende district, Uganda. PLoS One. 8:e647452013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Awasthi S and Moin S: Effectiveness of BCG
vaccination against tuberculous meningitis. Indian Pediatr.
36:455–460. 1999.
|
|
5
|
Nuttall JJ, Davies MA, Hussey GD and Eley
BS: Bacillus Calmette-Guérin (BCG) vaccine-induced complications in
children treated with highly active antiretroviral therapy. Int J
Infect Dis. 12:e99–e105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bolger T, O’Connell M, Menon A and Butler
K: Complications associated with the bacille Calmette-Guérin
vaccination in Ireland. Arch Dis Child. 91:594–597. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McShane H and Hill A: Prime-boost
immunisation strategies for tuberculosis. Microbes Infect.
7:962–967. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dean G, Whelan A, Clifford D, et al:
Comparison of the immunogenicity and protection against bovine
tuberculosis following immunization by BCG-priming and boosting
with adenovirus or protein based vaccines. Vaccine. 32:1304–1310.
2014. View Article : Google Scholar
|
|
9
|
Hoft DF, Blazevic A, Stanley J, et al: A
recombinant adenovirus expressing immunodominant TB antigens can
significantly enhance BCG-induced human immunity. Vaccine.
30:2098–2108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perez de Val B, Villarreal-Ramos B,
Nofrarías M, et al: Goats primed with Mycobacterium bovis BCG and
boosted with a recombinant adenovirus expressing Ag85A show
enhanced protection against tuberculosis. Clin Vaccine Immunol.
19:1339–1347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dou J, Wang Y, Yu F, et al: Protection
against Mycobacterium tuberculosis challenge in mice by DNA vaccine
Ag85A-ESAT-6-IL-21 priming and BCG boosting. Int J Immunogenet.
39:183–190. 2012. View Article : Google Scholar
|
|
12
|
Cervantes-Villagrana AR, Hernández-Pando
R, Biragyn A, et al: Prime-boost BCG vaccination with DNA vaccines
based in β-defensin-2 and mycobacterial antigens ESAT6 or Ag85B
improve protection in a tuberculosis experimental model. Vaccine.
31:676–684. 2013. View Article : Google Scholar
|
|
13
|
Xing Z and Lichty BD: Use of recombinant
virus-vectored tuberculosis vaccines for respiratory mucosal
immunization. Tuberculosis. 86:211–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lasaro MO and Ertl HC: New insights on
adenovirus as vaccine vectors. Mol Ther. 17:1333–1339. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Thorson L, Stokes RW, et al:
Single mucosal, but not parenteral, immunization with recombinant
adenoviral-based vaccine provides potent protection from pulmonary
tuberculosis. J Immunol. 173:6357–6365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Deng G, Li M, Liu X and Wang Y:
Roles of mucosal immunity against Mycobacterium tuberculosis
infection. Tuberc Res Treat. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cripps AW, Kyd JM and Foxwell AR: Vaccines
and mucosal immunisation. Vaccine. 19:2513–2515. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Deng G, Li M, et al: A recombinant
adenovirus expressing CFP10, ESAT6, Ag85A and Ag85B of
Mycobacterium tuberculosis elicits strong antigen-specific immune
responses in mice. Mol Immunol. 62:86–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H, Peng P, Miao S, et al:
Recombinant Mycobacterium smegmatis expressing an ESAT6-CFP10
fusion protein induces anti-mycobacterial immune responses and
protects against Mycobacterium tuberculosis challenge in mice.
Scand J Immunol. 72:349–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang Y, Wu X, Zhang J, et al:
Immunogenicity and therapeutic effects of Ag85A/B chimeric DNA
vaccine in mice infected with Mycobacterium tuberculosis. FEMS
Immunol Med Microbiol. 66:419–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pydi SS, Bandaru AR, Venkatasubramanian S,
et al: Vaccine for tuberculosis: up-regulation of IL-15 by Ag85A
and not by ESAT-6. Tuberculosis (Edinb). 91:136–139. 2011.
View Article : Google Scholar
|
|
22
|
Sibley L, Reljic R, Radford DS, et al:
Recombinant Bacillus subtilis spores expressing MPT64 evaluated as
a vaccine against tuberculosis in the murine model. FEMS Microbiol
Lett. 358:170–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi S, Yu L, Sun D, Liu J and Hickey AJ:
Rational design of multiple TB antigens TB10.4 and TB10.4-Ag85B as
subunit vaccine candidates against Mycobacterium tuberculosis.
Pharm Res. 27:224–234. 2010. View Article : Google Scholar
|
|
24
|
Berthet FX, Rasmussen PB, Rosenkrands I,
Andersen P and Gicquel B: A Mycobacterium tuberculosis operon
encoding ESAT-6 and a novel low-molecular-mass culture filtrate
protein (CFP-10). Microbiology. 144(Pt 11): 3195–3203. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gordon SV, Brosch R, Billault A, Garnier
T, Eiglmeier K and Cole ST: Identification of variable regions in
the genomes of tubercle bacilli using bacterial artificial
chromosome arrays. Mol Microbiol. 32:643–655. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Behr MA, Wilson MA, Gill WP, et al:
Comparative genomics of BCG vaccines by whole-genome DNA
microarray. Science. 284:1520–1523. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lewis KN, Liao R, Guinn KM, et al:
Deletion of RD1 from Mycobacterium tuberculosis mimics Bacille
Calmette-Guerin attenuation. J Infect Dis. 187:117–123. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pym AS, Brodin P, Brosch R, Huerre M and
Cole ST: Loss of RD1 contributed to the attenuation of the live
tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium
microti. Mol Microbiol. 46:709–717. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
You Q, Wu Y, Jiang D, et al: Immune
responses induced by heterologous boosting of recombinant bacillus
Calmette-Guerin with Ag85B-ESAT6 fusion protein in levamisole-based
adjuvant. Immunol Invest. 41:412–428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan W, Dong N, Zhang L, et al:
Immunogenicity and protective efficacy of a tuberculosis DNA
vaccine expressing a fusion protein of Ag85B-Esat6-HspX in mice.
Vaccine. 30:2490–2497. 2012. View Article : Google Scholar
|
|
31
|
Esparza-González SC, Troy A, Troudt J, et
al: Recombinant adenovirus delivery of calreticulin-ESAT-6 produces
an antigen-specific immune response but no protection against a
Mycobacterium tuberculosis challenge. Scand J Immunol. 75:259–265.
2012. View Article : Google Scholar
|
|
32
|
Lin CW, Su IJ, Chang JR, Chen YY, Lu JJ
and Dou HY: Recombinant BCG coexpressing Ag85B, CFP10 and
interleukin-12 induces multifunctional Th1 and memory T cells in
mice. APMIS. 120:72–82. 2012. View Article : Google Scholar
|
|
33
|
Betts G, Poyntz H, Stylianou E, et al:
Optimising immunoge-nicity with viral vectors: mixing MVA and
HAdV-5 expressing the mycobacterial antigen Ag85A in a single
injection. PLoS One. 7:e504472012. View Article : Google Scholar
|
|
34
|
Dietrich J, Andersen C, Rappuoli R,
Doherty TM, Jensen CG and Andersen P: Mucosal administration of
Ag85B-ESAT-6 protects against infection with Mycobacterium
tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J
Immunol. 177:6353–6360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dou J, Tang Q, Yu F, et al: Investigation
of immunogenic effect of the BCG priming and Ag85A-GM-CSF boosting
in Balb/c mice model. Immunobiology. 215:133–142. 2010. View Article : Google Scholar
|
|
36
|
Lu D, Garcia-Contreras L, Muttil P, et al:
Pulmonary immunization using antigen 85-B polymeric microparticles
to boost tuberculosis immunity. AAPS J. 12:338–347. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wakeham J, Wang J, Magram J, et al: Lack
of both types 1 and 2 cytokines, tissue inflammatory responses and
immune protection during pulmonary infection by Mycobacterium bovis
bacille Calmette-Guerin in IL-12-deficient mice. J Immunol.
160:6101–6111. 1998.PubMed/NCBI
|
|
38
|
Williams A, Reljic R, Naylor I, et al:
Passive protection with immunoglobulin A antibodies against
tuberculous early infection of the lungs. Immunology. 111:328–333.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Borrero R, García Mde L, Canet L, et al:
Evaluation of the humoral immune response and cross reactivity
against Mycobacterium tuberculosis of mice immunized with liposomes
containing glycolipids of Mycobacterium smegmatis. BMC Immunol.
14(Suppl 1): 132013. View Article : Google Scholar
|
|
40
|
Rodrigues LC, Pereira SM, Cunha SS, et al:
Effect of BCG revaccination on incidence of tuberculosis in
school-aged children in Brazil: the BCG-REVAC cluster-randomised
trial. Lancet. 366:1290–1295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dantas OM, Ximenes RA, de Albuquerque Mde
F, et al: A case-control study of protection against tuberculosis
by BCG revaccination in Recife, Brazil. Int J Tuberc Lung Dis.
10:536–541. 2006.PubMed/NCBI
|
|
42
|
Basaraba RJ, Izzo AA, Brandt L and Orme
IM: Decreased survival of guinea pigs infected with Mycobacterium
tuberculosis after multiple BCG vaccinations. Vaccine. 24:280–286.
2006. View Article : Google Scholar
|
|
43
|
Buddle B, Wedlock D, Parlane N, et al:
Revaccination of neonatal calves with Mycobacterium bovis BCG
reduces the level of protection against bovine tuberculosis induced
by a single vaccination. Infect Immun. 71:6411–6419. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Seder RA and Hill AV: Vaccines against
intracellular infections requiring cellular immunity. Nature.
406:793–798. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Flynn JL and Chan J: Immunology of
tuberculosis. Annu Rev Immunol. 19:93–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Woodworth JS, Wu Y and Behar SM:
Mycobacterium tuberculosis-specific CD8+T cells require perforin to
kill target cells and provide protection in vivo. J Immunol.
181:8595–8603. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Szabo SJ, Sullivan BM, Stemmann C,
Satoskar AR, Sleckman BP and Glimcher LH: Distinct effects of T-bet
in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T
cells. Science. 295:338–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharma M, Sharma S, Roy S, Varma S and
Bose M: Pulmonary epithelial cells are a source of interferon-γ in
response to Mycobacterium tuberculosis infection. Immunol Cell
Biol. 85:229–237. 2007.PubMed/NCBI
|
|
49
|
Bean AG, Roach DR, Briscoe H, et al:
Structural deficiencies in granuloma formation in TNF gene-targeted
mice underlie the heightened susceptibility to aerosol
Mycobacterium tuber- culosis infection, which is not compensated
for by lymphotoxin. J Immunol. 162:3504–3511. 1999.PubMed/NCBI
|
|
50
|
Mazanec MB, Nedrud JG, Kaetzel CS and Lamm
ME: A three-tiered view of the role of IgA in mucosal defense.
Immunol Today. 14:430–435. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Williams R and Gibbons R: Inhibition of
bacterial adherence by secretory immunoglobulin A: a mechanism of
antigen disposal. Science. 177:697–699. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tjärnlund A, Rodríguez A, Cardona PJ, et
al: Polymeric IgR knockout mice are more susceptible to
mycobacterial infections in the respiratory tract than wild-type
mice. Int Immunol. 18:807–816. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Olivares N, Marquina B, Mata-Espinoza D,
et al: The protective effect of immunoglobulin in murine
tuberculosis is dependent on IgG glycosylation. Pathog Dis.
69:176–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mosmann T and Coffman R: TH1 and TH2
cells: Different patterns of lymphokine secretion lead to different
functional properties. Annu Rev Immunol. 7:145–173. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lamichhane A, Azegamia T and Kiyonoa H:
The mucosal immune system for vaccine development. Vaccine.
32:6711–6723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Goonetilleke NP, McShane H, Hannan CM,
Anderson RJ, Brookes RH and Hill AV: Enhanced immunogenicity and
protective efficacy against Mycobacterium tuberculosis of bacille
Calmette-Guérin vaccine using mucosal administration and boosting
with a recombinant modified vaccinia virus Ankara. J Immunol.
171:1602–1609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen L, Wang J, Zganiacz A and Xing Z:
Single intranasal mucosal Mycobacterium bovis BCG vaccination
confers improved protection compared to subcutaneous vaccination
against pulmonary tuberculosis. Infect Immun. 72:238–246. 2004.
View Article : Google Scholar :
|
|
58
|
Santosuosso M, McCormick S, Zhang X,
Zganiacz A and Xing Z: Intranasal boosting with an
adenovirus-vectored vaccine markedly enhances protection by
parenteral Mycobacterium bovis BCG immunization against pulmonary
tuberculosis. Infect Immun. 74:4634–4643. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yu F, Wang J, Dou J, et al:
Nanoparticle-based adjuvant for enhanced protective efficacy of DNA
vaccine Ag85A-ESAT-6-IL-21 against Mycobacterium tuberculosis
infection. Nanomedicine. 8:1337–1344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ndiaye BP, Thienemann F, Ota M, et al:
Safety, immunogenicity, and efficacy of the candidate tuberculosis
vaccine MVA85A in healthy adults infected with HIV-1: A randomised,
placebo-controlled, phase 2 trial. Lancet Respir Med. 3:190–200.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Croyle MA, Patel A, Tran KN, et al: Nasal
delivery of an adenovirus-based vaccine bypasses pre-existing
immunity to the vaccine carrier and improves the immune response in
mice. PLoS One. 3:e35482008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lemiale F, Kong WP, Akyurek LM, et al:
Enhanced mucosal immunoglobulin A response of intranasal adenoviral
vector human immunodeficiency virus vaccine and localization in the
central nervous system. J Virol. 77:10078–10087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Richardson JS, Abou MC, Tran KN, Kumar A,
Sahai BM and Kobinger GP: Impact of systemic or mucosal immunity to
adenovirus on Ad-based Ebola virus vaccine efficacy in guinea pigs.
J Infect Dis. 204(Suppl 3): 1032–1042. 2011. View Article : Google Scholar
|
|
64
|
Santosuosso M, Zhang X, McCormick S, Wang
J, Hitt M and Xing Z: Mechanisms of mucosal and parenteral
tuberculosis vaccinations: adenoviral-based mucosal immunization
preferentially elicits sustained accumulation of immune protective
CD4 and CD8 T cells within the airway lumen. J Immunol.
174:7986–7994. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shim BS, Stadler K, Nguyen HH, et al:
Sublingual immunization with recombinant adenovirus encoding
SARS-CoV spike protein induces systemic and mucosal immunity
without redirection of the virus to the brain. Virol J. 9:2152012.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kaufman DR, Bivas-Benita M, Simmons NL,
Miller D and Barouch DH: Route of adenovirus-based HIV-1 vaccine
delivery impacts the phenotype and trafficking of vaccine-elicited
CD8+ T lymphocytes. J Virol. 84:5986–5996. 2010. View Article : Google Scholar : PubMed/NCBI
|