Introduction
Bone marrow contains a rare population of
mesenchymal stem cells (BMSCs), derived from the mesodermal layer
during embryologic development, and represents a multilineage
potential to differentiate into mesodermal lineages of mesenchymal
tissues, including cartilage, bone, fat, muscle and tendon
(1,2). Previous studies have demonstrated
that BMSCs even differentiate into tissues in various germ layers
beyond the normal embryonic limitation (mesodermal layer). For
example, BMSCs have the potential to differentiate into neuronal
(ectoderm) (3), pancreatic
(4) and hepatic (entoderm)
(5) tissue.
In the last decades, the isolation and culture of
BMSCs has been well illustrated (6), and they can be further purified by
their cell surface expression as specific markers (7). Moreover, the particular techniques to
induce BMSC differentiation into chondrocytes (8), osteocytes (9), adipocytes (10) and myocytes (11) have been described. BMSCs provide
excellent candidates for cell-based tissue engineering due to their
self-renewal and multipotent differentiation capacity (12).
Mesenchymal stem cells derived from the bone marrow
differentiate along tissue-specific lineages and microenvironments
when transplanted to an organ defect. Guan et al developed a
specific peptidomimetic ligand to direct BMSCs to the defect bone
surface and induced bone regeneration and bone strength (9). Additionally, cartilage defects have
been enhanced and regenerated by the transplantation of BMSCs in a
rabbit model (13) and clinical
patients (14). Furthermore,
Horwitz et al demonstrated that transplantation of BMSCs in
children with osteogenesis and imperfecta allogeneic increased bone
marrow density (15).
There have been great achievements in tissue
engineering regeneration in BMSCs, and the molecular changes in
gene expression profiles during proliferation, differentiation and
redifferentiation are crucial to explain the mechanisms of the
multipotent differentiation process, which depends on reliable,
accurate, fast and sensitive methods. As a powerful technique to
rapidly quantify gene expression, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) is
likely to play a vital role in deciphering the cellular and
molecular properties of BMSC differentiation with high sensitivity.
As a quantitative analysis, however, RT-qPCR depends on an
appropriate reference gene to normalize cell number, RNA
extraction, sample-to-sample variations, reverse transcription and
amplification efficiency differences (16). The appropriate reference genes are
required to be constitutively expressed in the cells or tissue for
the various investigations, generally undergoing basic cellular
functions and being expressed at abundant levels (17). However, evidence has revealed that
the commonly used reference gene expression levels, although
occasionally constant in certain cell types and experimental
conditions, varied considerably in different tissue, BMSC
proliferation and differentiation (18). There are still no ideal and
universal reference genes suitable for all experimental conditions
(17). As a result, the presumed
stability of the reference gene expression must be validated under
the particular experimental conditions being investigated, although
validation is lacking in most current research (19). Much research has been carried out
to identify a suitable reference gene for BMSCs; however, no
comprehensive conclusion has been drawn due to the few reference
genes and experimental conditions studied (20).
In the present study, we selected a series of
well-known and commonly used reference genes, and validated the
gene expression during BMSC differentiation to osteogenic and
chondrogenic lineages.
Materials and methods
Isolation and culture of rabbit
BMSCs
The present study was approved by the Ethics
Committee of the First Hospital of Jilin University (Changchun,
Jilin). Rabbit BMSCs were isolated and cultured according to
Haynesworth et al (21).
Briefly, 4-week-old New Zealand rabbits from the animal center of
Jilin University, China were anesthetized and the bone marrow was
washed out by a needle with low-glucose Dulbecco’s modified Eagle’s
medium (DMEM) containing 10% fetal bovine serum (FBS; Gibco Life
Technologies, Carlsbad, CA, USA), penicillin (50 U/ml) and
streptomycin (50 U/ml). Cells were incubated at 37°C with 5%
CO2. Non-adherent cells were removed on day 3 at the
first change of medium. When the adherent mesenchymal stem cells
became confluent, the BMSCs were digested, detached and
continuously passaged for subculture.
Chondrogenic differentiation
The BMSCs were seeded in six-well plates at 100,000
cells per well in 2 ml chondrogenic medium. Chondrogenic medium was
composed of high-glucose DMEM with 10% FBS, 10 ng/ml transforming
growth factor β-1 (Peprotech, Inc., Rocky Hill, NJ, USA) and 50 mM
ascorbate-2-phosphate (Sigma-Aldrich, St. Louis, MO, USA). The
differentiation medium was replaced twice a week for 14 days.
Osteogenic differentiation
Generally, the BMSCs were seeded in six-well plates
at 100,000 cells per well in 2 ml osteogenic medium, composed of
high-glucose DMEM with 10% FBS, 10 nM dexamethasone
(Sigma-Aldrich), 50 mM ascorbate-2-phosphate (Sigma-Aldrich) and 2
mM β-glycerophosphate (Sigma-Aldrich). The differentiation medium
was replaced twice a week for 14 days.
Selection of potential reference genes
and design of reference primers
In the present study, 12 candidate reference genes
were selected for investigation in accordance with a previous study
(16). Primer pairs of the 12
candidate reference genes were designed by the Primer Premier 5.0
software (Premier Biosoft, Palo Alto, CA, USA). The high scored
primer pairs were checked using the Primer-BLAST tool from NCBI to
ensure the specificity of amplification. The primer pair sequences
of the 12 candidate reference genes are listed in Table I. Prior to the expression analysis
of the reference genes, the specificity of the primers was
evaluated by the melting curve method following amplification with
qPCR, where a single high and thin peak was considered as good
specificity. Furthermore, the end-products were electrophoresed
with 1.5% agarose gel and stained with ethidium bromide. The single
product appeared at the expected site, suggesting good
specificity.
 | Table IPrimer sequences of candidate
reference genes for the differentiation of bone marrow mesenchymal
stem cells. |
Table I
Primer sequences of candidate
reference genes for the differentiation of bone marrow mesenchymal
stem cells.
| Symbol | Genebank | Gene name | Primer sequence
(forward/reverse) | Polymerase chain
reaction product length (bp) |
|---|
| ACTB | NM_001101683.1 | β-actin | F:
ATCAGCAAGCAGGAGTATGAC
R: GCCAATCTCGTCTCGTTTCT | 135 |
| GAPDH | NM_001082253.1 |
Glyceraldehyde-3-phosphate
dehydrogenase | F:
ATGGTGAAGGTCGGAGTGAA
R: GGGTGGAATCATACTGGAACA | 151 |
| PPIA | NM_001082057.1 | Peptidylprolyl
isomerase | F:
TCTCACCCACCTGACCATTC
R: GCAGACACGGAACCAAAGAC | 107 |
| RPL13a | XM_002723915.1 | Ribosomal protein
L13a | F:
CCGCCCTACGACAAGAAA
R: TACTTCCAGCCCACCTCAT | 119 |
| YWHAZ | XM_002721227.1 | Tyrosine
3-monooxygenase/tryptophan
5-monooxygenase activation protein, zeta | F:
CCAGGGAGATGAAGGAGATG
R: TCGCACAAAGGGATGTATGT | 106 |
| SDHA | XM_002723194.1 | Succinate
dehydrogenase complex, subunit A, flavoprotein | F:
GGAGACCCACAGGCTACAGA
R: AAGAAGGAAGCAAAGGGACAG | 115 |
| EF-1A | NM_001082339.1 | Eukaryotic
translation elongation factor 1 alpha 1 | F:
TTGGCTACAACCCTGACACA
R: GGTGACTTTCCATCCCTTGA | 110 |
| 18S rRNA | NR_033238.1 | 18S ribosomal
RNA | F:
ATCAGATACCGTCGTAGTTC
R: TTCCGTCAATTCCTTTAAG | 155 |
| B2M | XM_002717921.1 |
Beta-2-microglobulin-like | F:
AACGTGGAACAGTCAGACC
R: AGTAATCTCGATCCCATTTC | 157 |
| HPRT1 | NM_001105671.1 | Hypoxanthine
phosphoribosyltransferase 1 | F:
TGATTAGTGATGATGAACCG
R: CACACAGAGGGCTACAATG | 176 |
| RP-II | XM_002717160.1 | DNA directed RNA
polymerase II polypeptide B | F:
GCAAAATAAGGGTACGCTCTG
R: TGAAAGGCATGTCCTCTTGTC | 114 |
| TBP | XM_002723497.1 | TATA box binding
protein | F:
GAGAGCCACGAACCACAG
R: ACTTCACGTCACAGCTCCC | 183 |
Total RNA isolation and cDNA
synthesis
On days 1, 7 and 14 of BMSC chondrogenic and
osteogenic differentiation, cells were washed twice in
phosphate-buffered saline and total RNA was immediately isolated
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions. RNA
concentration was evaluated with a microplate reader (Tecan,
Männedorf, Switzerland) and the 260/280 ratio was between 1.8 and
2.0, which indicates pure RNA that is suitable for qPCR analysis.
Then 2 μg isolated total RNA was reverse transcribed to cDNA
according to the manufacturer’s instructions of the reverse
transcription kit (Takara Biotechnology Co., Ltd., Dalian, China).
Oligo dT and random primers were used to ensure that total mRNA was
transcribed to cDNA.
RT-qPCR
RT-qPCR was performed using the Mx3005P Multiplex
Quantitative PCR system (Stratagene, La Jolla, CA, USA), with qPCR
SYBR-Green qPCR reagents (Takara Biotechnology Co., Ltd.).
Approximately 100 ng cDNA and a final concentration of 200 nM
forward and reverse primer pairs were used for each qPCR reaction,
with ROX as the reference dye. The cycling conditions were as
follows: an initial cycle at 95°C for 15 sec, followed by 40 cycles
of 95°C degeneration for 5 sec, 60°C annealing and extension for 33
sec. The melting curve analysis was carried out on each sample to
ensure a single amplicon. Results were exported to Microsoft Excel
for analysis.
Evaluation of gene stability and
statistical analysis
The stability of candidate reference genes was
evaluated by various algorithms, including the geNorm (17), NormFinder and BestKeeper programs
and the comparative ΔCt method (22).
Normalization of collagen I, collagen II
and aggrecan as target genes
The effects of the reference gene variance on the
expression of collagen I, collagen II and aggrecan were analyzed
with the 2−ΔΔCt method, as markers of chondrogenic and
osteogenic differentiation of BMSCs. The primer pair sequence
target genes are listed in Table
II. The expression of genes on day 1 was designated as the
calibrator, and the target genes were normalized by the 12
reference genes and the recommended combination of geNorm,
Normfinder and BestKeeper.
 | Table IIPrimer sequences of target genes
collagen II, aggrecan and collagen I as markers of chondrogenic and
osteogenic differentiation of bone marrow mesenchymal stem
cells. |
Table II
Primer sequences of target genes
collagen II, aggrecan and collagen I as markers of chondrogenic and
osteogenic differentiation of bone marrow mesenchymal stem
cells.
| Symbol | Genebank | Gene name | Primer sequence
(forward/reverse) | Polymerase chain
reaction product length (bp) |
|---|
| Collagen II | NM_001195671.1 | Collagen type
II | F:
CTGTCCTGTGCGACGACATA
R: TCCTTTCTGCCCCTTTGGTC | 140 |
| Aggrecan | NW_003159560.1 | Aggrecan | F:
TGGAGAAGCCCTTGCATCTG
R: TGGGACGGAGGATGCTTCTA | 82 |
| Collagen I | AY633663.1 | Collagen type
I | F:
GAGGTGGACACCACCCTCAA
R: CCAGTGTCCATGTCGCAGAA | 200 |
Results
Isolation, culture and differentiation of
rabbit BMSCs
The techniques for the isolation of rabbit BMSCs
have been well investigated and are well established. In accordance
with a previous study (21), the
rabbit BMSCs were isolated after three passages. The cells are
shown in Fig. 1, and morphological
analysis clearly suggests highly purified BMSCs.
Expression profiles of candidate
reference genes
The Ct values of 12 candidate reference genes in the
chondrogenic and osteogenic differentiation of BMSCs were
calculated (Fig. 2) to compare the
various gene expression profiles. The Ct values for the 12
reference genes ranged from 10.18 (18s rRNA) to 28.29 (GAPDH),
demonstrating a wide variation. As shown in Fig. 2, 18s rRNA was highly expressed and
GAPDH had the lowest transcription value, with Ct values ranging
from 26.77 to 28.29. The individual reference genes had different
Ct value ranges and no single reference gene had a constant
expression level in the chondrogenic and osteogenic differentiation
of BMSCs.
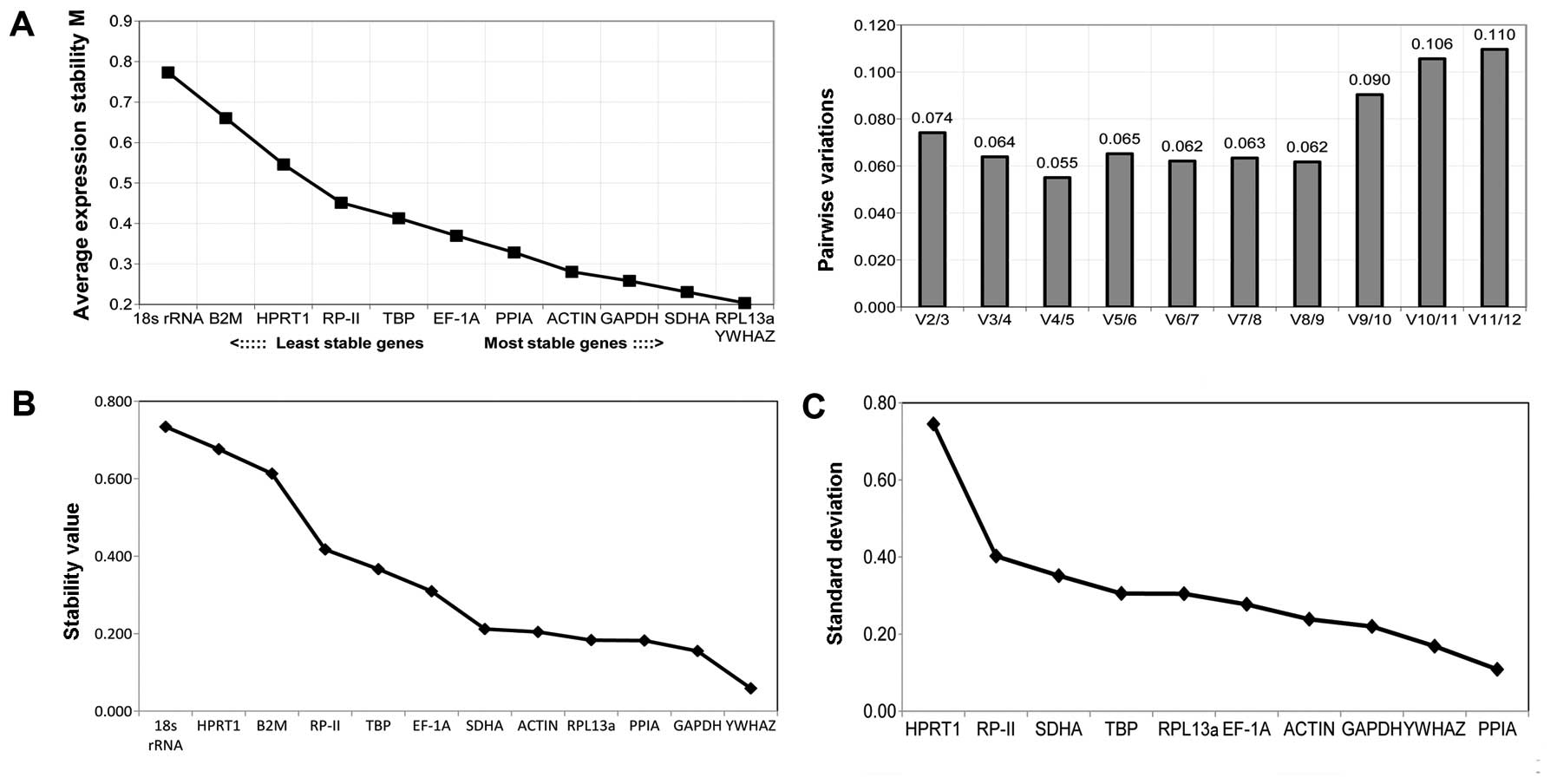
Osteogenic differentiation
The stability of reference genes during osteogenic
differentiation was evaluated by the geNorm, Normfinder and
BestKeeper software. As shown in Fig.
3A, geNorm assessed PPIA and HPRT1 as being the most stable
genes, followed by the genes TBP, RP-II and EF-1A, while the most
unstable genes were B2 M, YWHAZ and 18s rRNA. PPIA and HPRT1 were
sufficient as reference genes, with the V2/3 value of 0.071 much
lower than the suggested cut-off value of 0.15. Additionally,
similar results on gene stability were obtained by Normfinder
(Fig. 3B) and BestKeeper (Fig. 3C), which demonstrated that RPL13a
was the most stable reference gene, with a stability value of 0.091
and a standard deviation of 0.06. It was clearly demonstrated that
RPL13a, ACTB and EF-1A were moderately stable, while the best
combination of two reference genes was 18s rRNA and HPRT1, with a
stability value of 0.055 in Normfinder. Conversely, the stability
of B2M, YWHAZ and 18s rRNA was inconsistent with the geNorm
results, with higher stability values and standard deviation than
the other genes.
Chondrogenic differentiation
The stability of reference genes during chondrogenic
differentiation was also evaluated by the geNorm, Normfinder and
BestKeeper software. As shown in Fig.
4A, geNorm revealed RPL13 and YWHAZ to be the most stable
genes, followed by SDHA, GAPDH and ACTB. The most unstable genes
were B2 M, HPRT1 and 18s rRNA, with stability M-values higher than
0.5. Additionally, the V2/3 value was 0.074, much lower than the
suggested cut-off value, demonstrating that RPL13 and YWHAZ were
sufficient as reference genes to evaluate the chondrogenic
differentiation of BMSCs. Moreover, Normfinder (Fig. 4B) and BestKeeper (Fig. 4C) exhibited similar results on gene
stability in the chondrogenic differentiation, which were a little
different from the results of geNorm. Normfinder assessed YWHAZ as
the most stable gene, followed by GAPDH, PPIA and RPL13. However,
the value of the best combination of the two genes PPIA and RPL13
was 0.078, which was much larger than the value for YWHAZ alone,
suggesting that YWHAZ alone is sufficient for normalization.
Similar results were observed with BestKeeper, demonstrating PPIA,
YWHAZ, GAPDH and ACTB to be the most stable genes. Conversely, B2M,
HPRT1 and 18s rRNA were revealed to be the most unstable genes
during chondrogenic differentiation, with higher M-values,
stability values and standard deviation than the other genes.
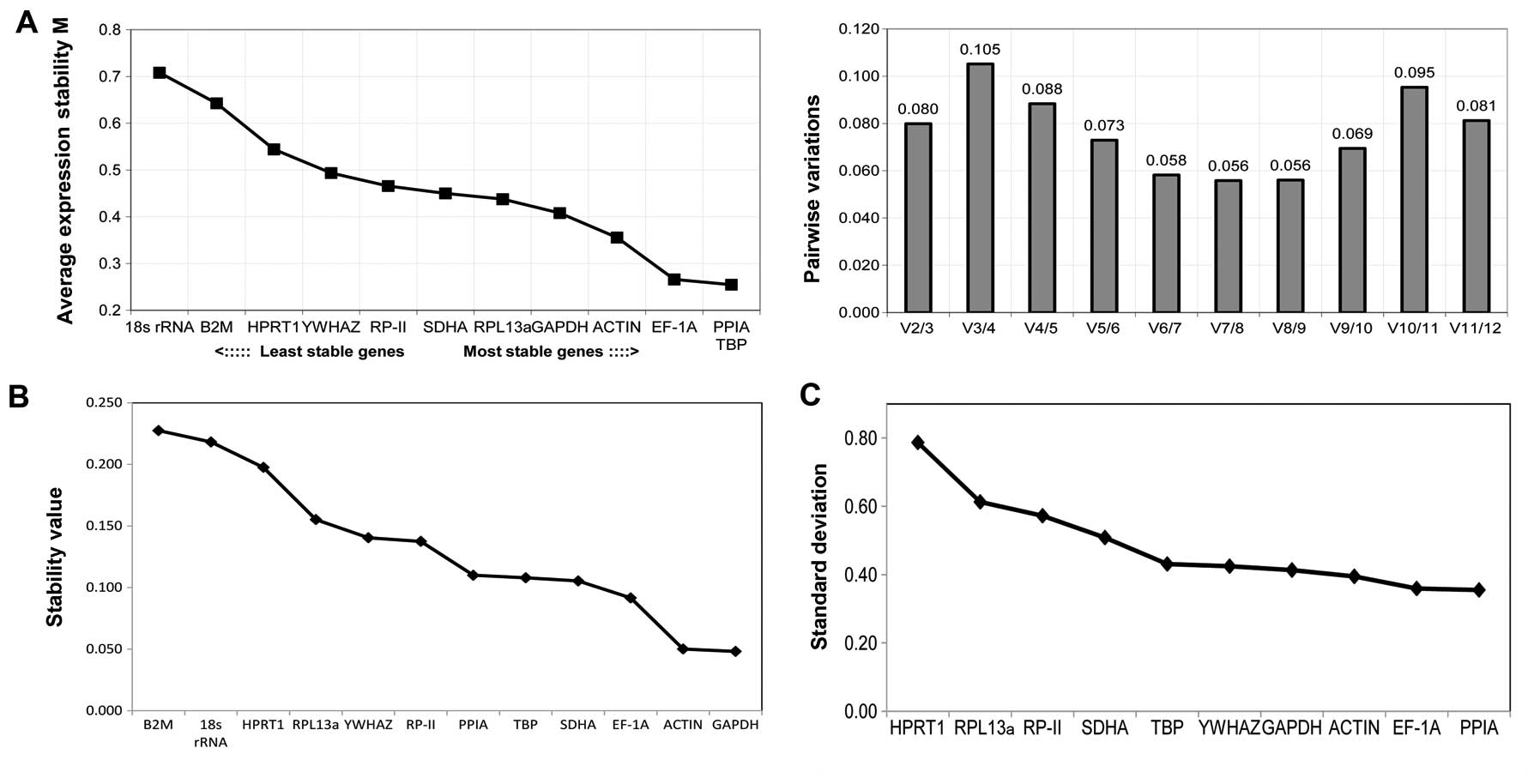
Osteogenic and chondrogenic
differentiation
The stability of reference genes during combined
osteogenic and chondrogenic differentiation was also evaluated
using the geNorm, Normfinder and BestKeeper software. As shown in
Fig. 5A, PPIA and TBP were
demonstrated to be the most stable genes in the geNorm analysis,
followed by EF-1A, ACTB and GAPDH, while the most unstable genes
were 18s rRNA, B2 M and HPRT1, with stability M-values higher than
0.5. Additionally, the V2/3 value was 0.080, much lower than the
suggested cut-off value, demonstrating that PPIA and TBP were
sufficient as reference genes to evaluate the chondrogenic and
osteogenic differentiation of BMSCs. Moreover, the results of
BestKeeper (Fig. 5C) were similar
to those of geNorm, with PPIA being the most stable gene followed
by EF-1A, ACTB and GAPDH. However, little difference existed with
Normfinder. It assessed GAPDH as being the most stable gene rather
than PPIA, followed by ACTB, EF-1A and SDHA, and the best
combination of two genes was ACTB and GAPDH, presenting a higher
stability value than the single genes. Conversely, B2M, HPRT1 and
18s rRNA were the most unstable genes during chondrogenic and
osteogenic differentiation, with higher M-values, stability values
and standard deviation than the other genes, which was inconsistent
with the results from geNorm.
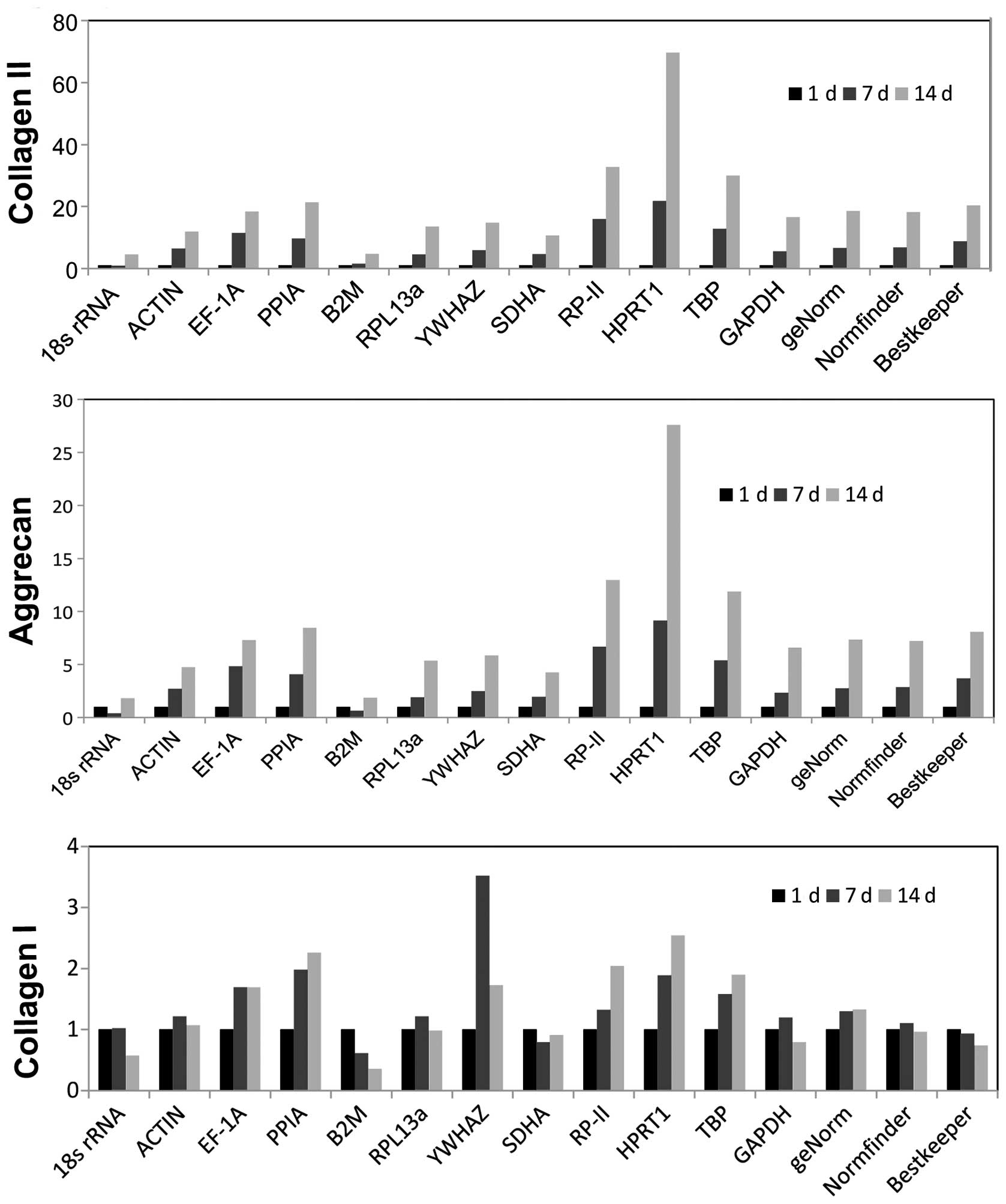
Expression profiles of marker genes
normalized against various reference genes in the differentiation
of BMSCs
The effects of reference gene variance on the
expression profiles of the chondrogenic and osteogenic
differentiation markers were investigated (Fig. 6). For clarity, the relative
expression of the marker genes for chondrogenic and osteogenic
differentiation-related genes collagen II, aggrecan and collagen I
was obtained with the 2−ΔΔCt method normalized by the 12
reference genes and the best reference combination recommended by
geNorm, Normfinder and BestKeeper. The results revealed that the
expression of collagen II and aggrecan increased with time by
during the chondrogenic differentiation of BMSCs, while collagen I
increased during osteogenic differentiation. As shown in Fig. 6A, the increasing trends of collagen
II and aggrecan were almost the same during chondrogenic
differentiation when the genes were normalized by the best
reference combination recommended by geNorm, Normfinder and
BestKeeper. However, the expression trends were quite different
when the genes were normalized by B2M, HPRT1 and 18s rRNA, the most
unstable genes during chondrogenic differentiation. The result is
in accordance with the validation of reference genes analysis.
Moreover, as for the osteogenic differentiation of BMSCs (Fig. 6B), the increased expression trends
were observed when the genes were normalized by PPIA, RP-II, HPRT1,
TBP and the combination of PPIA and HPRT1. However, results were
different based on other reference genes, demonstrating the
significance of suitable reference genes in the analysis of
osteogenic differentiation, and the results were consistent with
the geNorm analysis.
Discussion
Bone marrow mesenchymal stem cells are considered as
multipotent cells (23), existing
in the bone marrow, which represent a multilineage potential to
differentiate into mesodermal lineages (1,2). It
is the adult bone mesenchymal stem cells that contribute to the
regeneration of mesenchymal tissues following trauma or disease or
during aging (24–26). Evidence indicates the osteogenic
and chondrogenic differentiation ability of BMSCs in vitro
and in vivo. BMSCs exhibited a stable phenotype and remained
as a monolayer in vitro, maintaining their multilineage
differentiation potential (1).
Moreover, particular techniques to induce BMSC differentiation into
chondrogenic (8,27), osteogenic (9), adipogenic (10) and myogenic cells (11) have been well investigated.
Furthermore, implantation of BMSCs to defect organs or tissues has
proven successful in a variety of animal models, where the BMSCs
differentiated into the appropriate phenotypes according to the
local stimulating factors (28).
Im et al transported the cultured BMSCs to full-thickness
defect rabbit models and achieved a satisfactory result (29). In the study of Fuchs et al,
ovine BMSCs were harvested and seeded onto biodegradable scaffolds,
then transplanted into the cartilage defects of ewe femoral
trochlea. Three months after the transplantation, the cartilage
defects were repaired completely, with high proteoglycan and type
II collagen content (30). As for
bone regeneration, Niemeyer et al accomplished the repair of
sheep tibia defects through implantation of ovine BMSCs seeded on
mineralized collagen sponges (31). Liu et al demonstrated that a
BMSC sheet as a 3D scaffold material was a promising strategy in
healing a large bone defect area in osteoporosis through the
implantation of BMSCs to the defect part of the bone (4). In conclusion, BMSCs demonstrate
notable qualities as seed cells in cartilage and bone regeneration.
Tissue engineering in BMSCs has made great advances; however, the
mechanisms of the differentiations of BMSCs are still unclear.
Molecular changes in gene expression profiles during BMSC
differentiation are crucial to explain the mechanisms of the
multipotent differentiation process.
qPCR is a known, reliable, accurate, fast and
sensitive method to quantify gene expression and decipher the
properties of BMSC multipotent differentiation. However, an
appropriate reference gene is required to be constitutively
expressed to eliminate the errors caused by the cell number,
reverse transcription and amplification efficiency differences
(16). However, evidence has
revealed that the expression of widely used reference genes,
although occasionally constant in certain experimental conditions,
varies considerably in BMSC proliferation and differentiation
(18). Research has been carried
out to attempt to validate an appropriate reference gene in BMSC
differentiation (22), although no
conclusion has been reached. Amable et al studied the
appropriate reference genes for human mesenchymal cells during
expansion and differentiation, demonstrating that HPRT1 was the
most stably expressed gene for BMSCs, while the most unstable gene
was GAPDH (32). Studer et
al (20) and Ragni et
al (33) established RPL13a as
the most stable reference gene during the chondrogenic and
osteogenic differentiation of BMSCs. Quiroz et al also
validated RPL13a, GAPDH and ACTB as stable reference genes for
osteogenic differentiation of BMSCs (34). Curtis et al demonstrated
that EF-1a, RPL13a and YWHAZ were the most stable reference genes
for MSC differentiation in an animal model of global cerebral
ischemia (18). In conclusion, the
recommended reference genes varied according to the different
studies, and no consensus was reached, which was consistent with
our results. In the present study, the suitable reference genes
varied due to the differentiation microenvironments. For example,
YWHAZ was the most stable reference gene for the chondrogenic
differentiation of BMSCs, while RPL13a (Normfinder and BestKeeper),
PPIA and HPRT1 (geNorm) acted as the most efficient reference
genes. However, 18s rRNA, HPRT1 and B2M were considered to be the
most unstable reference genes in both chondrogenic and osteogenic
differentiation of BMSCs, which is in accordance with a number of
previous studies (18,22).
ACTB and GAPDH, the most popular reference genes in
use worldwide, are not the most effective reference genes under all
experimental conditions. Foldager et al reported that the
variations of ACTB and GAPDH for hypoxia-cultured human
chondrocytes were much larger than for other genes (35), underlining their ineffectiveness as
reference genes. The same results were obtained in the propagation,
differentiation and hypoxic exposure of adipose-derived stem cells,
where the expression of GAPDH was downregulated during
chondrogenesis and upregulated under hypoxic conditions, leading to
erroneous results in the expression of target genes (36). In the present study, ACTB and GAPDH
were not the most stable genes in all experimental conditions, when
the BMSCs underwent osteogenic differentiation.
In conclusion, our results demonstrated that the
expression of commonly used reference genes varies in the
chondrogenic and osteogenic differentiation of BMSCs. We identified
YWHAZ, PPIA and GAPDH as suitable reference genes for chondrogenic
differentiation, while RPL13a allowed an efficient normalization
expression value of interest genes for osteogenic differentiation
of BMSCs. Taking both chondrogenic and osteogenic differentiation
into consideration, GAPDH, ACTB, PPIA and EF-1A were the most
stable genes, which was a little different from the results for
chondrogenic and osteogenic differentiation alone, indicating that
reference gene stability varied according to the different
experimental conditions. However, the most unstable reference genes
were 18s rRNA, B2 M and HPRT1 in all analyses, and should be
avoided in studies on the differentiation of BMSCs. Our results
highlight the significance of using appropriate reference genes in
RT-qPCR normalization.
Acknowledgments
This study was financially supported by the National
Natural Science Foundation of China (project 51273081) and
Changchun Science and Technology (project 2012092).
References
|
1
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang Y, Jahagirdar BN, Reinhardt RL, et
al: Pluripotency of mesenchymal stem cells derived from adult
marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanchez-Ramos J, Song S, Cardozo Pelaez F,
et al: Adult bone marrow stromal cells differentiate into neural
cells in vitro. Exp Neurol. 164:247–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin G, Wang G, Liu G, et al: Treatment of
type 1 diabetes with adipose tissue-derived stem cells expressing
pancreatic duodenal homeobox 1. Stem Cells Dev. 18:1399–1406. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosová I, Dao M, Capoccia B, et al:
Hypoxic preconditioning results in increased motility and improved
therapeutic potential of human mesenchymal stem cells. Stem Cells.
26:2173–2182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang W, Zhang F, Shi H, et al:
Comparisons of rabbit bone marrow mesenchymal stem cell isolation
and culture methods in vitro. PloS One. 9:e887942014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lv FJ, Tuan RS, Cheung KM and Leung VY:
Concise review: the surface markers and identity of human
mesenchymal stem cells. Stem Cells. 32:1408–1419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jungebluth P, Alici E, Baiguera S, et al:
Tracheobronchial transplantation with a stem-cell-seeded
bioartificial nanocomposite: a proof-of-concept study. Lancet.
378:1997–2004. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guan M, Yao W, Liu R, et al: Directing
mesenchymal stem cells to bone to augment bone formation and
increase bone mass. Nat Med. 18:456–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karagianni M, Brinkmann I, Kinzebach S, et
al: A comparative analysis of the adipogenic potential in human
mesenchymal stromal cells from cord blood and other sources.
Cytotherapy. 15:76–88. 2013. View Article : Google Scholar
|
|
11
|
Salem HK and Thiemermann C: Mesenchymal
stromal cells: current understanding and clinical status. Stem
Cells. 28:585–596. 2010.
|
|
12
|
Gerson SL: Mesenchymal stem cells: no
longer second class marrow citizens. Nat Med. 5:262–264. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao L, Yang F, Liu G, et al: The promotion
of cartilage defect repair using adenovirus mediated Sox9 gene
transfer of rabbit bone marrow mesenchymal stem cells.
Biomaterials. 32:3910–3920. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grayson WL, Bunnell BA, Martin E, et al:
Stromal cells and stem cells in clinical bone regeneration. Nat Rev
Endocrinol. Jan 6–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Horwitz EM, Prockop DJ, Fitzpatrick LA, et
al: Transplantability and therapeutic effects of bone
marrow-derived mesenchymal cells in children with osteogenesis
imperfecta. Nat Med. 5:309–313. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haller F, Kulle B, Schwager S, et al:
Equivalence test in quantitative reverse transcription polymerase
chain reaction: confirmation of reference genes suitable for
normalization. Anal Biochem. 335:1–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vandesompele J, De Preter K, Pattyn F, et
al: Accurate normalization of real-time quantitative RT-PCR data by
geometric averaging of multiple internal control genes. Genome
Biol. 3:RESEARCH00342002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Curtis KM, Gomez LA, Rios C, et al:
EF1alpha and RPL13a represent normalization genes suitable for
RT-qPCR analysis of bone marrow derived mesenchymal stem cells. BMC
Mol Biol. 11:612010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Radonić A, Thulke S, Mackay IM, et al:
Guideline to reference gene selection for quantitative real-time
PCR. Biochem Biophys Res Commun. 313:856–862. 2004. View Article : Google Scholar
|
|
20
|
Studer D, Lischer S, Jochum W, et al:
Ribosomal protein l13a as a reference gene for human bone
marrow-derived mesenchymal stromal cells during expansion, adipo-,
chondro- and osteogenesis. Tissue Engineering Part C Methods.
18:761–771. 2012. View Article : Google Scholar
|
|
21
|
Haynesworth S, Goshima J, Goldberg V, et
al: Characterization of cells with osteogenic potential from human
marrow. Bone. 13:81–88. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Silver N, Best S, Jiang J and Thein SL:
Selection of housekeeping genes for gene expression studies in
human reticulocytes using real-time PCR. BMC Mol Biol. 7:332006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Owen M: Lineage of osteogenic cells and
their relationship to the stromal system. Bone and Mineral
Research. Peck WA: 3. Elsevier; New York, NY: pp. 1–25. 1985
|
|
24
|
Bianco P, Riminucci M, Gronthos S and
Robey PG: Bone marrow stromal stem cells: nature, biology and
potential applications. Stem Cells. 19:180–192. 2001. View Article : Google Scholar
|
|
25
|
Jones E and Yang X: Mesenchymal stem cells
and bone regeneration: current status. Injury. 42:562–568. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sethe S, Scutt A and Stolzing A: Aging of
mesenchymal stem cells. Ageing Res Rev. 5:91–116. 2006. View Article : Google Scholar
|
|
27
|
Winter A, Breit S, Parsch D, et al:
Cartilage-like gene expression in differentiated human stem cell
spheroids: A comparison of bone marrow-derived and adipose
tissue-derived stromal cells. Arthritis Rheum. 48:418–429. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Im GI, Kim DY, Shin JH, et al: Repair of
cartilage defect in the rabbit with cultured mesenchymal stem cells
from bone marrow. J Bone Joint Surg Br. 83:289–294. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fuchs JR, Hannouche D, Terada S, et al:
Fetal tracheal augmentation with cartilage engineered from bone
marrow-derived mesenchymal progenitor cells. J Pediatr Surg.
38:984–987. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Niemeyer P, Fechner K, Milz S, et al:
Comparison of mesenchymal stem cells from bone marrow and adipose
tissue for bone regeneration in a critical size defect of the sheep
tibia and the influence of platelet-rich plasma. Biomaterials.
31:3572–3579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amable PR, Teixeira MVT, Carias RBV, et
al: Identification of appropriate reference genes for human
mesenchymal cells during expansion and differentiation. PloS One.
8:e737922013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ragni E, Viganò M, Rebulla P, et al: What
is beyond a qRT-PCR study on mesenchymal stem cell differentiation
properties: how to choose the most reliable housekeeping genes. J
Cell Mol Med. 17:168–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Quiroz FG, Posada OM, Gallego-Perez D, et
al: Housekeeping gene stability influences the quantification of
osteogenic markers during stem cell differentiation to the
osteogenic lineage. Cytotechnology. 62:109–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Foldager CB, Munir S, Ulrik-Vinther M, et
al: Validation of suitable house keeping genes for hypoxia-cultured
human chondrocytes. BMC Mol Biol. 10:942009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fink T, Lund P, Pilgaard L, et al:
Instability of standard PCR reference genes in adipose-derived stem
cells during propagation, differentiation and hypoxic exposure. BMC
Mol Biol. 9:982008. View Article : Google Scholar : PubMed/NCBI
|