Introduction
Hypertensive disorders of pregnancy exert profound
effects on maternal and infant health. Together, gestational
hypertension, preeclampsia, eclampsia, chronic hypertension
complicated by preeclampsia and chronic hypertension have an
incidence of 9.4% in China and a worldwide incidence of 7–12%
(1,2). Pregnancy-induce hypertension (PIH)
disorders are a leading cause of morbidity and mortality in
pregnant and parturient women, and in perineonates, and an
understanding of their etiology is a significant concern within
obstetrics. A number of studies have suggested roles for the
placenta and the immune system in the development of these
disorders (3–6).
One candidate protein that functions in the placenta
as well as in the regulation of the immune system, is vascular
endothelial growth factor (VEGF). VEGF is the most active vascular
growth factor in the vascularization of the placenta (7), and has been shown to be involved in
the physiological and pathological conditions of hypertension in
pregnancy (8). VEGF also
contributes to the development of dendritic cells (DCs), which are
initiators of the immune response that play an important role in
regulating the innate immune system (9). DCs are a heterologous population of
cells, which are differentiated from CD34+ hematopoietic progenitor
cells (10). Immature DCs (iDCs)
have the ability to migrate and, during this process, to achieve
activation and functional maturation. It has been reported that the
VEGF receptor, fms-related tyrosine kinase 1 (FLT-1), is present on
the surface of CD34+ cells (11,12),
and that VEGF binds to FLT-1, kinase insert domain receptor/fetal
liver kinase 1 (KDR/FLK-1), and FLT-4 receptors (13). Upon binding to FLT-1, VEGF inhibits
the activity of the transcription factor, nuclear factor κB
(NF-κB), blocking the differentiation of hematopoietic stem cells
into DCs. Thus, VEGF may contribute to the development of PIH by
blocking the differentiation of stem cells into DCs.
The present study sought to investigate the
correlation between VEGF expression and DCs in the pathogenesis of
hypertensive disorders in pregnancy, by comparing levels of VEGF
and DCs in the peripheral blood of patients with hypertensive
disorders, as well as evaluating the effect of VEGF on DC
phenotypes, their ability to secrete cytokines and the stimulation
of primary T-cell activation.
Materials and methods
Study participants
The current study recruited 112 patients undergoing
treatment in the Division of Obstetrics between December 2012 and
December 2013 (mean age, 27.8±2.5 years; mean gestational age,
34.1±2.3 weeks). Of these, 46 patients were diagnosed with
gestational hypertension, 41 were diagnosed with preeclampsia and
25 were diagnosed with eclampsia. The study included 50 healthy
pregnant women (mean age, 27.7±3.1 years; mean gestational age,
33.7±1.1 weeks) as a control group. Among the observation groups
(pregnancy-induced hypertension, preeclampsia, and eclampsia),
differences in maternal and gestational ages were not statistically
significant, and patients had no other obstetric or medical
complications, or histories of autoimmune disorders. The present
study was approved by the Ethics Committee of Nantong Women and
Children Health Care Hospital (Nantong, China) and all patients
provided informed consent.
Antibodies and reagents
The antibodies used in the present study were mouse
monoclonal antibodies (mAb) used at a dilution of 1:20 and provided
by eBioscience Inc. (San Diego, CA, USA). The antbodies were as
follows: Phycoerythrin (PE)-labeled anti-human CD123 antibodies
(IgG1, cat. no. 12-1239), fluoresein isothiocyanate (FITC)-labeled
anti-human lineage cocktail 1 (Lin 1; IgG2b, cat. no.22-7778),
FITC-labeled anti-human CD80 (IgG1, cat. no. 11-0809), anti-human
interferon-γ (IFN-γ; IgG1, cat. no. 53-7319), anti-human CD86
(IgG2b, cat. no. 14-0869); FITC-labeled anti-human CD83 (IgG1, cat.
no. 11-0839), anti-human CD14 (IgG1, cat. no. 14-0149),
PerCy5.5-labeled anti-human HLA-DR antibody (IgG2b, cat. no.
45-9956), allophycocyanin (APC)-labeled anti-human CD11C antibody
(IgG1, cat. no. 17-0116), Isotype Control PerCP-Cy5.5 (mAb IgG2b,
cat. no. 45-4732), Isotype Control APC (mAb IgG1, cat. no.
17-4717), Isotype Control PE (mAb IgG1, cat. no. 12-4717), Isotype
Control FITC (mAb IgG2b, cat. no. 11-4732). Monensin, ionomycin,
and phorbol-12-my-ristate-13-acetate (PMA; Sigma-Aldrich, St.
Louis, MO, USA) were used for blocking and washing and the cells
were cultured in RPMI-1640 culture medium (Gibco Life Technologies,
Gaithersburg, MD, USA).
Detection of serum VEGF
Serum VEGF was detected using an enzyme-linked
immunosorbent assay (ELISA), according to the manufacturer's
instructions. The absorbance at 450 nm was measured using an ELISA
microplate reader (iMark; Bio-Rad, Hercules, CA, USA) in order to
determine the concentration of VEGF in each sample.
Detection of dendritic cells
Whole blood (50 µl) was obtained from each
subject and anticoagulated with heparin. PerCy5.5-labeled
anti-human HLA-DR, FITC-labeled anti-human Lin 1, APC-labeled
anti-human CD11C, PE-labeled anti-human CD123, APC-labeled IgG1
isotype control, and PE-labeled IgG1 isotype control antibodies
were added to each sample. After hybridizing in darkness for 30
min, 500 µl hemolytic agent was added and mixed, and samples
were placed in darkness for five minutes. Samples were then washed
three times using PBS, following which 300 µl of 20 g/l
paraformaldehyde (PFA; Sigma-Aldrich) was added. Samples were then
processed by flow cytometry in order to detect plasmacytoid
dendritic cells (pDC; Lin 1-HLA-DR+CD123+) and myeloid dendritic
cells (mDC; Lin1-HLA-DR+CD11C+). Data were analyzed using CellQuest
Software (Beckton, Dickinson and Company, Franklin Lakes, NJ,
USA).
VEGF treatment of dendritic cells
PBMCs from healthy individuals were separated by
Ficoll-Hypaque (Sigma-Aldrich), then washed three times using
complete RPMI-1640 medium, placed in 6-well plates at a density of
5×106 cells/l, and cultured at 37°C in 5% CO2
for 2 h. Suspended cells were removed and adherent cells were
collected after they had been rinsed with RPMI-1640. Adherent cells
were treated with culture medium containing cytokines (100 g/l
rhIL-4 and 100 g/l rhGM-CSF). After 3 days, the cytokines were
added again and half of the medium was renewed. On the sixth day,
half of the medium was renewed, and immature dendritic cells (iDCs)
were obtained and cultured as follows: Group A was treated with 1
mg/l LPS; and groups B, C and D were treated with 1 mg/l LPS plus
50 ng/l, 100 ng/l, and 150 ng/l VEGF, respectively. Following 8
days of culture, suspended cells were collected. The concentration
was adjusted to 1×109 cells/l using RPMI-1640 medium,
and DCs were collected and placed into test tubes. PerCy5.5-labeled
anti-human HLA-DR antibodies; FITC-labeled anti-human CD14 and CD83
antibodies; in addition to PerCy5.5-labeled IgG1 isotype control
antibodies and FITC-labeled IgG1 isotype control antibodies were
added. Following hybridization in darkness for 30 min, DCs were
washed three times with PBS and then added to 300 µl of 20
g/l PFA. Flow cytometry was used to measure the mean fluorescence
intensity (MFI) of the different dendritic cell types. CellQuest
Software was used to obtain and analyze data.
DC-induced differentiation of autologous
Th0 cells
Peripheral blood mononuclear cells (PBMCs) from
healthy individuals were separated and adherent cells were removed.
Nylon wool columns were used to separate CD4+ and T cells. Cells
were then incubated with a CD45RA antibody (mAb; IgG2b; cat. no.
14-0458-80; eBioscience; 1:20), and immunomagnetic bead separation
was performed in order to obtain CD4+CD45RA+Th0 cells. Cells were
resuspended in complete medium, then added to DCs at a ratio of
100:1 and placed into wells of a 24-well plate (each well had a
total volume of 1 ml). The concentration was adjusted to
1×109 cells/l using RMPI-1640 medium, and on day four of
cell culture. The cells (DC + Th0) were collected and analyzed
using flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Flow cytometry
Lymphocytes were collected after 4 days of growth
and cultured in 96-well plates at a concentration of
2×106 cells/ml. Ionomycin and PMA were added to bring
the volume in each well to 200 µl. Cells were then incubated
at 37°C in 50 ml/l CO2 for 3 h, then blocked with
monensin and cultured again for 2 h. The supernatant was removed by
centrifugation at 22,000 × g for 5 min, and 50 µl of the
remaining liquid was placed into test tubes. CD8-PerCP (BD
Pharmingen, San Diego, CA, USA) was then added. After culture in
darkness at 4°C for 30 min, cell surface molecules were stained
with a monoclonal antibody. The mixture was washed two times using
1 ml staining buffer, and the cells were fixed in 500 µl of
40 g/l PFA at 4°C for 30 min. The mixture was washed two times
using 1 ml staining buffer, and 500 µl of 1 g/l saponin-PBS
was then added to each tube. Following cell membrane permeation at
4°C for 15 min, the supernatant was removed by centrifugation at
22,000 × g for 5 min. The cells were then sealed with 20 µl
of 100 ml/l bovine serum albumin. IL-4-APC (mAb; IgG1, cat. no.
17-7049; eBioscience, 1:20) and IFN-γ-PE (mAb; IgG1; cat. no.
BMS107; eBioscience; 1:20) were added to test tubes to serve as an
isotype control, and intracellular cytokine staining was performed,
according to the manufacturer's instructions. Samples were washed
with PBS, 300 µl of 40 g/l PFA was added, and detection was
performed using a FACSCalibur flow cytometer (Beckton, Dickinson
and Company). Cell Quest Software (Beckton, Dickinson and Company)
was used to obtain and analyze data.
Statistical analysis
Data were analyzed using SAS 9.2 (SAS Institute
Inc., Cary, NC, USA). Sample groups were compared using Student's
t-test and linear correlation analysis. The means of multiple
samples were compared using analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
VEGF levels and DC profiles are
significantly altered in the peripheral blood of hypertensive
pregnant women
VEGF levels in each hypertensive group were lower
than those in the control group (Table
I, Fig. 1). In addition, VEGF
levels incrementally decreased in samples from each group of
hypertensive patients in accordance with disease severity. Analysis
of variance and a q-test showed that the differences in VEGF levels
among the four groups were statistically significant
(P<0.05).
 | Table IComparison of peripheral blood DC and
VEGF levels between observation groups and the control group. |
Table I
Comparison of peripheral blood DC and
VEGF levels between observation groups and the control group.
| Group | n | VEGF (ng/l) | pDC (%) | mDC (%) |
|---|
| Normal pregnancy | 50 | 156.31±14.11 | 0.21±0.09 | 0.25±0.10 |
| Pregnancy-induced
hypertension | 46 | 91.70±18.08a,c | 0.20± 0.08 | 0.27±0.10c |
| Preeclampsia | 41 | 65.30±16.14a,b | 0.15±0.11a,b | 0.33±0.13a,b |
| Eclampsia | 25 | 45.66±12.25a,bc | 0.18±0.08 | 0.35±0.20a,b |
The percentage of pDCs in the preeclampsia group was
significantly lower than that in the control and pregnancy-induced
hypertension groups (Table I,
Fig. 2). The percentage of mDCs in
each observation group was significantly higher than that of the
control group, and the mDC levels in the preeclampsia and eclampsia
groups were significantly higher than those in the
pregnancy-induced hypertension groups (Table I, Fig.
3).
VEGF expression and mDC levels are
negatively correlated in preeclamptic and eclamptic patients
In order to determine whether the observed
differences in circulating VEGF and in the population of DCs in
patients with PIH are associated, the correlation between VEGF and
DCs was analyzed. In the control and pregnancy-induced hypertension
groups, VEGF levels were not correlated with the percentage of
mDCs. However, in the preeclampsia and the eclampsia groups, VEGF
levels were significantly negatively correlated with the percentage
of mDCs (r=−0.34 and −0.42, respectively; P<0.05; Table II). There was no significant
correlation between VEGF levels and the percentage of pDCs among
any of the groups.
 | Table IICorrelation between VEGF level and
percentage of mDCs in hypertensive patients. |
Table II
Correlation between VEGF level and
percentage of mDCs in hypertensive patients.
| Group | VEGF (ng/l) | mDC (%) | r | P-value |
|---|
| Preeclampsia | 65.30±16.14 | 0.33±0.13 | −0.34 |
0.029a |
| Eclampsia | 45.66±12.25 | 0.35±0.20 | −0.42 |
0.034a |
VEGF affects the maturation and
differentiation of DCs
DCs cultured with LPS matured, and consequently
exhibited increased expression of CD83, as well as the
co-stimulatory molecules CD80 and CD86, while the expression of
CD14 decreased. Fig. 4 shows a
sample expression profile of CD14, CD80, CD83 and CD86 from cells
in group A. The MFI levels of DC surface molecules in the three
groups treated with VEGF were lower than those in group treated
with LPS, and their differences were statistically significant
(P<0.05). The differences in the expression of CD14, CD80, CD83
and CD86 between groups C and D were not statistically significant.
However, there was a significant difference between these groups
and group B (Table III, Fig. 5).
 | Table IIIComparison of MFI of DC surface
molecules among four different groups. |
Table III
Comparison of MFI of DC surface
molecules among four different groups.
| Group (n=5) | CD14 | CD80 | CD83 | CD86 |
|---|
| A | 11.45±5.12 | 60.05±32.53 | 138.27±69.41 | 89.91±38.30 |
| B | 21.45±8.83a | 34.31±19.99a | 93.13±41.92a | 50.26±43.77a |
| C | 24.79±7.08a | 24.88±8.23a,b | 53.89±34.45a,b |
31.86.30±25.31a,b |
| D | 23.11±7.34a | 23.22±13.18a,b | 63.55±23.36a,b | 29.53±25.18a,b |
Cytokines are produced during Th0
differentiation induced by DCs
A typical cytokine profile is shown in Fig. 6. The expression of IFN-γ in the
group treated with LPS alone was the highest, but there were not
statistically significant differences in the expression levels of
IL-4 among the four groups and the ratios of IFN-γ/IL-4 between the
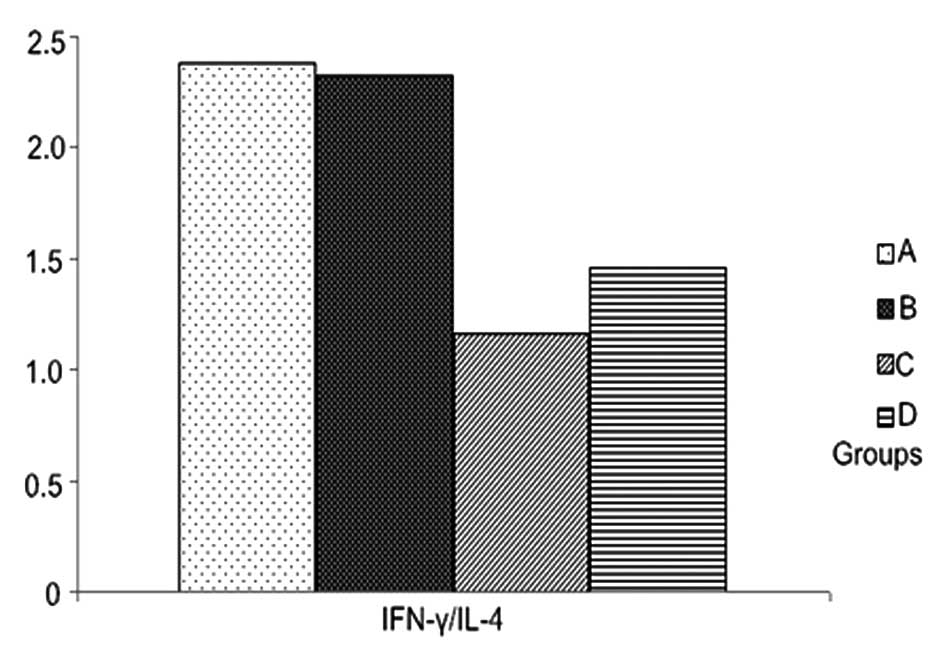
group treated with 50 ng/l VEGF and with LPS. IFN-γ/IL-4 ratios in
the groups treated with 100 ng/1 and 150 ng/l VEGF were
significantly lower than those treated with LPS alone (Table IV, Figs. 7 and 8).
 | Table IVComparison of IFN-γ, IL-4 and
IFN-γ/IL-4 between different groups. |
Table IV
Comparison of IFN-γ, IL-4 and
IFN-γ/IL-4 between different groups.
| Group (n=5) | IFN-γ (%) | IL-4 (%) | IFN-γ/IL-4 |
|---|
| A | 7.51±1.39 | 3.59±0.86 | 2.38±0.74 |
| B | 5.77±0.85a | 3.14±0.85 | 2.32±0.49 |
| C | 2.82±0.76a | 2.96±0.74 | 1.16±0.60a |
| D | 2.72±0.97a | 2.83±0.74 | 1.46±0.92a |
Discussion
The present study showed that VEGF levels in each
hypertensive group were lower than those in the control group, and
that the more severe hypertensive cases were associated with lower
VEGF levels. The expression of mDCs in peripheral blood samples was
higher in the preeclampsia and eclampsia groups than that in the
control pregnancy-induced hypertension groups, and was negatively
correlated with VEGF levels. Only in the preeclampsia group were
pDCs found to be lower than those in the control and
pregnancy-induced hypertension groups, and both differences were
statistically significant. However, the correlation of pDCs with
VEGF levels was not significant. These results suggest that, at a
high concentration, VEGF may inhibit differentiation into mDCs,
while it appears to have no inhibitory effects on the level of
pDCs.
Notably, Schonkeren et al (14) found that soluble FLT-1 (sFLT-1)
expression on the surface of CD14+ macrophages in patients with
preeclampsia was increased as a result of the action of VEGF on
sFLT-1, thereby inhibiting the differentiation of CD14+ precursor
cells into DCs. Further, Stober et al (15) demonstrated that mDCs stimulate and
regulate T cells to secrete large quantities of the cytokines
IL-12, IL-18, TNF-α and IFN-γ, activating T cell proliferation and
promoting the differentiation of Th0 cells into Th1 cells.
Therefore, VEGF may reduce the function and number of mDCs and
inhibit the appearance of Th1-type cytokines, thus encouraging a
normal pregnancy.
The present study also demonstrated that VEGF may
inhibit DC maturation, and that VEGF levels most similar to those
observed in the normal pregnancy group, exerted the strongest
inhibitory effect. When VEGF was given to autologous primary T
cells, stimulated by DCs, it inhibited the activation of primary T
cells stimulated by mDCs. Furthermore, lower VEGF levels resulted
in stronger MFI on the surface of DCs, increased DC maturity, and
an enhanced ability to produce Th1-type cytokines in stimulated
primary T cells. In addition, the ratio of Th1/Th2 also rose. This
is in accordance with a study by Block et al (16) which showed that VEGF reduced the
expression of IL-12β, which causes CD4 T cells to differentiate
into Th1 cells in response to DCs. Chen et al (17) also reported that highly expressed
VEGF may reduce the function and number of mDCs, and contribute to
immunosuppression.
Numerous studies have illustrated the interaction
between VEGF and DCs (18–21), in which VEGF expression leads to
abnormal DC phenotypes and functions. The present study suggests
that VEGF levels in normal pregnancies may inhibit the maturation
of DCs and inhibit the differentiation of Th0 cells into Th1 cells,
indicating that the interaction between VEGF and DCs is important
in maintaining the maternal-fetal immune balance. These results
provide a basis for a greater understanding of the molecular
pathogenesis of hypertensive disorders of pregnancy.
Acknowledgments
This study was supported by Jiangsu Provincial
Health Department (grant no. F 201213).
References
|
1
|
Gaio DS, Schmidt MI, Duncan BB, et al:
Hypertensive disorders in pregnancy: Frequency and associated
factors in a cohort of Brazilian women. Hypertens Pregnancy.
20:269–281. 2001. View Article : Google Scholar
|
|
2
|
Lykke JA, Langhoff-Roos J, Sibai BM, et
al: Hypertensive pregnancy disorders and subsequent cardiovascular
morbidity and type 2 diabetes mellitus in the mother. Hypertension.
53:944–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nahar L, Nahar K, Hossain MI, Jahan S and
Rahman MM: Placental changes in pregnancy induced hypertension.
Mymensingh Med J. 22:684–693. 2013.PubMed/NCBI
|
|
4
|
Nahar L, Nahar K, Hossain MI, Yasmin H and
Annur BM: Placental changes in pregnancy induced hypertension and
its impacts on fetal outcome. Mymensingh Med J. 24:9–17.
2015.PubMed/NCBI
|
|
5
|
LaMarca B, Cornelius D and Wallace K:
Elucidating immune mechanisms causing hypertension during
pregnancy. Physiology (Bethesda). 28:225–233. 2013.
|
|
6
|
Cao X, Wang LL and Luo X: Expression of
regulatory T and helper T cells in peripheral blood of patients
with pregnancy-induced hypertension. Clin Exp Obstet Gynecol.
40:502–504. 2013.PubMed/NCBI
|
|
7
|
Kalkunte SS, Mselle TF, Norris WE, et al:
Vascular endothelial growth factor C facilitates immune tolerance
and endovascular activity of human uterine NK cells at the
maternal-fetal interface. J Immunol. 182:4085–4092. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tripathi R, Ralhan R, Saxena S, et al:
Soluble VEGFR-1 in pathophysiology of pregnancies complicated by
hypertensive disorders: The Indian scenario. J Human Hypertens.
27:107–114. 2012. View Article : Google Scholar
|
|
9
|
Geissmann F, Auffray C, Palframan R, et
al: Blood monocytes: Distinct subsets, how they relate to dendritic
cells and their possible roles in the regulation of T-cell
responses. Immunol Cell Biol. 86:398–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van de Laar L, Buitenhuis M, Wensveen FM,
et al: Human CD34-derived myeloid dendritic cell development
requires intact phosphatidylinositol 3-kinase-protein kinase
B-mammalian target of rapamycin signaling. J Immunol.
184:6600–6611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Laxmanan S, Robertson SW, Wang E, et al:
Vascular endothelial growth factor impairs the functional ability
of dendritic cells through Id pathways. Biochem Biophys Res Comm.
334:193–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oyama T, Ran S, Ishida T, et al: Vascular
endothelial growth factor affects dendritic cell maturation through
the inhibition of nuclear factor-kappa B activation in hemopoietic
progenitor cells. J Immunol. 160:1224–1232. 1998.PubMed/NCBI
|
|
13
|
Seetharam L, Gotoh N, Maru Y, et al: A
unique signal transduction from FLT tyrosine kinase, a receptor for
vascular endothelial growth factor VEGF. Oncogene. 10:135–147.
1995.PubMed/NCBI
|
|
14
|
Schonkeren D, van der Hoorn ML, Khedoe P,
et al: Differential distribution and phenotype of decidual
macrophages in preeclamptic versus control pregnancies. Am J
Pathol. 178:709–717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stober D, Schirmbeck R and Reimann J:
IL-12/IL-18-dependent IFN-γ release by murine dendritic cells. J
Immunol. 167:957–965. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Block MS, Nevala WK, Leontovich AA, et al:
Differential response of human and mouse dendritic cells to VEGF
determines interspecies discrepancies in tumor-mediated TH1/TH2
polarity shift. Clin Cancer Res. 17:1776–1783. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Z, Varney ML, Backora MW, et al:
Down-regulation of vascular endothelial cell growth factor-C
expression using small interfering RNA vectors in mammary tumors
inhibits tumor lymphangiogenesis and spontaneous metastasis and
enhances survival. Cancer Res. 65:9004–9011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vicari AP, Treilleux I and Lebecque S:
Regulation of the trafficking of tumour-infiltrating dendritic
cells by chemokines. Semin Cancer Biol. 14:161–169. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berger S, Dyugovskaya L, Polyakov A, et
al: Short-term fibronectin treatment induces endothelial-like and
angiogenic properties in monocyte-derived immature dendritic cells:
involvement of intracellular VEGF and MAPK regulation. Eur J Cell
Biol. 91:640–653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mahnke K, Schmitt E, Bonifaz L, et al:
Immature, but not inactive: The tolerogenic function of immature
dendritic cells. Immunol Cell Biol. 80:477–483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sugiyama M, Kakeji Y, Tsujitani S, et al:
Antagonism of VEGF by genetically engineered dendritic cells is
essential to induce antitumor immunity against malignant ascites.
Mol Cancer Ther. 10:540–549. 2011. View Article : Google Scholar : PubMed/NCBI
|