Introduction
The synuclein family consists of synuclein-α (SNCA),
synuclein-β (SNCB) and synuclein-γ (SNCG). SNCA has been widely
investigated due to its role in pathogenesis of neurodegenerative
diseases, such as Alzheimer's disease and Parkinson's disease
(1,2). SNCB assumes a neuroprotective role by
inhibiting SNCA expression and aggregation (3,4).
SNCG was initially termed breast cancer specific gene 1 on account
of its high levels in advanced infiltrating breast carcinoma,
compared with those in normal or benign breast lesions (5).
Elevated level of SNCG is detected in a wide range
of cancer types, including gastric, lung, liver, colon, ovarian,
cervical and esophageal cancer, and glioma (6–10).
In addition, it correlates with adverse outcomes in breast cancer
(11,12) and colon cancer (10). Downregulation of SNCG by small
interfering (si)RNA decreased migration and invasion of HCT116
colorectal cancer cells (13). A
moderate elevation of matrix metalloproteinase (MMP)2 and
significant upregulation of MMP9 occurred in Y79 retinoblastoma
cells overexpressing SNCG (14).
SNCG was shown to promote migration of MDA-MB-435 breast cancer,
and A2780 and OVCAR5 ovarian cancer cells by modulating RHO and Erk
pathways. Inhibition of the RHO pathway by C. difficile
Toxin B or of the Erk pathway by U0126 blocked cell migration in
cells overexpressing SNCG (15).
However, the exact mechanism of how SNCG promotes cell motility
remains unclear.
A previous study showed that SNCA promoted the
phos-phorylation of Akt (16).
Since SNCA shares homologous sequences and similar functions with
SNCG, it was proposed that there is a correlation between SNCG and
the Akt pathway. Epithelial to mesenchymal transition (EMT), is
currently the focus of cancer cell migration, invasion and
metastasis investigation (17).
During EMT, non-motile, polarized epithelial cells dissolve their
cell-cell junctions and convert into individual, non-polarized,
motile and invasive mesenchymal cells (17). Considering the role of SNCG in
promoting cell invasiveness (13–15),
it would be interesting to evaluate the correlation between SNCG
and EMT. Based on the fact that EMT is prompted by various
signaling pathways, including Erk (18) and Akt (19), while SNCG activated Erk pathway
(15,20), we explored the possible connection
between SNCG and EMT in this study.
Materials and methods
Materials and cell lines
The stable cell line MCF7-green fluorescent protein
(GFP)-SNCG, which stably overex-presses GFP-SNCG, and the MCF7-GFP
control cell line were constructed by respectively transfecting
MCF7 cells (American Type Culture Collection, Manassas, VA, USA)
with pEGFP-SNCG (kindly provided by Dr. Erich Shi; Albert Einstein
College of Medicine, Bronx, NY, USA) and pEGFP-N1 (Promega
Corporation, Madison, WI, USA). MCF7-GFP-SNCG and MCF7-GFP were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS) (all from Bioroc Pharmaceutical & Biotec Co., Ltd.,
Tianjin, China). Transwell chambers with 8 µm polycarbonate
membranes in 24-well dishes were purchased from Corning Inc.
(Corning, NY, USA). Anti-E-cadherin (cat. no. R868) was purchased
from Bioworld (Nanjing, China). Anti-p-Erk (cat. no. 9101),
anti-Erk (cat. no. 9107), anti-p-Akt (cat. no. 12694), anti-Akt
(cat. no. 2920), anti-ZO-1 (cat. no. 8193), anti-Vimentin (cat. no.
3390), anti-β-catenin (cat. no. 2698) and anti-TCF8/ZEB1 (cat. no.
3396) antibodies, and U0126, a MEK inhibitor, were purchased from
Cell Signaling Technology Inc. (Danvers, MA, USA).
Cell proliferation analysis
Cells were seeded in 96-well plates in triplicate
with RPMI-1640 and 10% FBS. The confluence values were measured
using the CloneSelect Imager system (Molecular Devices Ltd., New
Milton, UK) at 0, 24, 48, 72 and 96 h. The confluence values were
positively correlated with cell numbers. Experiments were repeated
twice.
Cell migration assay
Cells (4×104) were suspended in
serum-free RPMI-1640 and added to the upper chamber. RPMI-1640 (700
µl) with 10% FBS was added to the lower chamber at the same
time. Chambers were incubated at 37°C in the 5% CO2
incubator for 24 h. Following incubation, the chambers were fixed
with pre-cooled methanol for 30 min and stained with 0.1% crystal
violet (Sigma-Aldrich, St. Louis, MO, USA) for 30 min. Non-migrated
cells on the upper surface of filters were removed with cotton
swabs. Cell numbers were counted in five random fields using light
microscopy (Eclipse 80i; Nikon Corporation, Tokyo, Japan).
Experiments were repeated twice.
Wound healing assay
Cells were grown to 95% confluency in a 24-well
plate in RPMI-1640 medium with 10% FBS. A wound was created by
scratching monolayer cells with a sterile 200 µl pipette
tip. Cells were washed three times with phosphate-buffered saline
to remove non-adherent cells. RPMI-1640 medium with 2% fetal bovine
serum were added. Images of the wound were captured at different
time points. The recovery rate of the wound was measured and
quantified using Image Pro Plus version 7.0 software (Media
Cybernetics, Warrendale, PA, USA) and calculated using the
equation: % Cell migration = [1-(scratch area at
Tx)/scratch area at T0]. Where the
Tx is the respective time point and T0 is the
time after the scratch. Experiments were repeated twice.
Western blot analysis
For western blot analysis, cells were harvested with
2X sodium dodecyl sulfate and boiled for 10 min, then loaded on 12%
polyacrylamide gels. After electrophoresis, proteins were
transferred onto a nitrocellulose membrane (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), blocked for 2 h in 5% skimmed milk.
Subsequently, membranes were probed with primary antibodies diluted
in 5% bovine serum albumin (Sigma-Aldrich) overnight at 4°C,
followed by incubation with secondary antibodies for 45 min at room
temperature. The enhanced chemiluminesence system (Pierce
Biotechnology, Inc., Rockford, IL, USA) was used for analysis.
GAPDH was used as a loading control.
Statistical analysis
SPSS software, version 17.0 (SPSS Inc., Chicago, IL,
USA) was used for statistical analysis. The two-sided t-test method
was used for analyzing the difference between the experimental
group and the control group. P<0.05 was considered to indicate a
statistically significant difference.
Results
SNCG has no effect on the proliferation
of MCF7 breast cancer cells
To investigate the function of SNCG, MCF7-GFP-SNCG
breast cancer cells and MCF7-GFP control cells were selected for
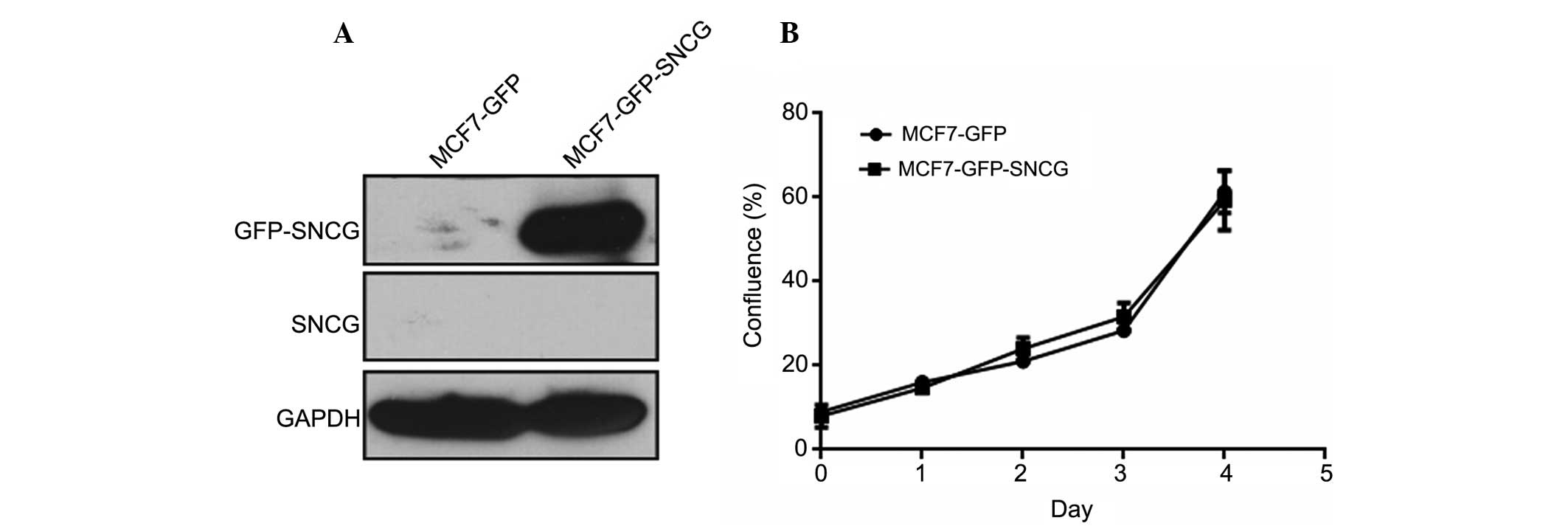
the study. As detected by SNCG monoclonal antibodies, which weres
prepared by our lab in a previous study (21), endogenous SNCG was undetectable in
MCF7-GFP-SNCG and MCF7-GFP cells, while the fusion protein GFP-SNCG
was strongly expressed in MCF7-GFP-SNCG cells (Fig. 1A). In the proliferation assay, no
significant difference between MCF7-GFP-SNCG and MCF7-GFP cells was
found (P=0.711), suggesting that SNCG has a minimal effect on the
proliferation of MCF7 breast cancer cells (Fig. 1B).
SNCG increases the migration of MCF7
breast cancer cells in vitro
To investigate whether SNCG promoted migration of
MCF7 cells, Transwell and wound healing assays were performed. In
the Transwell assay, the number of migrated MCF7-GFP-SNCG cells was
almost twice that of MCF7-GFP, and the difference between them was
statistically significant (P<0.05, Fig. 2A). In addition, MCF7-GFP-SNCG cells
also migrated faster than MCF7-GFP cells in the wound healing assay
(Fig. 2B). Therefore, SNCG
significantly promoted the mobility of MCF7 breast cancer
cells.
Erk pathway mediates SNCG-promoted
migration
SNCG had been demonstrated to be associated with Erk
and constitutively activated Erk (15,20).
The Akt pathway was also shown to be activated by SNCA (16). Considering that Erk and Akt
pathways have essential roles in regulating pathways related to
cancer malignant phenotypes, it was hypothesized that SNCG may
enhance the mobility of MCF7 cells via Erk or Akt pathways. To test
this hypothesis, western blotting was performed to detect the
expression of p-Erk and p-Akt. As shown in Fig. 3A, SNCG upregulated p-Erk and had no
effect on p-Akt, which indicated SNCG activated the Erk pathway
rather than the Akt pathway in MCF7 cells. To further investigate
whether the Erk pathway contributes to SNCG-induced mobility in
MCF7 cells, U0126, a MEK inhibitor, was used. Cells were cultured
in 10 µM U0126 1 h prior to performing Transwell and wound
healing assays. The results revealed that U0126 significantly
reduced migration and healing of MCF7-GFP-SNCG and MCF7-GFP cells
(P<0.05, Fig. 3B and C).
Therefore, SNCG promoted migration of MCF7 by activating downstream
Erk pathway.
SNCG promotes migration by breaking cell
adherens junctions and tight junctions, which is not triggered by
the Erk pathway
EMT is key in cancer cell metastasis, and is
regulated by various pathways, including Erk and Akt (18,19).
According to the fact that SNCG may promote cancer cell migration
by activating the Erk pathway, the correlation between SNCG and EMT
was then examined. To identify whether SNCG had an impact on EMT,
western blotting was conducted to check the relevant protein
expression levels in MCF7-GFP-SNCG and MCF7-GFP cells. The data
revealed that SNCG downregulated EMT-related proteins, such as
E-cadherin and ZO-1 (Fig. 4A).
E-cadherin is the major component of epithelial adherens junctions
(22), while ZO-1 is important in
cell tight junctions (23). Thus,
the results implied that SNCG may promote the migration of MCF7 by
breaking cell adherens junctions and tight junctions. Certain
studies reported that Erk pathway could induce EMT (18). However, although it was
demonstrated that U0126 did block the Erk pathway, the expression
levels of E-cadherin and ZO-1 were not changed (Fig. 4B), implying that SNCG-activated Erk
had no effect on E-cadherin or ZO-1 in MCF7 cells.
Discussion
SNCG shares the conserved N-terminal domain with
SNGA and SNGB, and the homologous identities are 54 and 56%,
respectively. Their main differences are in their acidic C-terminal
domain, which is presumably where their functional differences
arise (24). Various studies have
shown that SNCG promoted migration, invasion and metastasis of
cancer cells (13–15,25),
and SNCG was associated with recurrence and poor prognosis of
multiple types of cancer. Our previous results also indicated that
SNCG is an independent predictor for poor prognosis in patients
with breast cancer and colon adenocarcinoma (8,11).
In order to evaluate the function of SNCG on cancer
cells, a stable MCF7-GFP-SNCG cell line overexpressing SNCG and
MCF7-GFP controls were constructed. A proliferation assay was
conducted and determined that SNCG had no effect on the
proliferative ability of MCF7 cells, which was in accordance with
the results of a previous study (26), but was contradictory to the results
of others (13,27). It was speculated that these
differences were due to the different type of cells used for
experiments. Transwell and wound healing assays were also performed
to test the effect of SNCG on migration. The results revealed that
SNCG accelerated migration of MCF7 breast cancer cells, which
coincided with the results of numerous studies (13,15,28).
Taking the results of the proliferation assay into consideration,
the significant difference in the migration experiment was not
caused by the difference in proliferation. Thus, SNCG indeed
promoted the motility of MCF7 cells.
Next, the mechanism of how SNCG promote motility of
MCF7 cells was explored. It was found that SNCG activated the Erk
pathway but not the Akt pathway, suggesting that SNCG has
functional difference with its homologous proteins. Furthermore, it
was demonstrated that U0126 blocked migration of MCF7-GFP-SNCG and
MCF7-GFP cells, which further identified the role of the Erk
pathway in enhancing the motility of MCF7 cells. Recently, several
studies have shown that EMT is involved in the development and
progression of cancer (17,29).
EMT is an essential way for cancer cells to gain migratory and
invasive properties (29). The
correlation between SNCG and EMT has not been reported previously.
According to the fact that SNCG modulated the Erk pathway and the
Erk pathway promotes EMT (18),
this correlation was explored. Western blotting was performed to
detect the expression levels of EMT-associated proteins, such as
E-cadherin, ZO-1 and Vimentin. It was demonstrated that SNCG
down-regulated E-cadherin and ZO-1, while had no impact on other
EMT-related proteins, such as Vimentin, β-catenin or TCF8/ZEB1.
E-cadherin and ZO-1 are all essential factors in cell adherens
junctions and tight junctions. The results indicated that SNCG may
break these junctions. Considering that EMT could be modulated by
the Erk pathway, it was assumed that SNCG may downregulate the
expression level of E-cadherin and ZO-1 by activating the Erk
pathway. U0126 was used again to confirm this hypothesis; however,
the results indicated that the expression levels of E-cadherin and
ZO-1 were not regulated by Erk pathway. Due to the complex
signaling pathways existing in the cells, it was assumed that SNCG
utilizes other pathways to downregulate E-cadherin and ZO-1, which
requires further investigation in the future.
In conclusion, overexpression of SNCG promoted
migration of MCF7 breast cancer cells; however, had no effect on
proliferation. The data also demonstrated that SNCG promoted
migration by upregulating the Erk pathway and downregulating
E-cadherin and ZO-1 in manner independent of Erk. The results
provided novel evidence of a correlation between SNCG and cancer,
and confirmed the association between SNCG and the EMT-associated
proteins E-cadherin and ZO-1, thus highlighting the effects of SNCG
on the malignant phenotype of cancer cells.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81272410) and National 973
Program (grant no. 2015CB553906).
References
|
1
|
Ueda K, Fukushima H, Masliah E, et al:
Molecular cloning of cDNA encoding an unrecognized component of
amyloid in alzheimer disease. Proc Natl Acad Sci USA.
90:11282–11286. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spillantini MG, Schmidt ML, Lee VM, et al:
Alpha-synuclein in lewy bodies. Nature. 388:839–840. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hashimoto M, Rockenstein E, Mante M, et
al: Beta-synuclein inhibits alpha-synuclein aggregation: a possible
role as an anti-parkinsonian factor. Neuron. 32:213–223. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JY and Lansbury PT: Beta-synuclein
inhibits formation of alpha-synuclein protofibrils: a possible
therapeutic strategy against Parkinson's disease. Biochemistry.
42:3696–3700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji HJ, Liu YE, Jia T, et al:
Identification of a breast cancer specific gene, BCSG1, by direct
differential cDNA sequencing. Cancer Res. 57:759–764.
1997.PubMed/NCBI
|
|
6
|
Guo J, Shou C, Meng L, et al: Neuronal
protein synuclein-γ predicts poor clinical outcome in breast
cancer. Int J Cancer. 121:1296–1305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bruening W, Giasson BI, Klein-Szanto AJ,
et al: Synucleins are expressed in the majority of breast and
ovarian carcinomas and in preneoplastic lesions of the ovary.
Cancer. 88:2154–2163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu H, Liu W, Wu Y, et al: Loss of
epigenetic control of synu-clein-gamma gene as a molecular
indicator of metastasis in a wide range of human cancers. Cancer
Res. 65:7635–7643. 2005.PubMed/NCBI
|
|
9
|
Iwaki H, Kageyama S, Isono T, et al:
Diagnostic potential in bladder cancer of a panel of tumor markers
(calreticulin, gamma-synuclein and catechol-o-methyltransferase)
identified by proteomic analysis. Cancer Sci. 95:955–961. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu C, Dong B, Lu AP, et al: Synuclein
gamma predicts poor clinical outcome in colon cancer with normal
levels of carcino-embryonic antigen. BMC Cancer. 10:3592010.
View Article : Google Scholar
|
|
11
|
Guo J, Shou C, Meng L, et al: Neuronal
protein synuclein-γ predicts poor clinical outcome in breast
cancer. Int J Cancer. 121:1296–1305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu K, Quan Z, Weng Z, et al: Expression of
neuronal protein synuclein gamma gene as a novel marker for breast
cancer prognosis. Breast Cancer Res Treat. 101:259–267. 2007.
View Article : Google Scholar
|
|
13
|
Ye Q, Feng B, Peng YF, et al: Expression
of γ-synuclein in colorectal cancer tissues and its role on
colorectal cancer cell line HCT116. World J Gastroenterol.
15:5035–5043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Surgucheva IG, Sivak JM, Surguchov AP, et
al: Effect of gamma-synuclein overexpression on matrix
metalloproteinases in retinoblastoma Y79 cells. Arch Biochem
Biophys. 410:167–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan ZZ, Bruening W and Godwin AK:
Involvement of RHO GTPases and ERK in synuclein-gamma enhanced
cancer cell motility. Int J Oncol. 29:289–295. 2006.
|
|
16
|
Seo JH, Rah JC, Choi SH, et al:
Alpha-synuclein regulates neuronal survival via Bcl-2 family
expression and PI3/Akt kinase pathway. FASEB J. 16:1826–1828.
2002.PubMed/NCBI
|
|
17
|
Grunert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie L, Law BK, Chytil AM, Brown KA, Aakre
ME and Moses HL: Activation of the Erk pathway is required for
TGF-β1-induced EMT in vitro. Neoplasia. 6:603–610. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Wang HS, Zhou BH, et al:
Epithelial-mesenchymal transition (EMT) induced by TNF-α requires
AKT/GSK-3β-mediated stabilization of snail in colorectal cancer.
PLoS One. 8:e566642013. View Article : Google Scholar
|
|
20
|
Pan ZZ, Bruening W, Godwin AK, et al:
Gamma-synuclein promotes cancer cell survival and inhibits stress-
and chemotherapy drug-induced apoptosis by modulating MAPK
pathways. J Biol Chem. 277:35050–35060. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu C, Guo J, Qu L, et al: Applications of
novel monoclonal antibodies specific for synuclein-gamma in
evaluating its levels in sera and cancer tissues from colorectal
cancer patients. Cancer Lett. 269:148–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cavallaro U and Christofori G: Cell
adhesion and signaling by cadherins and Ig-CAMs in cancer. Nat Rev
Cancer. 4:118–132. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elizabeth M, Christopher TC and Ian GM:
Zonula occludens-a function in the assembly of tight junctions in
Madin-Darby canine kidney epithelial cells. Mol Biol Cell.
17:1922–1932. 2006. View Article : Google Scholar
|
|
24
|
Surguchov A: Molecular and cellular
biology of synucleins. Int Rev Cell Mol Biol. 270:225–317. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye Q, Huang F, Wang XY, et al: Effects of
γ-synuclein on the tumorigenicity and metastasis of colon cancer
SW116 cells in vitro and in vivo. Oncol Rep. 30:2161–2170.
2013.PubMed/NCBI
|
|
26
|
Jiang Y, Liu YE, Lu A, et al: Stimulation
of estrogen receptor signaling by γ-synuclein. Cancer Res.
63:3899–3903. 2003.PubMed/NCBI
|
|
27
|
Chen JY, Jiao L, Xu CL, et al: Neural
protein gamma-synuclein interacting with androgen receptor promotes
human prostate cancer progression. BMC Cancer. 12:5932012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia T, Liu YE, Liu J and Shi YE:
Stimulation of breast cancer invasion and metastasis by synuclein
γ. Cancer Res. 59:742–747. 1999.PubMed/NCBI
|
|
29
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|