Introduction
Depression is a common mental health concern
(1) and electroconvulsive therapy
(ECT) is the preferred treatment option for patients diagnosed with
major depression. Electroconvulsive seizure for a duration of
between 120 and 180 sec can lead to cognitive disorders (2) due to pathological dysfunction of the
glutamic acid (Glu) signalling system (3–5). Glu
is the predominant excitatory neurotransmitter, which transmits
~40% of synapses, causes oxidative stress (6) resulting in hippocampal
indiscriminations and saturated long-term potentiation (LTP)
(4), and causes synaptic
plasticity impairment (5,7).
Tau proteins or τ proteins are low-molecular weight
microtubule-associated proteins (8). These asymmetric phosphoproteins are
distributed in the frontal and temporal lobes of the brain, axons
and dendrons of the hippocampus and neurons in the entorhinal area.
Their primary structures include the prominent feature of amino
acid residue repeats in a 'tandem repeat' form, with the major
Pro-Gly-Gly-Gly-structure at the carboxyl terminus. These repeats
are involved in the microtubule-associated region of the Tau
proteins, promoting the assembly and stabilisation of axon
microtubules (9), maintaining the
space between microtubules (10),
affecting the axonal material transport of nerve cells, promoting
neuronal growth and development, inhibiting lipid peroxidation and
tubulin aggregation, and facilitating learning and memory (11). Tau protein phosphorylation is one
of the predominant mechanisms regulating neuronal functions
(12). The abnormal
phosphorylation of Tau proteins induces misfolding and molecular
aggregation (13), which
consequently weakens their ability to stabilise microtubules and
decrease axonal transfer efficiency, resulting in transmitter
transport, storage and release disorders and synapse degeneration
or changes in the distribution and activity of prion proteins
(14). These phenomena can also
cause neuronal apoptosis or death and lead to learning and memory
impairment. Based on the presumed mechanism, Tau proteins can exert
physiological functions as soluble DNA molecular chaperones. In
addition, these proteins cannot bind with DNA during
formaldehyde-induced degeneration or misfolding, causing a series
of signal transduction impairments (15,16).
Tau protein hyperphosphorylation involves two
mechanisms: Endogenous and exogenous. The former is triggered by
the abnormal hyperphosphorylation of Tau protein in the brain,
whereas the latter is triggered by dysequilibrium between protein
kinases and phosphotases (17).
GSK-3β is currently the Tau protein kinase with the most marked
effect (18–20). It can catalyse Tau protein
phosphorylation at multiple sites (21,22),
including Thr181, Ser199, Ser202,
Thr205, Thr212, Thr217,
Thr231, Ser396 and Ser404. In
addition, GSK-3β phosphorylates 9G8 and affects the alternative
splicing of Tau exon 10, therefore, affecting the physiological
function of the Tau proteins (23). Akt is at the core of the PIK/Akt
signal transduction pathway (24)
and is an upstream regulating factor of GSK-3β (25). Glu inhibits the activity of various
protein kinases, including Akt (26).
The present study aimed to determine the effects of
ECT at different electrical currents and for different durations on
the hyperphosphorylation of Tau protein in depressed rats. The
results may serve as a basis for further investigation of the
molecular biological mechanisms underlying neural protection and
clinical intervention therapy.
Materials and methods
Reagents and apparatus
The following reagents and apparatus were used
throughout the present study: Pure
5-methyl-dihydro-propylcyclohepten-imine maleate (NMDA receptor
antagonist or dizocilpine or MK-801 (Sigma-Aldrich, St. Louis, MO,
USA), 2,6-diisopropylphenol (AstraZeneca Pharmaceuticals, Waltham,
MA, USA), pure L-glutamic acid (Sigma-Aldrich), rabbit anti-human
p-AT8Ser202 monoclonal antibody (GTX128164; GeneTex,
Irvine, CA, USA), mouse anti-human GSK-3β1H8 monoclonal
antibody (sc-377213; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA), diaminobenzidine (DAB) colour development kit (Beyotime
Institute of Biotechnology, Shanghai, China), Bicinchoninic acid
(BCA) Protein Assay kit (Beyotime Institute of Biotechnology),
o-phthalaldehyde (OPA) domestic analytical reagent (Beyotime
Institute of Biotechnology), β-mercaptoethanol (Amresco LLC, Solon,
OH, USA), chromatography-grade methanol (Beyotime Institute of
Biotechnology), HPD-25D oil-free diaphragm vacuum pump (Sherali
Seok Industrial Co., Ltd., Shanghai, China), YDJZ-II medical micro
electric grinder (Huien Medical Instrument and Device Co., Ltd.,
Shanghai, China), Harvard sine-wave shock generator (NatureGene
Corp., Medford, NJ, USA), Morris water maze video analysis system
(Academy of Military Medical Sciences, Beijing, China), 5810R
low-temperature high-speed centrifuge (Eppendorf, Hamburg,
Germany), HPLC system (Waters, Milford, MA, USA), 18-ODS
chromatography column (Dima Glass, Richmond Hill, ON, Canada),
protein electrophoresis system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), Goldisc multimedia image processing system
(Chengdu Goldisc UESTC Multimedia Technology Co., Ltd., China) and
Olympus-45 optical photomicrography system (Olympus, Tokyo,
Japan).
Animals
Healthy male Sprague-Dawley rats (24 weeks-old),
weighing between 250 and 300 g were provided by the Department of
Laboratory Animal Science, Tianjin Medical University (Tianjin,
China). The rats were housed in a well-ventilated environment with
free access to food and water, were subjected to an alternating 12
h light/dark cycle and were handled for 2 min daily to allow for
acclimation.
Following 1 week of adaptive breeding, models of
depressed rats without olfactory bulbs were established, as
described previously (27,28). The rats were anaesthetised with an
intraperitoneal injection of 2.75% sodium pentobarbital (55 mg/kg;
Biyuntian Biotech Co., Ltd., Shanghai, China). The skin at the
midpoint of the two ears was incised to expose the skull, and 2 mm
intersections were cut between 7 and 8 mm in front of the anterior
fontanels and on the two sides of the median raphes. Subsequently,
two holes, 2 mm in diameter, were drilled using an electric
grinder, and the olfactory bulb tissues were removed. The incisions
were washed with penicillin solution (200,000 U/ml) and the skin
was sutured. The rats were injected with 40,000 units penicillin
sodium/day for three consecutive days.
The rats were handled and weighed each day following
surgery, and the animals were subjected to open field assessment at
9 am for 2 weeks following recuperation from surgery. The open
field assessment involved a box divided into 25 cells. The
laboratory personnel placed the rats in the central square of the
open field box. The rats were observed for 5 min to observe their
movement, whereby 1 square was 1 point on the scoring system. The
open field test mainly reflects the activity and the curiosity of
the rats to novel environments therefore the open field test can
evaluate the degree of depression in rats. A total of 48 rats with
total horizontal and vertical scores ranging between 30 and 120 in
the open field assessment were included in the experimental
group.
Principles for animal
experimentation
The experiments in the present study were approved
by the Hospital Ethics Committee of the First Affiliated Hospital
of Chongqing Medical University (Chongqing, China). All animal
experiments were performed according to the Principle for Treatment
of Laboratory Animals issued by the American Medical Association
and the Guide for the Care and Use of Laboratory Animals by the
American Society of Animal Science and National Institutes of
Health. All experiments and analyses were performed in a
double-blinded manner, and the rats were raised in separate cages
throughout the experiment.
Grouping of laboratory animals
For the factorial design in analysis of variance
(ANOVA), two intervention factors were included: ECT, comprising
the ECT and without ECT groups; and drugs, comprising three groups
(i.p injection of saline, 2,6-diisopropylphenol and MK-801).
Intervention measures for laboratory
animals
A total of 48 adult depressed rats without olfactory
bulbs were randomly divided into six experimental groups (n=8 per
group): I.p injection of 5 ml saline; ii) i.p injection of 5 ml of
10 mg/kg NMDA receptor antagonist MK-801 (29); iii) i.p injection of 5 ml 10 mg/kg
MK-801 and a course of ECT; iv) i.p injection of 5 ml 200 mg/kg
2,6-diisopropylphenol (5); v) i.p
injection of 5 ml 200 mg/kg 2,6-diisopropylphenol and a course of
ECT; and vi) i.p injection of 5 ml saline and a course of ECT.
ECT
Each group of corresponding drugs were administered
by i.p injection 15 min prior to each ECT. The electrodes were
placed at the bilateral temporal areas of the rats and a Harvard
sine-wave ECT apparatus was used to provide electrical stimulation
with a square wave (a single half sine wave of 20 ms) at a current
of 50 mA and a frequency of 50 Hz for 1 sec. The occurrence of a
tonic-clonic convulsion seizure was considered to indicate
successful treatment (30). ECT
was performed seven times at 9 am once every 2 days. The drugs were
also injected into the rats in the experimental groups without
ECT.
Assessment of the learning and memory
functions of experimental rats using a the Morris water maze video
analysis system
The Morris water maze assessment was performed
within 24 h after the rats had received ECT. The Morris water maze
was equally divided into four quadrants: I, II, III and IV. Prior
to training, the water maze was filled with tap water and ink was
added to produce cloudy water, and a platform was placed 2 cm below
the water surface in quadrant I. All experiments were performed
between 9 and 3 pm in a quiet room with consistent article
placement, lighting and a water temperature of 24±1°C. Morris 1.0
software (Academy of Military Medical Science) was used to track
and record the data analyses. Place navigation assessments were
performed on days 1–6. Briefly, the rats were placed in the water,
facing the pool wall from quadrants I, II, III and IV in
counterclockwise direction and observed for 120 sec. Prior to
assessment, a platform was placed 2 cm under the water surface in
the centre of quadrant I. The escape latency, which was the time
during which the rats searched for and climbed the platform, was
detected using a camera system (Olympus-45, Olympus, Tokyo, Japan).
When the rats failed to find the platform within 120 sec, they were
led back to the platform and the escape latency was recorded as 120
sec. Following assessment, the mean escape latency on days 1–6 was
determined to indicate learning. The shorter the escape latency,
the better the learning capacity of the rats. A space probe trial
was performed on day 7. Briefly, the platform was removed and the
rats were placed in the water facing the pool wall from quadrant
III, furthest from the original platform. The duration of swimming
of the rats in each quadrant within 60 was recorded using a camera
system. The duration of swimming to the original platform in
quadrant I (space probe duration) was used to determine the memory
performance. The longer the duration, the better the memory
capacity of the rats.
Sample collection
As an important region closely associated with
learning and memory functions in the brain, the hippocampus is
involved in information acquisition, preservation and extraction,
and is the predominant target area subject to injury by stress.
Therefore, the hippocampus was selected as the region of
investigation in the present study. The hippocampal tissues of the
rats were extracted within 24 h after the Morris water maze
assessment. The rats were fasted without water deprivation 8 h
prior to sample collection and were subsequently anaesthetised by
i.p injection of 20% ethyl carbamate (1.5 g/kg; Biyuntian Biotech
Co., Ltd.). They were quickly sacrificed by decapitation to remove
the brain tissues. The blood stains were soaked in ice-cold
de-diethyl pyrocarbonate (Biyuntian Biotech Co., Ltd.) water to
separate the bilateral hippocampal tissues. The left hippocampus
was divided into sections A and B. Section A was frozen in liquid
nitrogen overnight at −80°C in an ultra-low-temperature
refrigerator for western blot analysis. Following weighing, section
A was added to a 1 ml methanol-water centrifugate and subsequently
homogenised at a low temperature. A sample of the homogenate was
centrifuged at 10,000 × g at 4°C for 15 min and the supernatant was
collected, filtered with a filter membrane and maintained at −80°C
to measure the Glu content (µg/g). The right hippocampus was
fixed in 10% poly-formaldehyde (Biyuntian Biotech Co., Ltd.) at 4°C
for 3 days, dehydrated, paraffin embedded, sectioned (thickness 1
µm) and subsequently equipped for immunity organisation
using the SP method.
Determination of hippocampal Glu content
of rats using HPLC (31)
The samples assessed were the treated supernatant of
the hippocampal tissues of the rats. Reagents used in HPLC included
Glu standard, OPA domestic analytical reagent (Beyotime Institute
of Biotechnology), β-mercaptoethanol (Amresco LLC) and
chromatography-grade methanol (Beyotime Institute of
Biotechnology). Glu standard solutions at concentrations of 0.15,
0.30, 0.735, 1.47, 2.94, 3.675 and 5.88 mg/l were prepared and
determined following derivatisation. The equipment used for
chromotography included a 5810R low-temperature high-speed
centrifuge, HPLC system equipped with a 600-series pump, a model
2475 fluorescence detector and an Empower chromatographic
workstation and 18-ODS chromatographic column (Dima Glass) at 35°C.
Mobile phase A contained 0.1 mol/l potassium acetate and mobile
phase B contained methanol undergoing binary gradient elution. The
mobile phase was filtered using a 0.45 µm microporous filter
membrane and subjected to ultrasonic degassing at a flow rate of
1.0 ml/min, excitation wavelength of 250 nm and emission wavelength
of 410 nm. Quantification was determined based on the Glu peak
area. For preparation of the derivatisation reagent, OPA (20 mg)
was dissolved in 500 µl methanol for ultrasonic dissolution,
500 µl β-mercaptoethanol and 9 ml boric acid buffer solution
(pH 10.0; Biyuntian Biotech Co., Ltd.) was added, and was
subsequently stored between 0°C and 4°C. For preparation of the
amino acid standard solution, standard solution (100 µmol/l)
was prepared using the Glu standard and was diluted prior to
assessment.
Derivatisation and analysis
Either the standard solution (100 µl) or
tissue sample solution was placed in an microcentrifuge tube and
subsequently reacted with 100 µl derivatisation reagent for
2 min, with 20 µl of sample injected at 20°C.
Establishment of the Glu standard
curve
Glu standard solutions at concentrations of 0.15,
0.30, 0.735, 1.47, 2.94, 3.675 and 5.88 mg/l were prepared and
determined following derivatisation. Quantitative analysis was
performed using the external standard method, and the
concentrations (X) and peak areas (Y) were subjected to linear
regression to obtain a linear equation.
Determination of hippocampal Glu
content
The homogenate supernatant from the hippocampal
tissue was thawed and added to 2 ml frozen formic acid (1 mol/l;
Biyuntian Biotech Co., Ltd.), following which the mixture was
homogenised manually in an ice bath. The homogenate was centrifuged
at 8×1012 × g for 30 min at 4°C and the supernatant was
maintained at −20°C for subsequent use. The homogenate supernatant
(1 ml) of the brain tissue was added to 0.75 ml 4% sodium
bicarbonate solution (Biyuntian Biotech Co., Ltd.) and subsequently
centrifuged at 3.5×1012 × g for 5 min at 4°C. The
supernatant was filtered using a 0.45 µm filter membrane and
then loaded. Subsequently, 24 µl loaded solution was
collected and 12 µl derivatisation reagent and 960 µl
sodium tetraborate buffer solution (pH 9.18; Biyuntian Biotech Co.,
Ltd.) was added to the sample and allowed to stand at 20°C for 3
min. Sampling and gradient elution were then performed to determine
the Glu content. The sampling was performed using the sampling tube
of the HPLC chromatographic system and the gradient elution was
performed using an 18 ODS chromatographic column (Dima Glass).
Assessment of the protein expression
levels of p-AT8Ser202 and GSK-3β1H8 in the
hippocampus of rats using the SP method
The parrafin embedded hippocampal tissues were
dewaxed, hydrated with ethanol at 20°C, flushed with distilled
water and subsequently immersed in 0.01 mol/l phosphate-buffered
saline (PBS; Biyuntian Biotech Co., Ltd.) for 5 min, 3%
H2O2 for 15 min at 20°C and 0.01 M citric
acid buffer (Biyuntian Biotech Co., Ltd., Shanghai, China) with a
liquid hydrogen ion index of 6.0 for 15 min. The samples were
subsequently boiled for 15–20 min for antigen repair, cooled for 20
min at room temperature and incubated in 10% sheep serum albumin
(Biyuntian Biotech Co., Ltd., Shanghai, China) for 15 min at 20°C.
The regular SP method was performed as follows: 50 µl
antibodies, including rabbit anti-human p-AT8Ser202
monoclonal antibody and mouse anti-human GSK-3β1H8
monoclonal antibody, at a dilution of 1:400, at 37°C for 2 h.
Subsequently, 50 µl immunoglobulin G and 50 µl S-A
(horseradish peroxidase) were added to each slide. A
3,3′-diaminobenzidine (DAB) colour reaction was then performed. For
the negative control, the primary antibodies were replaced with
0.01 mol/l PBS. The number of positive cells under each high power
field (10 rats/group, 10 slides/rat, 10 high power fields/slide)
were determined under a light microscope (BM-E biological
microscope; Leica Microsystems GmbH, Wetzlar, Germany) and the
average optical density value of the positive cells was measured
using a multimedia image handling system.
Determination of the protein expression
levels of p-AT8Ser202 and GSK-3β1H8 in the
rat hippocampus using western blot analysis
The hippocampal tissues of the rats were
homogenised, and 0.2 g homogenate was placed in cell lysis buffer
(Biyuntian Biotech Co., Ltd., Shanghai, China) for western blot
analysis, and immunoprecipitation buffer (Biyuntian Biotech Co.,
Ltd., Shanghai, China) to extract proteins. The protein
concentration was determined using a BCA protein assay kit and
adjusted for consistency. Equal quantities (30 µg) of the
extracted protein samples were diluted with 5X sodium dodecyl
sulphate (SDS) sample loading buffer solution (Biyuntian Biotech
Co., Ltd., Shanghai, China) at a ratio of 1:1 (v/v) and boiled at
100°C for 5 min. The mixture of the pre-stained protein molecular
weight markers was further dissolved in 1X SDS sample loading
buffer solution and then boiled at 100°C for 3 min. The samples (15
µl aliquot of each) were loaded, using
glyceraldehydes-3-phosphate dehydrogenase for calibration, and
subjected to SDS-polyacrylamide gel electrophoresis (PAGE) until
the target molecular weight was achieved. The protein bands were
electrically transferred to polyvinylidine fluoride membranes
(Bio-Rad Laboratories, Inc.) using a wet transfer process. The
membrane was then blocked with 50 g/l non-fat milk powder for 3 h
and subsequently incubated with rabbit anti-human
p-AT8Ser202 monoclonal antibody (1:400) and mouse
anti-human GSK-3β1H8 monoclonal antibody (1:400) at 4°C
overnight. The immunoglobulin G (1:200) was marked using the
corresponding horseradish peroxidase and then incubated at 37°C for
2 h. A DAB kit was used for colour development and a Goldisc
multimedia image processing system was used to determine the
integral absorbance value of the positive bands.
Statistical analysis
The data are expressed as the mean ± standard
deviation. Homogeneity of variances for each sample group were
assessed using SPSS 19.0 statistical software (SPSS, Inc., Chicago,
IL, USA). Each group was subjected to factorial design and one-way
analysis of variance (ANOVA) to determine the predominant effects
and interaction effects of each treatment factor. The effect of
each treatment factor was analysed using one-way ANOVA, and
multiple comparisons were determined using the least significant
difference test and Student Newman-Kuels-q-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Detection of learning and memory
functions of the rats using the Morris water maze video analysis
system: Escape latency and space probe time
The ECT and drug (2,6-diisopropylphenol and NMDA
receptor antagonist) treatments resulted in learning and memory
impairment in the rats, with prolonged escape latency (ECT,
F=148.986 and P<0.001; NMDA receptor antagonist and
2,6-diisopropylphenol, F=3.809 and P=0.030) and a shortened
space probe time (ECT, F=4.376 and P=0.043; NMDA receptor
antagonist and 2,6-diisopropylphenol, F=17.863 and
P<0.001). However, these effects presented a negative
association (escape latency, F=32.870 and P<0.001; space
probe time, F=98.938 and P<0.001). The combination of ECT
and Glu receptor antagonist or 2,6-diisopropylphenol alleviated
learning and memory impairment in rats (Tables I and II).
 | Table IMorris water maze assessment of
escape latency. |
Table I
Morris water maze assessment of
escape latency.
| Group | Saline | NMDA receptor
antagonist |
2,6-diisopropylphenol | Total | F-statistic | P-value |
|---|
| Control | 26.65±3.39 | 36.35±3.30 | 34.59±3.91 | 32.53±5.48 | 17.009 | 0.000 |
| ECT | 61.68±8.26 | 45.77±5.53 | 43.93±4.34 | 50.46±10.10 | 19.425 | 0.000 |
| Total | 44.17±19.09 | 41.06±6.56 | 39.26±6.26 | 41.49±12.11 | 3.809a | 0.030a |
| F-statistic | 123.107 | 17.102 | 20.463 | 148.986a | – | – |
| P-value | 0.000 | 0.001 | 0.000 | 0.000a | – | – |
 | Table IIMorris water maze assessment of space
probe duration. |
Table II
Morris water maze assessment of space
probe duration.
| Group | Saline | NMDA receptor
antagonist |
2,6-diisopropylphenol | Total | F-statistic | P-value |
|---|
| Control | 26.36±4.21 | 11.17±1.69 | 11.86±1.51 | 16.46±7.62 | 77.237 | 0.000 |
| ECT | 10.92±2.30 | 16.38±2.16 | 17.67±1.65 | 14.99±3.58 | 24.363 | 0.000 |
| Total | 18.64±8.61 | 13.78±3.28 | 14.76±3.37 | 15.73±5.94 | 17.863a | 0.000a |
| F-statistic | 82.814 | 28.928 | 54.291 | 4.376a | – | – |
| P-value | 0.000 | 0.000 | 0.000 | 0.043a | – | – |
Detection of Glu content in rat
hippocampus using HPLC
ECT (F=277.841 and P<0.001) and
2,6-diisopropylphenol (F=21.320 and P<0.001)
significantly increased the concentration of Glu in the
hippocampus, and these effects were negatively associated. The NMDA
receptor antagonist had no significant effect on the concentration
of Glu in the hippocampus (Table
III).
 | Table IIIGlu content in the rat hippocampus
(µmol/gprot). |
Table III
Glu content in the rat hippocampus
(µmol/gprot).
| Group | Saline | NMDA receptor
antagonist |
2,6-diisopropylphenol | Total | F-statistic | P-value |
|---|
| Control | 46.51±9.35 | 49.43±9.77 | 36.90±6.25 | 44.28±9.87 | 4.652 | 0.021 |
| ECT | 162.16±31.89 | 149.93±24.86 | 92.32±16.34 | 134.80±39.32 | 17.553 | 0.000 |
| Total | 104.34±63.89 | 70.88±25.69 | 64.61±31.02 | 89.54±53.82 | 21.320a | 0.000a |
| F-statistic | 96.903 | 113.283 | 80.278 | 277.841a | – | – |
| P-value | 0.000 | 0.000 | 0.000 | 0.000a | – | – |
Determination of the protein expression
levels of p-AT8Ser202 and GSK-3β1H8 in the
rat hippocampus using the SP method
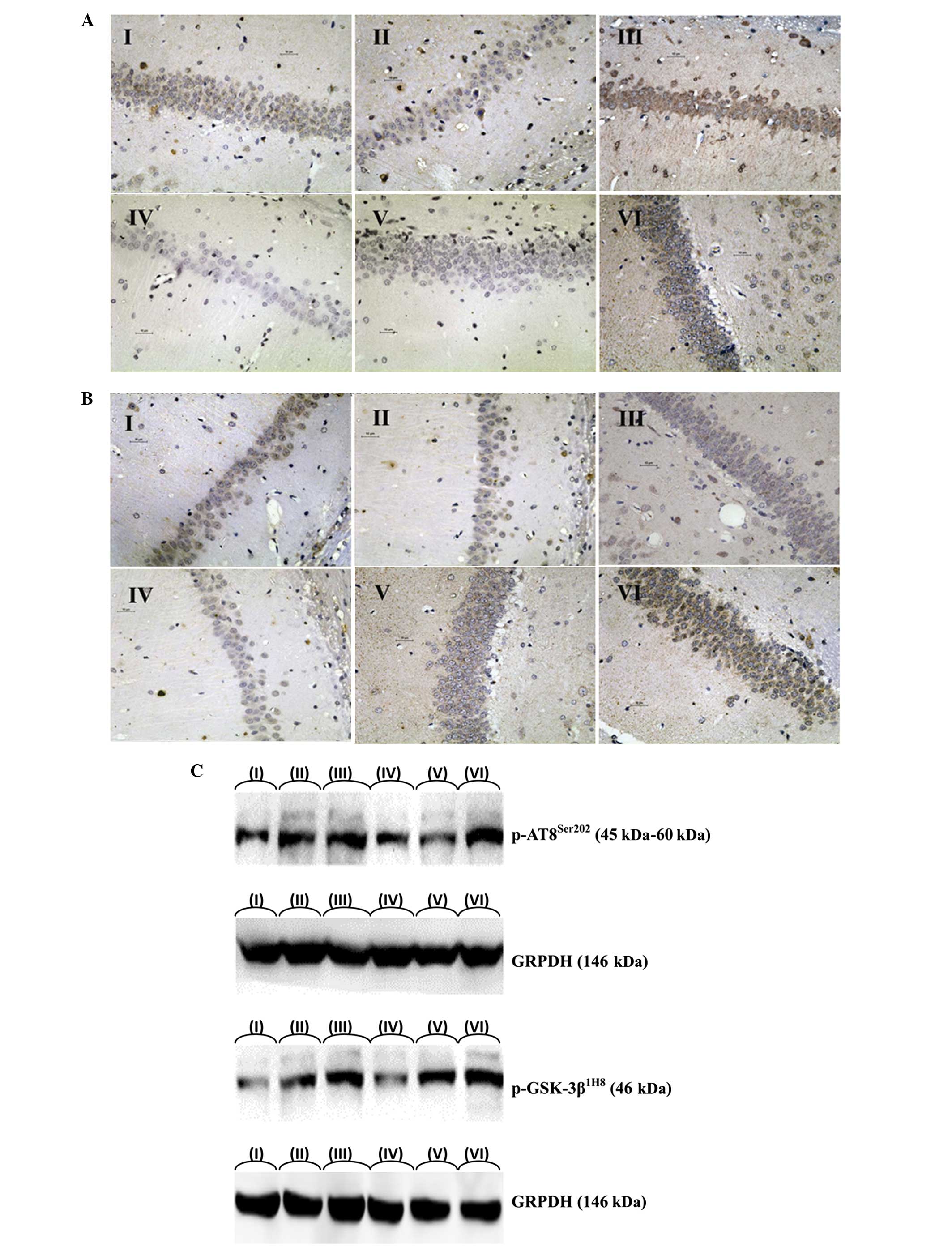
ECT increased the protein expression levels of
p-AT8Ser202 and GSK-3β1H8 in the hippocampus
of rats, as indicated by the quantity of IR-positive cells
(p-AT8Ser202, F=255.037 and P<0.001;
GSK-3β1H8, F=98.216 and P<0.000) and the
integral absorbance value of the IR-positive cells
(p-AT8Ser202, F=366.698 and P<0.001;
GSK-3β1H8, F=167.764 and P<0.001). However,
the NMDA receptor antagonist and 2,6-diisopropylphenol reduced the
expression levels of p-AT8Ser202 and
GSK-3β1H8 in the hippocampus of the rats, as indicated
by the quantity of IR-positive cells (p-AT8Ser202,
F=56.003 and P<0.001; GSK-3β1H8,
F=71.848 and P<0.001) and the integral absorbance value
of the IR-positive cells (p-AT8Ser202, F=56.003
and P<0.001; GSK-3β1H8, F=71.848 and
P<0.001). These effects presented a negative association. The
NMDA receptor antagonist and 2,6-diisopropylphenol slowed the
ECT-induced increase of the protein expression of Tau in the
hippocampus of the rats (Fig. 1A and
B; Tables IITable III–IV), as indicated by the quantity of
IR-positive cells (p-AT8Ser202, F=3.507 and
P=0.039; GSK-3β1H8, F=3.651 and P=0.035) and the
integral absorbance value of the IR-positive cells
(p-AT8Ser202, F=40.174 and P<0.001;
GSK-3β1H8, F=11.247 and P<0.001). Therefore,
the NMDA receptor antagonist and 2,6-diisopropylphenol slowed the
ECT-induced increase in protein expression of phosphorylated Tau in
the hippocampus, (Fig. 1A and B;
Tables IVTable V–VII).
 | Table IVNumber of p-AT8Ser202
IR-positive cells the in rat hippocampus. |
Table IV
Number of p-AT8Ser202
IR-positive cells the in rat hippocampus.
| Group | Saline (n) | NMDA receptor
antagonist (n) |
2,6-diisopropylphenol (n) | Total (n) | F-statistic | P-value |
|---|
| Control | 39.13±6.94 | 21.50±3.59 | 20.25±2.76 | 26.96±9.92 | 38.964 | 0.000 |
| ECT | 80.13±11.63 | 48.88±6.77 | 53.13±8.48 | 60.71±16.64 | 27.257 | 0.000 |
| Total | 59.63±23.11 | 35.19±15.07 | 36.69±18.04 | 43.83±21.78 | 56.003a | 0.000a |
| F-statistic | 73.329 | 102.138 | 108.781 | 255.037a | – | – |
| P-value | 0.000 | 0.000 | 0.000 | 0.000a | – | – |
 | Table VIntegral absorbance value of positive
cells in rat hippocampus (p-AT8Ser202-IR). |
Table V
Integral absorbance value of positive
cells in rat hippocampus (p-AT8Ser202-IR).
| Group | Saline | MDA receptor
antagonist |
2,6-diisopropylphenol | Total | F-statistic | P-value |
|---|
| Control | 0.1807±0.0135 | 0.0727±0.0119 | 0.0644±0.0114 | 0.1060±0.0554 | 222.375 | 0.000 |
| ECT | 0.4095±0.0521 | 0.1585±0.0200 | 0.1634±0.0125 | 0.2438±0.1238 | 150.951 | 0.000 |
| Total | 0.2951±0.1238 | 0.1156±0.0471 | 0.1139±0.0524 | 0.1749±0.1177 | 279.136a | 0.000a |
| F-statistic | 144.283 | 108.566 | 273.455 | 366.698a | – | – |
| P-value | 0.000 | 0.000 | 0.000 | 0.000a | – | – |
 | Table VIIIntegral absorbance value of positive
cells in rat hippocampus (GSK-3β1H8-IR). |
Table VII
Integral absorbance value of positive
cells in rat hippocampus (GSK-3β1H8-IR).
| Group | Saline | NMDA receptor
antagonist |
2,6-diisopropylphenol | Total | F-statistic | P-value |
|---|
| Control | 0.1012±0.1169 | 0.0645±0.0043 | 0.0634±0.0063 | 0.0815±0.0267 | 106.291 | 0.000 |
| ECT | 0.1673±0.0132 | 0.0873±0.0072 | 0.0899±0.0062 | 0.1148±0.0389 | 187.156 | 0.000 |
| Total | 0.1421±0.0288 | 0.0758±0.0132 | 0.0767±0.0150 | 0.0982±0.0371 | 291.269a | 0.000a |
| F-statistic | 62.186 | 60.189 | 71.389 | 167.764a | – | – |
| P-value | 0.000 | 0.000 | 0.000 | 0.000a | – | – |
Determination of the protein expression
levels of p-AT8Ser202 and GSK-3β1H8 in the
rat hippocampus using western blot analysis
ECT increased the protein expression levels of
p-AT8Ser202 and GSK-3β1H
(p-AT8Ser202, F=350.725 and P<0.001;
GSK-3β1H8, F=35.412 and P<0.001), whereas
2,6-diisopropylphenol and the NMDA receptor antagonist reduced
their expression levels (p-AT8Ser202, F=73.129
and P<0.001; GSK-3β1H8, F=68.465 and
P<0.001). These effects presented a negative association
(p-AT8Ser202, F=4.580 and P=0.016;
GSK-3β1H8, F=5.698 and P=0.006). For example,
2,6-diisopropylphenol and the NMDA receptor antagonist slowed the
ECT-induced increase in the protein expression of Tau, and the key
regulatory protein may be GSK-3β1H8 (Fig. 1C; Tables VIII and IX).
 | Table VIIIWestern blot analysis and integral
absorbance value of p-AT8Ser199/202 protein content in
rat hippocampus. |
Table VIII
Western blot analysis and integral
absorbance value of p-AT8Ser199/202 protein content in
rat hippocampus.
| Group | Saline | NMDA receptor
antagonist |
2,6-diisopropylphenol | Total | F-statistic | P-value |
|---|
| Control | 695.38±151.65 | 405.25±74.39 | 399.88±89.92 | 500.17±176.16 | 23.879 | 0.000 |
| ECT | 1354.38±94.42 | 913.13±76.87 | 888.13±71.15 | 1051.88±232.17 | 50.175 | 0.000 |
| Total | 1024.88±361.52 | 659.19±272.26 | 644.00±264.02 | 776.02±345.37 | 73.129a | 0.000a |
| F-statistic | 119.457 | 101.646 | 149.425 | 350.725a | – | – |
| P-value | 0.000 | 0.000 | 0.000 | 0.000a | – | – |
 | Table IXWestern blot analysis and integral
absorbance value of GSK-3β1H8 protein content in rat
hippocampus. |
Table IX
Western blot analysis and integral
absorbance value of GSK-3β1H8 protein content in rat
hippocampus.
| Group | Saline | NMDA receptor
antagonist |
2,6-diisopropylphenol | Total | F-statistic | P-value |
|---|
| Control | 496.75±98.35 | 327.25±62.21 | 329.75±52.06 | 384.58±107.29 | 13.937 | 0.000 |
| ECT | 700.75±52.42 | 400.13±61.09 | 392.75±58.00 | 497.88±156.46 | 75.294 | 0.000 |
| Total | 598.75±129.98 | 363.69±70.45 | 361.25±62.39 | 441.23±144.53 | 68.465a | 0.000a |
| F-statistic | 26.806 | 5.589 | 5.227 | 35.412a | – | – |
| P-value | 0.000 | 0.033 | 0.038 | 0.000a | – | – |
Discussion
Glu and learning-memory impairment
In the present study, significant post-ECT increased
hippocampal Glu concentration was accompanied with decreased
learning and memory abilities, as indicated by prolonged escape
latency and shortened space probe duration, the former indicating
impaired learning ability and the latter indicating a decline in
explicit memory. These findings were in agreement with those of
previous studies (3–5), in which ECT was demonstrated to
induce Glu-associated excitotoxicity. In addition, this process was
revealed to be proportional to the current and duration of ECT,
which further confirmed the observation. The NMDA antagonist
alleviated the spatial learning and memory impairment induced by
the overexcitation of GluR, which was consistent with the
experimental result of Wu et al (32) that post-ECT decline in learning and
memory abilities are a result of oxidative stress, caused by the
overexcitation of GluR, and result in hippocampal LTP saturation
and synaptic plasticity impairment.
The hippocampus of the limbic system is important in
memory. The episodic memory in the explicit memory depends on the
hippocampus (33). The hippocampus
is not only closely associated with short-term memory, but also
with long-term spatial memory in rats (34). The water maze assessment was used
to determine the spatial memory in the episodic memory. The spatial
memory of humans or animals is summarised in the cognitive map
stored in the hippocampus (35).
Hippocampal cells can receive and process spatial information from
different sources, enabling cognitive map formation or increased
synaptic contact of cell assemblies in the association cortex to
form the permanent memory of spatial positions (36). The present study demonstrated that
increased hippocampal Glu concentration and the increased
hyperphosphorylation of the Tau protein caused impairment of the
spatial memory of the rats. By contrast, 2,6-diisopropylphenol
partially inhibited the excitotoxicity of Glu and further
alleviated the hyperphosphorylation of the Tau protein. These
results also confirmed that the hippocampal tissues function in the
spatial memory and explicit memory of rats.
Palmio et al (37) indicated that ECT was unable to
impair neurons, as the post-ECT neuron-specific enolase and S-100B
proteins in the serum were not significantly increased. These
findings are inconsistent with those of the present study. Whether
this result is associated with the limited sample size (10
individuals) requires further confirmation.
In the present study, 2,6-diisopropylphenol
decreased the post-ECT hippocampal Glu content and improved the
post-ECT learning and memory abilities of the rats. This finding
was consistent with those of previous studies (38). However, the NMDA receptor
antagonist caused learning and memory impairment and partially
alleviated post-ECT learning and memory impairment. The NMDA
receptor antagonist revealed no significant effect on hippocampal
Glu content. Therefore, the NMDA receptor antagonist may function
by inhibiting the excitability of Glu instead of decreasing the
excretion of Glu in the hippocampus. Additionally, the excitability
of the NMDA receptor at normal levels is essential for learning and
memory, which is also consistent with a previous study (39).
In comparative analysis of the present study with
previous reports, in contrast to the present study, Stover et
al (40) revealed that
2,6-diisopropylphenol increases the concentration of Glu in the
cerebrospinal fluid. This finding may be attributed to the patients
undergoing neurosurgery and the effect of the interference factor
(neurosurgery) on the concentration of Glu in the nervous system,
which is higher compared with the anesthesia treatment. Previous
studies also demonstrated that 2,6-diisopropylphenol failed to
protect the Glu content of rat cortex and hippocampal tissues from
injury (41). At high doses,
2,6-diisopropylphenol can aggravate injury and even increase the
release of Glu (42). This finding
is in disagreement with that of the present study, and the in
vitro brain slices used may not be appropriate to simulate the
in vivo environment. In contrast to the present study, Pesić
et al (43) revealed that
2,6-diisopropylphenol induces cortical neuron death. This
inconsistency in results may be attributed to the fact that the
rats used in this previous study were 7-day-old neonates,
therefore, their nervous system was immature and more sensitive to
drugs, compared with adult rats.
Learning and memory abilities are associated with
the hyperphosphorylation of Tau protein, in that learning and
memory abilities decrease as the phosphorylation of Tau protein is
upregulated (16). In the present
study, increased levels of phosphorylated hippocampal Tau proteins
prolonged the escape latency and shortened the space probe duration
of the rats, revealing impaired learning ability and explicit
memory, respectively. Therefore, Tau proteins may exert
physiological effects as soluble DNA molecular chaperones, which
cannot bind with DNA during formaldehyde induced degeneration or
misfolding (44).
Phosphorylation at the pAT8Ser202 site
was also observed to be closely associated with ECT intervention,
consistent with the results of previous studies (23,45,46).
However, the explanation of its signal transduction pathway and
action site differed. Jeon et al (47) indicated that the process is
performed by serine/threonine protein kinase-1 at the
Ser262 site. However, the present study demonstrated
that the Ser202 site and ECT-induced Tau
hyperphosphorylation may be more important.
The present study demonstrated that the expression
levels of all phosphorylated hippocampal Tau proteins were
upregulated as the post-ECT Glu concentration in the neurons
increased, and decreased axonal transport efficiency caused further
accumulation of Glu in the brain. However, Tau proteins cannot bind
with DNA and their function as molecular chaperones was affected,
resulting in neuronal dysfunction. This result is in agreement with
the results of Wu et al (32), who revealed that stress can
activate the excitatory neurotransmission system to induce
hippocampal Tau protein hyperphosphorylation. The results of the
present study are also consistent with those reported by Tan et
al (48), who demonstrated
that low body temperature, rather than 2,6-diisopropylphenol, can
induce the Tau protein hyperphosphorylation.
Vossel et al (49) demonstrated that NMDA receptor
excitation can activate the polymerisation of GSK-3β, casein kinase
2 and actin to increase the phosphorylation of Tau, which impairs
neurons. By contrast, NMDA receptor antagonists inhibit the
excitotoxicity of Glu at the receptor level to interrupt nerve
injury caused by GSK-3β-induced Tau protein hyperphosphorylation
(50,51).
The present study demonstrated that the increased
hippocampal Glu concentration was accompanied by a decline in
learning and memory. However, the learning and memory impairment
caused by the overexcitation of GluR was alleviated following
treatment with the GluR antagonist. This finding was similar to
that of previous studies (38).
The present study demonstrated that the ECT-induced
increase of hippocampal Glu concentration and increased hippocampal
Tau protein hyperphosphorylation were a dose- and time-dependent.
Among all the hyperphosphorylation sites, phosphorylation at the
pAT8Ser202 site was the most closely associated with ECT
stress, and may also have been involved in learning and memory
impairment caused by Tau protein hyperphosphorylation. This result
may be associated with Tau protein phosphorylation at the
Ser202 site, positioned in the microtubule binding
domain, being involved in regulating the binding activity of Tau
proteins and microtubules.
Following ECT, GSK-3β1H8, a key protein
which regulates Tau protein phosphorylation, was also upregulated,
together with p-AT8Ser202, and the upregulated
expression levels of GSK-3β1H8 and
p-AT8Ser202 were reversed following intervention of
2,6-diisopropylphenol and NMDA receptor antagonist. This result is
consistent with that of Muyllaert et al (52), which demonstrated that GSK-3β
activity is regulated by serine and tyrosine phosphorylation,
indicating that the relevant signal transduction pathways of
2,6-diisopropylphenol and the NMDA receptor antagonist are affected
by the key protein, GSK-3β1H8.
Therefore, the present study hypothesized the
following signal transduction pathways: ECT induces increases in
hippocampal Glu concentration, whereas Glu excites the ionotropic
receptor (GluR), inhibits the Akt signal channel, increases the
expression and activity of GSK-3β, and increasesthe phosphorylation
of Tau protein in the hippocampus, thereby decreasing axonal
transport efficiency, and promoting neural signal transmission
impairment and synaptic degeneration, causing neuronal apoptosis or
death. Hippocampal Tau protein hyperphosphorylation can induce
neurotransmitter transport impairment, further resulting in the
accumulation of Glu in the injured neuron, forming cycles that
aggravate neuronal injury.
Previous studies indicated that
2,6-diisopropylphenol can reduce the Glu content of the brain and
inhibit neuronal apoptosis by improving the activity of Akt
(53). In the present study,
2,6-diisopropylphenol lowered the post-ECT hippocampal
concentration of Glu, which was consistent with a study by Xu et
al (54). In addition,
2,6-diisopropylphenol decreased Tau protein hyperphosphorylation to
improve post-ECT learning and memory abilities, therefore it was
suggested that 2,6-diisopropylphenol affected the signaling pathway
of the enhancement of ECT-induced Tau protein phosphorylation in
two aspects: 2,6-diisopropylphenol reduced the Glu concentration of
the brain and improved Akt activity, the latter possibly being a
secondary effect of the former.
2,6-diisopropylphenol also reduced the expression of
GSK-3β1H8, decreasing hippocampal Tau protein
hyperphosphorylation and improving post-ECT spatial learning and
memory abilities. This result is similar to the findings of Straiko
et al (55), who revealed
that ketamine and 2,6-diisopropylphenol inhibits protein kinase
phosphorylation through the same pathway as that of lithium in
inhibiting enzyme phosphorylation.
Vossel et al (49) demonstrated that NMDA receptor
excitation activates the polymerisation of GSK-3β, casein kinase 2
and actin to increase the phosphorylation of Tau, thereby injuring
neurons. However, the NMDA receptor antagonist can inhibit the
excitotoxicity of Glu at the receptor level (50,51,56–62),
interrupting the neuronal injury caused by GSK-3β induced Tau
protein hyperphosphorylation. In the present study, the NMDA
receptor antagonist decreased the expression of
GSK-3β1H8, therefore, decreasing hippocampal Tau protein
hyperphosphorylation and improving post-ECT spatial learning and
memory abilities. This result is consistent with those of previous
studies (35,50,51,56–60).
However, there are different views on the specific site of Tau
protein phosphorylation, which has the closest association with
excitation by the NMDA receptor (61–63).
Kingston et al (64) revealed that 2,6-diisopropylphenol
can inhibit the phosphorylation of NMDA receptor NR1 subunits
through the signal transduction pathway of serine/threonine
phosphatase PP2A, thereby decreasing the activity of the NMDA
receptor (45). This conclusion
further demonstrated that 2,6-diisopropylphenol functions directly
as an NMDA receptor antagonist (65) and partially reverses post-ECT
learning and memory impairment (66).
The results of the present and previous studies
demonstrated that 2,6-diisopropylphenol functions directly as an
NMDA receptor antagonist and partially reverses post-ECT learning
and memory impairment (65). ECT,
as a marked inducer of stress, induces increased hippocampal Glu
concentration, while Glu excites the ionotropic receptor GluR,
therefore, inhibiting the Akt signaling pathway (67), decreasing the activity of Akt,
weakening the inhibition of GSK-3β by Akt (68), increasing the activity of GSK-3β,
increasing hippocampal protein Tau phosphorylation, decreasing
axonal transport efficiency, impairing neural signal transmission
and causing neuron apoptosis or death, leading to the learning and
memory impairment. Tau hyperphosphorylation resulted in
neurotransmitter transport impairment and further induced Glu
accumulation in injured neurons, forming cycles and aggravating
neuron injury. GluR and GSK-3β were key nodes in this mechanism. It
has been suggested that hippocampal Tau protein
hyperphosphorylation induced by ECT is slowed, and space learning
and memory impairment is alleviated correspondingly if one link is
inhibited, therefore, the use of 2,6-diisopropylphenol or NMDA
receptor antagonist to reduce Glu in brain and
2,6-diisopropylphenol can be used as an NMDA receptor
antagonist.
The present study demonstrated that the stress of
ECT induced increased hippocampal Glu concentration and Tau protein
phosphorylation and decreased learning and memory abilities, and
was positively associated with the current and duration of ECT. Glu
excites the NMDA receptor and AMPAR to increase hippocampal Tau
protein phosphorylation, affecting the alternative splicing of Tau
exon 10 and decreasing axonal transport efficiency, resulting in
neural signal transmission impairment and synaptic degeneration,
finally leading to learning and memory impairment (69). In addition, hippocampal Tau protein
hyperphosphorylation can result in neurotransmitter transport
impairment, causing further accumulation of Glu in the injured
neurons, forming cycles that aggravate neuronal injury (70).
As demonstrated in the present study, inhibition of
the 2,6-diisopropylphenol or NMDA receptor antagonist links, slowed
the ECT induced hippocampal Tau protein phosphorylation and
alleviated spatial learning and memory impairment. Furthermore, the
NMDA receptor was as important as the AMPA receptor for GluR in the
above process, indicating that the role of AMPA in the
neuromolecular biological mechanism of learning and memory is
similar to that of the NMDA receptor.
The present study demonstrated that GSK-3β was a key
protein in the signaling pathway regulating Tau phosphorylation.
ECT-induced increase of hippocampal Glu concentration increased the
hippocampal Tau protein phosphorylation, leading to learning and
memory impairment. Glu excites the iontropic receptors, NMDA and
AMPA, to inhibit the Akt signaling pathway, thereby decreasing the
activity of Akt activity, weakening GSK-3β inhibition by Akt,
increasing GSK-3β activity, increasing hippocampal Tau protein
phosphorylation and decreasing axonal transport efficiency. This
series of events resulted in neural signal transmission impairment,
synaptic degeneration and neuronal apoptosis or death, leading to
learning and memory impairment. Hippocampal Tau protein
hyperphosphorylation in the hippocampus resulted in
neurotransmitter transport impairment, further causing accumulation
of Glu in the injured neurons, forming cycles that aggravate
neuronal injury. GluR, Akt and GSK-3β are key factors in this
signal transduction pathway and, as demonstrated in the present
study, inhibiting 2,6-diisopropylphenol slowed ECT induced
hippocampal Tau protein phosphorylation and alleviated spatial
learning and memory impairment.
The findings indicate that increased apoptosis may
be an explanation for the reduced OB volume and olfactory
dysfunction in patients with depression. In addition, the
mitochondrial-dependent death pathway may be involved in apoptosis
in the OB of the rats.
Acknowledgments
This study was supported by a grant from the
National Natural Science Foundation of China (grant. no.
30972831).
References
|
1
|
Ebert MH: Current diagnosis and treatment
psychiatry. 2nd ed. Mc Graw-Hill Press; New York: 2008
|
|
2
|
Conrad MS and Richard A: The handbook for
electroconvulsive therapy. 1st ed. Eagle Race Medical Technologies
Company Press; California: 1999
|
|
3
|
Luo J, Mins, Wei K, Li P, Dong J and Liu
YF: Propofol protects against impairment of learning-memory and
imbalance of hippocampal Glu/GABA induced by electroconvulsive
shock in depressed rats. J Anesth. 25:657–665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andrade C, Singh NM, Thyagarajan S,
Nagaraja N, Sanjay Kumar Rao N and Suresh Chandra J: Possible
glutamatergic and lipid signalling mechanisms in ECT-induced
retrograde amnesia: experimental evidence for involvement of COX-2
and review of literature. J Psychiatr Res. 42:837–850. 2008.
View Article : Google Scholar
|
|
5
|
Dong J, Mins, Wei K, Li P, Cao J and Li Y:
Effects of electroconvulsive therapy and propofol on spatial memory
and glutamatergic system in hippocampus of depressed rats. J ECT.
26:126–130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kartalcis, Karabulut AB, Ozcan AC, Porgali
E and Unal S: Acute and chronic effects of electroconvulsive
treatment on oxidative parameters in schizophrenia patients. Prog
Neuropsychopharmacol Biol Psychiatry. 35:1689–1694. 2011.
View Article : Google Scholar
|
|
7
|
Kato N: Neurophysiological mechanisms of
electroconvulsive therapy for depression. Neurosci Res. 64:3–11.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weingarten MD, Lockwood AH, Hwos Y and
Kirschner MW: A protein factor essential for microtubule assembly.
Proc Natl Acad Sci USA. 72:1858–1862. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Osiecka KM, Nieznanska H, Skowronek KJ,
Jozwiak J and Nieznanski K: Tau inhibits tubulin oligomerization
induced by prion protein. Biochim Biophys Acta. 1813:1845–1853.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cleveland DW, Hwo SY and Kirschner MW:
Purification of tau, a microtubule-associated protein that induces
assembly of microtubules from purified tubulin. J Mol Biol.
116:207–225. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Onishi T, Matsumoto Y, Hattori M, Obayashi
Y, Nakamura K, Yano T, Horiguchi T and Iwashita H: Early-onset
cognitive deficits and axonal transport dysfunction in P301S mutant
tau transgenic mice. Neurosci Res. 80:76–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Calignon A, Fox LM, Pitstick R, et al:
Caspase activation precedes and leads to tangles. Nature.
464:1201–1204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Combs B, Voss K and Gamblin TC:
Pseudohyperphosphorylation has differential effects on
polymerization and function of tau isoforms. Biochemistry.
50:9446–9456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Canu N, Filesi I, Pristerà A, Ciotti MT
and Bioccas: Altered intracellular distribution of PrPC and
impairment of proteasome activity in tau overexpressing cortical
neurons. J Alzheimers Dis. 27:603–613. 2011.PubMed/NCBI
|
|
15
|
Onishi T, Iwashita H, Uno Y, et al: A
novel glycogen synthase kinase-3 inhibitor
2-methyl-5-(3-{4-[(S)-methylsulfinyl]phenyl}-1-benzofuran-5-yl)-1,3,4-oxadiazole
decreases tau phosphorylation and ameliorates cognitive deficits in
a transgenic model of Alzheimer's disease. J Neurochem.
119:1330–1340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kopeikina KJ, Carlson GA, Pitstick R, et
al: Tau accumulation causes mitochondrial distribution deficits in
neurons in a mouse model of tauopathy and in human Alzheimer's
disease brain. Am J Pathol. 179:2071–2082. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Annamalai B, Won JS, Choi S, Singh I and
Singh AK: Role of S-nitrosoglutathione mediated mechanisms in tau
hyper-phosphorylation. Biochem Biophys Res Commun. 458:214–219.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Q, Zhang JY, Liu SJ and Li HL:
Overactivated mitogen-activated protein kinase by anisomycin
induces tau hyperphosphorylation. Sheng Li Xue Bao. 60:485–491.
2008.PubMed/NCBI
|
|
19
|
Fu ZQ, Yang Y, Song J, et al: LiCl
attenuates thapsigargin induced tau hyperphosphorylation by
inhibiting GSK-3beta in vivo and in vitro. J Alzheimers Dis.
21:1107–1117. 2010.
|
|
20
|
De Vos A, Anandhakumar J, Van den Brande
J, et al: Yeast as a model system to study tau biology. Int J
Alzheimers Dis. 2011:4289702011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blazquez Llorca L, Garcia-Marin V,
Merino-Serrais P, Ávila J and DeFelipe J: Abnormal tau
phosphorylation in the thorny excrescences of CA3 hippocampal
neurons in patients with Alzheimer's disease. J Alzheimers Dis.
26:683–698. 2011.PubMed/NCBI
|
|
22
|
Bibow S, Ozenne V, Biernat J, Blackledge
M, Mandelkow E and Zweckstetter M: Structural impact of
proline-directed pseudophosphorylation at AT8, AT100 and PHF1
epitopes on 441 residue tau. J Am Chem Soc. 133:15842–15845. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ray P, Kar A, Fushimi K, Havlioglu N, Chen
X and Wu JY: PSF suppresses tau exon 10 inclusion by interacting
with a stem-loop structure downstream of exon 10. J Mol Neurosci.
45:453–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bibows, Mukrasch MD, Chinnathambis, et al:
The dynamic structure of filamentous tau. Angew Chem Int Ed Engl.
50:11520–11524. 2011. View Article : Google Scholar
|
|
25
|
Chin PC, Majdzadeh N and D'Mellos R:
Inhibition of GSK3beta is a common event in neuroprotection by
different survival factors. Brain Res Mol Brain Res. 137:193–201.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lehtihet M, Webb DL, Honkanen RE and
Sjöholm A: Glutamate inhibits protein phosphatases and promotes
insulin exocytosis in pancreatic beta-cells. Biochem Biophys Res
Commun. 328:601–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tasset I, Medina FJ, Peña J, et al:
Olfactory bulbectomy induced oxidative and cell damage in rat:
protective effect of melatonin. Physiol Res. 59:105–112. 2010.
|
|
28
|
Wang D, Noda Y, Tsunekawa H, et al:
Behavioural and neurochemical features of olfactory bulbectomized
rats resembling depression with comorbid anxiety. Behav Brain Res.
178:262–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mutlu O, Ulak G, Celikyurt IK, Akar FY,
Erden F and Tanyeri P: Effects of olanzapine, sertindole and
clozapine on MK-801 induced visual memory deficits in mice.
Pharmacol Biochem Behav. 99:557–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Altar CA, Laeng P, Jurata LW, et al:
Electroconvulsive seizures regulate gene expression of distinct
neurotrophic signaling pathways. J Neurosci. 24:2667–2677. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kato S, Kito Y, Hemmi H and Yoshimura T:
Simultaneous determination of D-amino acids by the coupling method
of D-amino acid oxidase with high-performance liquid
chromatography. J Chromatogr B Analyt Technol Biomed Life Sci.
879:3190–3195. 2011. View Article : Google Scholar
|
|
32
|
Wu FY, Feng Q, Cheng M, Yan J, Xu YX and
Zhu CQ: The activation of excitatory amino acid receptors is
involved in tau phosphorylation induced by cold water stress. Prog
Biochem Biophys. 37:510–516. 2010. View Article : Google Scholar
|
|
33
|
Choi BR, Kwon KJ, Park SH, et al:
Alternations of septal-hippocampal system in the adult wistar rat
with spatial memory impairments induced by chronic cerebral
hypoperfusion. Exp Neurobiol. 20:92–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Preissmann D, Bertholet L, Sierro G,
Cabungcal JH and Schenk F: Accurate performance of a rat model of
schizophrenia in the water maze depends on visual cue availability
and stability: a distortion in cognitive mapping abilities? Behav
Brain Res. 223:145–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carroll JC, Iba M, Bangasser DA, et al:
Chronic stress exacerbates tau pathology, neurodegeneration and
cognitive performance through a corticotrophin-releasing factor
receptor-dependent mechanism in a transgenic mouse model of
tauopathy. J Neurosci. 31:14436–14449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dumont JR, Amin E, Wright NF, Dillingham
CM and Aggleton JP: The impact of fornix lesions in rats on spatial
learning tasks sensitive to anterior thalamic and hippocampal
damage. Behav Brain Res. 278:360–374. 2015. View Article : Google Scholar
|
|
37
|
Palmio J, Huuhka M, Laines, et al:
Electroconvulsive therapy and biomarkers of neuronal injury and
plasticity: Serum levels of neuron-specific enolase and S-100b
protein. Psychiatry Res. 177:97–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu X, Hao X, Luo J, Min S, Xie F and
Zhang F: Propofol inhibits inflammatory cytokine-mediated glutamate
uptake dysfunction to alleviate learning/memory impairment in
depressed rats undergoing electroconvulsive shock. Brain Res.
1595:101–109. 2015. View Article : Google Scholar
|
|
39
|
Kloda A, Martinac B and Adams DJ:
Polymodal regulation of NMDA receptor channels. Channels (Austin).
1:334–343. 2007. View Article : Google Scholar
|
|
40
|
Stover JF and Kempski OS: Anesthesia
increases circulating glutamate in neurosurgical patients. Acta
Neurochir (Wien). 147:847–853. 2005. View Article : Google Scholar
|
|
41
|
Feiner JR, Bickler PE, Estradas, Donohoe
PH, Fahlman CS and Schuyler JA: Mild hypothermia, but not propofol,
is neuroprotective in organotypic hippocampal cultures. Anesth
Analg. 100:215–225. 2005. View Article : Google Scholar
|
|
42
|
Li KY, Guan YZ, Krnjević K and Ye JH:
Propofol facilitates glutamatergic transmission to neurons of the
ventrolateral preoptic nucleus. Anesthesiology. 111:1271–1278.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pesić V, Milanović D, Tanić N, et al:
Potential mechanism of cell death in the developing rat brain
induced by propofol anesthesia. Int J Dev Neurosci. 27:279–287.
2009. View Article : Google Scholar
|
|
44
|
Nie CL, Wang XS, Liu Y, Perretts and He
RQ: Amyloid-like aggregates of neuronal tau induced by formaldehyde
promote apoptosis of neuronal cells. BMC Neurosci. 8:92007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yao Z, Guo Z, Yang C, et al: Phenylbutyric
acid prevents rats from electroconvulsion-induced memory deficit
with alterations of memory-related proteins and tau
hyperphosphorylation. Neuroscience. 168:405–415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bulbarelli A, Lonati E, Cazzaniga E,
Gregori M and Masserini M: Pin1 affects Tau phosphorylation in
response to Abeta oligomers. Mol Cell Neurosci. 42:75–80. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jeon S, Kim YS, Park J and Bae CD:
Microtubule affinity-regulating kinase 1 (MARK1) is activated by
electroconvulsive shock in the rat hippocampus. J Neurochem.
95:1608–1618. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tan W, Cao X, Wang J, Lv H, Wu B and Ma H:
Tau hyper-phosphorylation is associated with memory impairment
after exposure to 1.5% isoflurane without temperature maintenance
in rats. Eur J Anaesthesiol. 27:835–841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vossel KA, Zhang K, Brodbeck J, et al: Tau
reduction prevents Abeta-induced defects in axonal transport.
Science. 330:1982010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Allyson J, Dontigny E, Auberson Y, Cyr M
and Massicotte G: Blockade of NR2A-containing NMDA receptors
induces Tau phosphorylation in rat hippocampal slices. Neural
Plast. 2010:3401682010.PubMed/NCBI
|
|
51
|
Sato S, Xu J, Okuyamas, et al: Spatial
learning impairment, enhanced CDK5/p35 activity and downregulation
of NMDA receptor expression in transgenic mice expressing
tau-tubulin kinase 1. J Neurosci. 28:14511–14521. 2008. View Article : Google Scholar
|
|
52
|
Muyllaert D, Kremer A, Jaworski T, et al:
Glycogen synthase kinase-3beta, or a link between amyloid and tau
pathology? Genes Brain Behav. 7(Suppl 1): 57–66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu C, Zhang XN, Liu D and Min S: Effects
of propofol, ginsenoside Rg-1, protein phosphatase-2a, and lithium
on the learning and memory in rats and the content of glutamic acid
in hippocampus after the electroconvulsive therapy. Zhongguo Yi Xue
Ke Xue Yuan Xue Bao. 36:234–240. 2014.PubMed/NCBI
|
|
54
|
Xu JJ and Wang YL: Propofol attenuation of
hydrogen peroxide-mediated oxidative stress and apoptosis in
cultured cardiomyocytes involves haeme oxygenase-1. Eur J
Anaesthesiol. 25:395–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Straiko MM, Young C, Cattano D, et al:
Lithium protects against anesthesia-induced developmental
neuroapoptosis. Anesthesiology. 110:862–868. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lesort M, Blanchard C, Yardin C, Esclaire
F and Hugon J: Cultured neurons expressing phosphorylated tau are
more resistant to apoptosis induced by NMDA or serum deprivation.
Brain Res Mol Brain Res. 45:127–132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Klein RC, Warder SE, Galdzicki Z,
Castellino FJ and Prorok M: Kinetic and mechanistic
characterization of NMDA receptor antagonism by replacement and
truncation variants of the conantokin peptides. Neuropharmacology.
41:801–810. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Amadoro G, Ciotti MT, Costanzi M, Cestari
V, Calissano P and Canu N: NMDA receptor mediates tau-induced
neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad
Sci USA. 103:2892–2897. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Paterlini M, Valerio A, Baruzzi F, Memo M
and Spano PF: Opposing regulation of tau protein levels by
ionotropic and metabotropic glutamate receptors in human NT2
neurons. Neurosci Lett. 243:77–80. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Elyaman W, Terro F, Wong NS and Hugon J:
In vivo activation and nuclear translocation of phosphorylated
glycogen synthase kinase-3beta in neuronal apoptosis: links to tau
phosphorylation. Eur J Neurosci. 15:651–660. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Burnouf S, Martire A, Derisbourg M, et al:
NMDA receptor dysfunction contributes to impaired brain-derived
neurotrophic factor-induced facilitation of hippocampal synaptic
transmission in a Tau transgenic model. Aging Cell. 12:11–23. 2013.
View Article : Google Scholar
|
|
62
|
Mondragón-Rodríguez S, Trillaud-Doppia E,
Dudilot A, et al: Interaction of endogenous tau protein with
synaptic proteins is regulated by N-methyl-D-aspartate receptor
dependent tau phosphorylation. J Biol Chem. 287:32040–32053. 2012.
View Article : Google Scholar
|
|
63
|
Chen NN, Luo DJ, Yao XQ, et al: Pesticides
induce spatial memory deficits with synaptic impairments and an
imbalanced tau phosphorylation in rats. J Alzheimers Dis.
30:585–594. 2012.PubMed/NCBI
|
|
64
|
Kingston S, Mao L, Yang L, Arora A, Fibuch
EE and Wang JQ: Propofol inhibits phosphorylation of
N-methyl-D-aspartate receptor NR1 subunits in neurons.
Anesthesiology. 104:763–769. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hama-Tomioka K, Kinoshita H, Nakahata K,
et al: Roles of neuronal nitric oxide synthase, oxidative stress
and propofol in N-methyl-D-aspartate-induced dilatation of cerebral
arterioles. Br J Anaesth. 108:21–29. 2012. View Article : Google Scholar
|
|
66
|
Li X, Li W, Luo J, et al: Effects of
propofol on the activation of hippocampal CaMKIIalpha in depressed
rats receiving electroconvulsive therapy. J ECT. 28:242–247. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang HY, Wang GL, Yu YH and Wang Y: The
role of phosphoinositide-3-kinase/Akt pathway in propofol-induced
postconditioning against focal cerebral ischemia-reperfusion injury
in rats. Brain Res. 1297:177–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Freitas AE, Machado DG, Budni J, et al:
Antidepressant-like action of the bark ethanolic extract from
Tabebuia avellanedae in the olfactory bulbectomized mice. J
Ethnopharmacol. 145:737–745. 2013. View Article : Google Scholar
|
|
69
|
Flores-Rodríguez P, Ontiveros Torres MA,
Cárdenas-Aguayo MC, et al: The relationship between truncation and
phosphorylation at the C-terminus of tau protein in the paired
helical filaments of Alzheimer's disease. Front Neurosci.
9:332015.PubMed/NCBI
|
|
70
|
Qu X, Xu C, Wang H, et al: Hippocampal
glutamate level and glutamate aspartate transporter (GLAST) are
up-regulated in senior rat associated with isoflurane induced
spatial learning/memory impairment. Neurochem Res. 38:59–73. 2013.
View Article : Google Scholar
|