Introduction
Oral cancer (OC) is the sixth most common cancer
worldwide and has one of the highest mortality rates, due to it
being largely asymptomatic until the latter stages of the disease.
Cancers of the oral cavity include cancers that occur in the
tongue, floor of the mouth, buccal mucosa, alveolus, retromolar
trigone, gingival, hard palate, and lips (1,2). A
total of 90% of OCs are squamous cell carcinomas (SCC) (3). SCC is caused by the presence of
malignant cells in the epithelium. Oral SCC (OSCC) accounts for
2–3% of all malignancies, the prevalence of which ranges between
1–10 cases per 100,000 people in the majority of countries
(4).
OSCC prognosis is poor, with a 5-year survival rate
of ~50%, according to the National Cancer Research Institute
(5). Treatments for OSCC are
usually limited to a combination of chemotherapy and radiation, in
order to reduce tumour size prior to the surgical removal of the
tumour margins. Two of the most common chemotherapeutic agents used
in the treatment of OSCC are cisplatin and 5-fluorouracil (6), and recurrent tumours are usually
treated with one of these two chemotherapy drugs. However,
resistance to these drugs may develop following treatment, as is
the case in numerous types of cancer (7,8).
Pyrrolo-1,5-benzoxazepines (PBOXs) are a novel
family of compounds that have been shown to induce cell cycle
arrest and apoptosis in numerous cancer cell lines, including
chemotherapy-resistant cell lines (9,10).
In addition, PBOXs have been shown to induce cell apoptosis in
ex vivo patient samples and in in vivo animal models
of breast cancer and chronic myeloid leukaemia (10,11).
Notably, PBOXs display minimal toxicity towards normal blood and
bone marrow cells (12). A
recently improved understanding regarding the molecular mechanisms
underlying the apoptotic effects of PBOX compounds has allowed
their development as anti-neoplastic therapeutic agents. Within the
PBOX family, two members exhibit markedly elevated activity, PBOX-6
and PBOX-15 (Fig. 1) (10).
Mulligan et al (13) previously demonstrated that
PBOX-induced apoptosis in cancer cells is preceded by a marked
G2/M phase cell cycle arrest, and that the cells
displayed morphological features that suggested an inhibition of
mitosis, notably in pro-metaphase. The effects of PBOXs on cell
morphology are similar to those induced by two
microtubule-targeting drugs, paclitaxel and nocodazole, which are
polymerising and depolymerising agents, respectively. These results
are concordant with previous studies that also demonstrated that
anti-microtubule agents arrest the cell cycle in pro-metaphase
(14,15).
Previous studies have suggested that PBOXs possess
anti-microtubule activity. Through indirect immuno-fluorescence
analysis, Mulligan et al (13) demonstrated that pro-apoptotic PBOX
compounds result in depolymerisation of the microtubule network,
and an inhibition of the assembly of purified tubulin in
vitro. Tubulin has therefore been identified as the molecular
target of the pro-apoptotic PBOX compounds (15).
In the present study the pro-apoptotic capabilities
of two representative members of the PBOX family, PBOX-6 and
PBOX-15, were examined in the TR146 (buccal mucosa) and Ca9.22
(gingival carcinoma) cell lines. The aim of the present study was
to investigate the potential of these compounds on inhibiting the
proliferation of OSCC cells. The present study also examined the
cell death mechanism and efficacy of the compounds and aimed to
determine the effectiveness of the compounds against OSCC cells
with differing genotypes. Together, the results from the present
study may indicate the potential of the PBOX compounds in the
treatment of OSCC, and whether there is in merit further
investigation, either alone, or in combination with other agents as
a potential treatment modality for OC.
Materials and methods
Reagents
All reagents were obtained from Sigma-Aldrich
(Arklow, Ireland) unless otherwise stated. The PBOX compounds,
7-[(N,N-dimethycarbomoyl) oxy]-6-(naphth-1-yl)
pyrrolo[2,1-d][1,5]benzoxazepine (PBOX-6), and 4-acetoxy-5-
(naphth-1-yl)naphtho[2,3-b] pyrrolo[2,1-d][1,4]oxazepine (PBOX-15),
were synthesized as previously described (16). The PBOX compounds were subsequently
dissolved in 1% ethanol, and stored at −20°C. The antibodies used
in the present study were as follows: Mouse anti-human/rat/mouse
anti-α-tubulin (cat. no. CP06; EMD Millipore, Billerica, MA, USA);
mouse anti-human anti-poly (ADP ribose) polymerase (PARP; cat. no.
MABC547; Merck Biosciences Ltd., Nottingham, UK); and mouse
anti-β-actin (cat. no MAB1501; EMD Millipore).
Cell culture
Both cell lines were maintained in a 95% humidified
atmosphere containing 5% CO2 at 37°C, and all cell
culture experiments were carried out under sterile conditions in a
laminar flow hood. Cell growth and viability were visually
monitored using a light microscope (Nikon Eclipse TS100;
MicronOptical, Wexford, Ireland) with 10 and 20× dry objectives.
The TR146 cell line was initially derived from the neck node of a
67 year-old female (the primary tumour was located in the buccal
mucosa) and was obtained from the Health Protection Agency Culture
Collection (Salisbury, UK). The TR146 cells were maintained in
Dulbecco's Modified Eagle's medium, supplemented with 10% v/v
foetal bovine serum (FBS), 10 U/ml penicillin, 0.1 mg/ml
streptomycin, and 2 mM glutamine. The Ca9.22 cell line was obtained
from the Japanese Collection of Research Bioresources Cell Bank
(Osaka, Japan). The Ca9.22 cells were maintained in Minimum
Essential medium, supplemented with 10% v/v FBS, 10 U penicillin,
0.1 mg/ml streptomycin, and 2 mM glutamine. All control cells were
treated with vehicle (1% ethanol) alone.
Cell proliferation
Cell proliferation was determined using AlamarBlue™
dye (Life Technologies, Grand Island, NY, USA), which allowed the
visualisation of changes in compound fluorescence that occur as a
consequence of the reduced number of viable, proliferating cells.
The cells were seeded in 96-well plates for each of the time points
with specified concentrations of PBOX-6 (10 nM-100 μM) or
PBOX-15 (1 nM-250 μM), and were incubated at 37°C in 5%
CO2. A final concentration of 10% (v/v) AlamarBlue™ was
added to the cells 4 h prior to the end of each time point.
Fluorescence was measured at an excitation wavelength of 544 nm and
at an emission wavelength of 590 nm, using a SpectraMax Gemini
spectrofluorometric plate reader (Molecular Devices (UK) Ltd,
Wokingham, UK). Cell viability was determined as a percentage of
the vehicle-only cells. The experimental results were displayed as
dose-response curves and half maximal effective concentration
(EC50) values, as determined using Prism GraphPad 5
(GraphPad Software, Inc., La Jolla, CA, USA).
Immunofluorescence
The cells were cultured for 24 h on 13 mm glass
coverslips with the following seed densities: Ca9.22,
3×104 cells/coverslip; and TR146, 4×104
cells/cover-slip. The cells were subsequently cultured in the
presence of either PBOX-6 or PBOX-15 for a further 24 h. Following
incubation, the cells were fixed with 4% paraformaldehyde in
phosphate-buffered saline (PBS) for 15 min, permeabilised with 0.2%
Triton in PBS for 10 min, rinsed with PBS, and blocked using 1%
bovine serum albumin (BSA) in PBS containing Tween® 20
(PBST) for 30 min. The cells were then incubated with the following
primary antibodies: Mouse anti-α-tubulin (1:1,000) in 1% BSA and
PBST for 1 h, prior to incubation with secondary goat anti-mouse
antibody 488 (cat. no. a-11001; Life Technologies; 1:500) in 1% BSA
and PBST for 1 h. The coverslips were then placed on glass slides
with 3.5 μl Vectashield™ mounting medium (Vector Labs,
Burlingame, CA, USA) containing the nuclear counterstain
4,6-diamidino-2-phenylindole (DAPI), and stored in the dark at 4°C
until imaging. The indirect fluorescence of the cells was examined
using a standard filter set for DAPI and blue/green, through 10 and
20× dry objectives, and a 60x oil objective using a Zeiss
Axiovert/Axiocam CCD system, and imaged using AxioVision AxioVs40
software (Carl Zeiss Ltd., Cambridge, UK).
DNA content
The cellular DNA content was determined using of
propidium iodide (PI), an intercalating fluorescent dye. The
fluorescence intensity is proportional to the quantity of DNA
present in the cell. Both cell lines were cultured in the presence
of PBOX-6 or PBOX-15 at the desired concentrations and time points.
The cells were then harvested by trypsinisation, prior to being
centrifuged at 220 × g for 5 min, washed with PBS, and centrifuged
once more. The supernatant was decanted and the cell pellets were
resuspended in 200 μl PBS, prior to the addition of 2 ml
ice-cold 70% ethanol in order to fix the cells. Following overnight
fixation at 4°C, the cells were centrifuged at 200 × g for 5 min in
order to remove the ethanol, and the pellet was resuspended in 400
μl PBS, followed by 25 μl RNase A, and 75 μl
PI. The cells were incubated in the dark at 37°C for 30 min. The
samples were subsequently transferred to appropriately labeled
fluorescence activated cell sorting (FACS) tubes, and were analysed
using a FACSCalibur flow cytometer (BD Biosciences, Oxford, UK).
The fluorescent signal was detected using a 630-22 nm band pass
filter (FL2). The cell lines were gated in order to prevent cell
debris and doublets from being counted. A total of >10,000 cells
were counted and analysed using Treestar FlowJo v10 (FlowJo,
Oregon, OR, USA).
Analysis of protein expression by western
blotting
The cells cultured in the presence of PBOX-6 or
PBOX-15 for 24, 48, and 72 h, were harvested and centrifuged at 220
× g for 5 min, following which the supernatants were discarded and
the cell pellets were resuspended in 2 ml ice-cold PBS. Following
further centrifugation at 220 × g for 5 min, the supernatant was
removed and the pellets were resuspended in 80 μl ice-cold
lysis buffer (150 mM sodium chloride, 1.0% Triton X-100, 50 mM
Tris; pH 8.0) containing Complete Protease Inhibitor Cocktail
(Roche Diagnostics Ltd., Burgess Hill, UK). The samples were
maintained under constant agitation at 1,000 rpm for 30 min at 4°C,
prior to being centrifuged at 13,000 × g for 20 min at 4°C. The
supernatants were aspirated and placed in an eppendorf tube on ice
until further experimentation. The protein concentrations were
deterxw-mined using a bicinchoninic acid assay. The protein samples
were separated by SDS-PAGE with an 8% resolving gel. The proteins
were then transferred to a polyvinylidene difluoride (PVDF)
membrane using a semi-dry blotter for 1 h. The PVDF membranes were
subsequently blocked with 5% non-fat milk, and probed with the
appropriate primary antibodies prior to being incubated with
horseradish peroxidase-conjugated goat anti-mouse secondary
antibody (cat. no. sc2031; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA). Protein expression was visualised through
chemiluminescence using an Immobilon Western Chemiluminescent HRP
substrate (Merck Millipore, Darmstadt, Germany).
Statistical analysis
Statistical significance was determined using Prism
GraphPad 5 (GraphPad Software, La Jolla, CA, USA). Analysis was
performed using an unpaired t-test with post-hoc Bonferroni
analysis. P<0.001 was considered to indicate a statistically
significant difference.
Results
PBOX compounds reduce OC cell
proliferation
The OSCC cell lines were treated for 72 h with
PBOX-6 or PBOX-15 at various concentrations. Cell proliferation was
subsequently measured using AlamarBlue™ assay. The results of the
assay indicated that PBOX-6 and PBOX-15 reduced proliferation of
both the Ca.922 and the TR146 cell lines in a dose-dependent manner
(Fig. 2). PBOX-15 was the more
potent of the two analogues. After 72 h, the EC50 values
of PBOX-6 and PBOX-15 were 35±2.7 μM and 470±86 nM for the
TR146 cells, and 2.6±1 μM and 83±32 nM for the Ca9.22 cells,
respectively (Table I). The drug
concentrations used for the remaining experiments were chosen
according to the results of this cell proliferation assay.
 | Table IHalf maximal effective concentration
(EC50) values obtained for each
pyrrolo-1,5-benzoxazepine (PBOX) compound in the TR146 and Ca9.22
oral squamous cell carcinoma cell lines. |
Table I
Half maximal effective concentration
(EC50) values obtained for each
pyrrolo-1,5-benzoxazepine (PBOX) compound in the TR146 and Ca9.22
oral squamous cell carcinoma cell lines.
| Cell line | EC50
|
|---|
| 24 h | 48 h | 72 h |
|---|
| TR146 |
| PBOX-6 | 9.2±2.3
μM | 8.1±0.3
μM | 35±2.7μM |
| PBOX-15 | 480±92 nM | 530±52 nM | 470±86 nM |
| Ca9.22 |
| PBOX-6 | 4.5±1.5
μM | 2.9±0.7
μM | 2.6±1 μM |
| PBOX-15 | 210±28 nM | 170±81 nM | 83±32 nM |
PBOX compounds destabilise and
depolymerise the micro-tubule network in OC cells
The Ca9.22 and TR146 cells were treated with either
PBOX-6 or PBOX-15 for 24 h. In addition, the cells were also
treated with the known tubulin polymeriser, paclitaxel (1
μmol; 24 h), and the tubulin depolymeriser, nocodazole (10
μmol; 24 h), both purchased from Sigma-Aldrich, which served
as positive controls. Immunofluorescent staining was used to detect
morphological changes in the microtubule network, such as
alterations in microtubule organisation and arrangement (Fig. 3). In the vehicle TR146 (Fig. 3A) and Ca9.22 (Fig. 3B) cells, the microtubule network
was organised as cytoplasmic tubulin filaments radiating from a
central point to the periphery. Exposure of the cells to the
tubulin polymerising agent paclitaxel resulted in a highly
concentrated accumulation of filaments in dense peripheral bundles,
indicative of microtubule stabilisation. Conversely, exposure to
the tubulin depolymerising agent nocodazole resulted in diffuse
tubule staining with no definition of structure caused by
microtubule disassembly. No alterations in microtubule structure
were evident following treatment with a low dose of 100 nM PBOX-6
and PBOX-15, whereas higher doses of 10 μM PBOX-6 and 1
μM PBOX-15 resulted in a change in tubulin morphology
resembling that induced by nocodazole. These results indicate that
PBOX compounds destabilise the microtubule network in a similar
manner to a depolymerising agent, in both TR146 and Ca9.22 cell
lines.
 | Figure 3Pyrrolo-1,5-benzoxazepines (PBOX)-6
and PBOX-15 induce microtubule disruption and depolymerisation of
oral squamous cell carcinoma (OSCC) cell lines. (A) TR146 and (B)
Ca9.22 cells were treated with either vehicle, 1 μM
paclitaxel (Tax), 10 μM nocodazole (Noc), 100 nM, 1
μM or 10 μM PBOX-15, or 100 nM, 1 μM or 10
μM PBOX-6 for 24 h. The medium was subsequently removed and
the cells were fixed with paraformaldehyde. The cells were then
incubated with monoclonal anti-α-tubulin antibody, followed by
further incubation with fluorescein isothiocyanate-conjugated
anti-mouse secondary antibody. The organization of the microtubule
network (green) and the cellular DNA (blue) was visualized using a
Zeiss Axiovert/Axiocam CCD system through 10× and 20× dry
objectives, and a 60× oil objective. Scale bar, 20 μm. (C)
In order to confirm tubulin depolymerisation in the TR146 and
Ca9.22 cell lines, the cells were treated for 4 h with either
vehicle (1% (v/v) ethanol), PBOX-6 (10 μM), PBOX-15 (1
μM), Tax (1 μM), or Noc (10 μM). The
polymerised (P) or unpolymerised (U) tubulin was then separated by
centrifugation in a microtubule-preserving buffer. The ratio of
polymerised, vs. unpolymerised tubulin was subsequently assessed by
western blot analysis using monoclonal antibodies directed against
tubulin and loading control β-actin, followed by incubation with
horseradish peroxidase-conjugated anti-mouse antibody. The images
are representative of experiments repeated three times. |
In order to confirm the results of previous studies,
namely that the disruption observed using a confocal microscope is
the result of depolymerisation of the tubulin network, tubulin
polymerisation assays were performed using western blotting
(Fig. 3C). Following 4 h treatment
with 10 μM PBOX-6 or 1 μM PBOX-15, tubulin was shown
to be completely depolymerised in both cell lines. Following 4 h
treatment with paclitaxel, tubulin was polymerised, whereas
treatment with nocozadole resulted in unpolymerised tubulin.
Treatment with the vehicle (1% EtOH) resulted in equal amounts of
polymerised to unpolymerised tubulin. These results indicate that
the disruption observed by confocal microscopy was indeed
depolymerisation of the tubulin network.
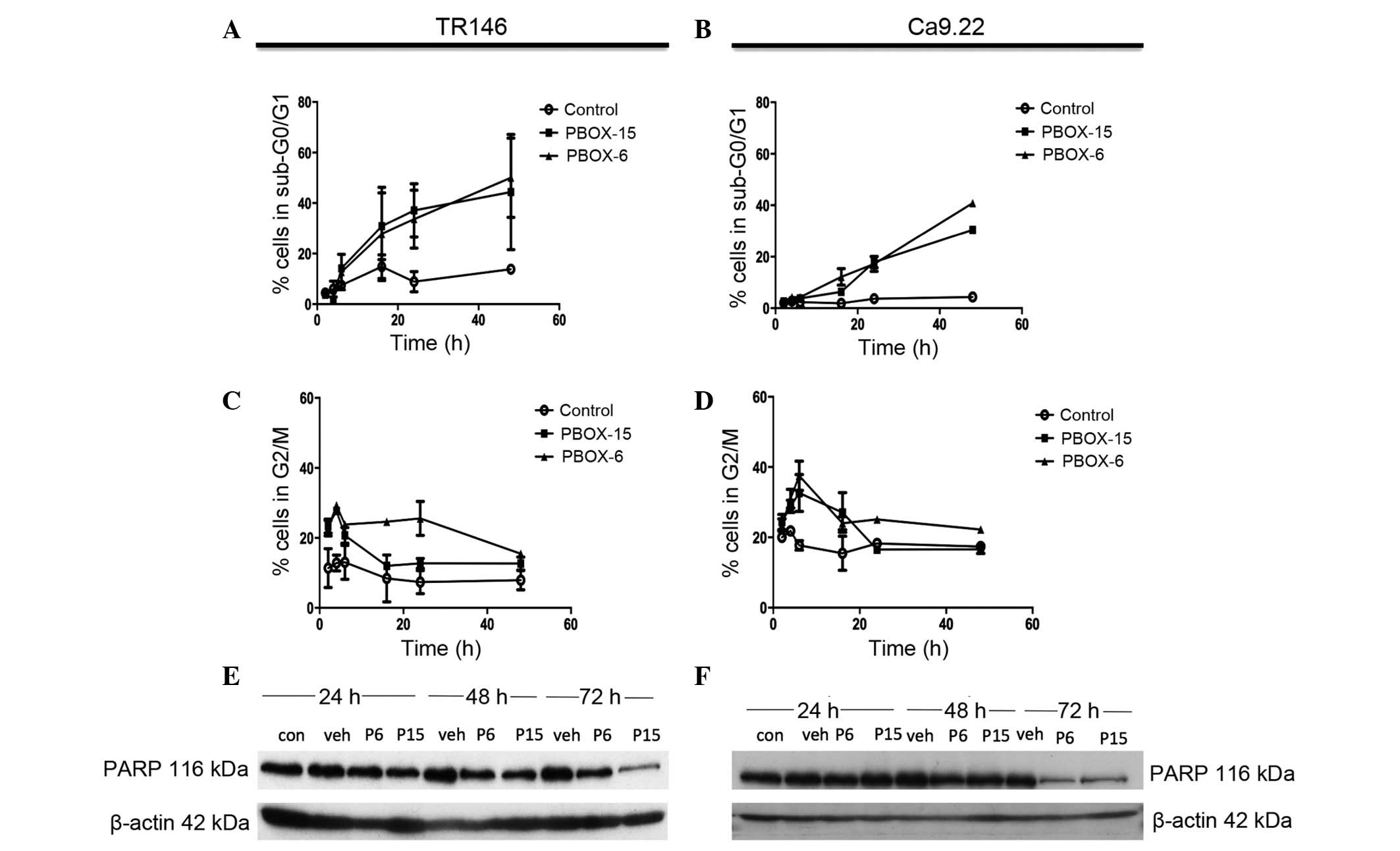
PBOX compounds induce G2/M
arrest and apoptosis in OC cells
The DNA content of the PI-stained TR146 and Ca9.22
cells was measured using flow cytometric analysis, thus allowing
the successful identification of cell cycle arrest or apoptosis. A
decrease in the number of cells in the G2/M phase of the
cell cycle was observed in both cell lines following 8h treatment
with PBOX-6 (10 μM) and PBOX-15 (1 μM) (Fig. 4C and 4D). A marked decrease was observed in the
number of TR146 cells arrested in G2/M phase following
72 h treatment, 15.4±1.07% and 12.6±1.87% for PBOX-6 and -15,
respectively. A significant decrease also occurred in the number of
Ca9.22 cells arrested in the G2/M phase following 72 h
of treatment, 22.23±0.95% and 16.6±1.25% for PBOX-6 and PBOX-15,
respectively. This decline in the levels of G2/M phase
cells correlated with a marked increase in the number of apoptotic
TR146 cells, with the levels of apoptosis rising to 4.4±1.210% in
the control cells, 57.6±8.1% in the cells treated with 10 μM
PBOX-6, and 39.1±6.6% in the cells treated with 1μM PBOX-15
(Fig 4A and 4B). Similarly, in the Ca9.22 cell line,
the number of cells in the sub-G0/G1 phase
following 72 h increased to 2.120±0.8% in the control cells,
40.84±0.8% in the cells treated with PBOX-6, and 30.45±1.56% in the
cells treated with PBOX-15. A decrease in the number of cells in
the G2/M phase of the cell cycle were observed in each cell line
following 8 h treatment with PBOX-6 (10 μM) and PBOX-15 (1
μM; Fig. 4C and 4D).
PBOX compounds induce degradation of PARP
in OC cell lines
In order to confirm that the cell death observed
during flow cytometric analysis was indeed cellular apoptosis, PARP
degradation, which is associated with controlled cell death, was
assessed. Concordant with the DNA content analysis results, both
PBOX-6 (10 μM) and PBOX-15 (1 μM) induced a decrease
in the expression levels of full length PARP in Ca9.22 and TR146
cells following 72 h treatment (Fig.
4E and 4F). Treatment with the
vehicle (1% (v/v) EtOH) did not affect PARP expression in either
cell line. In the TR146 cell line, PARP degradation was observed
following 24 h treatment with 1 μM PBOX-15, as compared with
the vehicle control. PARP degradation was clearly observed in the
TR146 cell line following 48 h treatment with both PBOX compounds.
In addition, PARP degradation was also observed in the Ca9.22 cell
line following 48 h treatment with both PBOX compounds. β-actin was
used as a loading control in all experiments. These results
indicate that the PBOX compounds induce apoptosis via tubulin
disassembly.
Discussion
The present study assessed a novel set of compounds
with regards to their potential therapeutic value in OC. PBOX-15
and PBOX-6 are potent pro-apoptotic members of the PBOX family, and
have previously been shown to induce apoptosis in numerous human
tumour cell lines, and to have anti-cancer properties in various
cell culture systems, animal models, and clinical samples (11,17).
In addition to inducing apoptosis in various cancer cell types,
PBOX-6 and -15 possess the added benefit of inducing cytotoxicity
in multi drug-resistant cancer cells (18).
The present study demonstrated that PBOX compounds
were capable of reducing the proliferation of OC cell lines TR146
and Ca9.22, with EC50 values of 35±2.7 μM and
2.6±1 μM for PBOX-6, and 470±86 nM and 83±32 nM for PBOX-15.
These values are within a range previously observed in other cancer
cell lines exposed to PBOX compounds, including K562 chronic
myeloid leukemia cells, A2780 ovarian carcinoma cells, and various
cancerous mammary cells (9,16,17).
PBOX-15, as previously reported, is the more potent of the two
compounds, a result that was confirmed in the present study in both
cell lines tested, as demonstrated by the order of magnitude of
difference in the calculated EC50 values. Significant
variation in the sensitivity of each cell type to PBOX treatment
was also observed. The Ca9.22 gingiva cell line was sensitive to
both PBOX-15 and PBOX-6, and had significantly lower
EC50 values, as compared with the TR146 buccal cell
line. The observed variation in efficacy of the PBOXs in the two
experimental cell lines suggests a phenotypic or genotypic factor
may be involved. In OSCC, ~50% of cases are associated with a
mutation in the p53 gene, which results in deleterious phenotypic
manifestations and impaired p53 function. The Ca9.22 cells have a
known p53 mutation (19), whereas
the TR146 cells have no known p53 mutation. Previous studies have
reported the influence of p53 in modifying drug efficacy and
function in cancer cells (20,21).
The disassembly of the microtubule network in other
cancer cell lines following treatment with PBOX-6 and PBOX-15
suggested that the compounds act as microtubule depolymerizing
agents (13). To examine this
hypothesis in OSCC, the Ca9.22 and TR146 cells were treated with
various concentrations of PBOX-6 and PBOX-15. The results of the
present study were concordant with previous studies, demonstrating
that both PBOX compounds caused disassembly of tubulin following 24
h treatment in vitro (13,22).
In the case of the control compounds, nocodazole depolymerised
tubulin, whereas treatment with paclitaxel resulted in tubulin
aggregation through polymerisation. PBOX-15, the more potent of the
two compounds, was effective at disrupting tubulin at 1 μM
in both cell lines, whereas PBOX-6-mediated depolymerisation was
only observed at concentrations of 10 μM.
This inhibition of tubulin assembly is likely to be
the cause of cell death following treatment with the PBOX compounds
(13). Tubulin is a key protein in
spindle formation during the cell cycle. A mechanism known as the
spindle assembly checkpoint is activated during metaphase in the
cell cycle (23). In the presence
of tubulin breakdown and loss of spindle tension, the cell will not
be able to continue into anaphase. In order to determine part of
the mechanism underlying the response of OSCC cells to PBOX
treatment, the effects of PBOX-6 and PBOX-15 on the cell cycle were
examined. The PBOXs caused a time-dependent accumulation of cells
in G2/M phase, as early as 4 h in TR146 cells, and 8 h
in Ca9.22 cells. This was followed by an increase in the number of
cells in sub-G0/G1 phase, indicative of
apoptosis and a concomitant decrease in the percentage of cells in
G2/M. To confirm the mechanism of cell death,
degradation of the DNA repair enzyme PARP, which is indicative of
apoptosis, was examined. A decrease in the expression levels of the
full-length 116 kDa PARP was evident in both cell lines following
treatment with both compounds after 72 h, suggesting PARP had been
cleaved. The decrease in full-length PARP in the Ca9.22 cell line
was more prominent than in the TR146 cell line, demonstrating an
increased sensitivity of the Ca9.22 cells to the compounds as it
also increased the EC50 values obtained using this cell
line.
The results of the present study indicated that
PBOX-6, and its more potent analogue PBOX-15, may prove effective
in the treatment of OSCC. The present study has shown that PBOX-6
and PBOX-15 depolymerise their molecular target, tubulin, resulting
in cell death through apoptosis in OSCC cell lines. Given the
prevalence of OSCC, the use of PBOX compounds as topical agents may
be a potential therapeutic strategy for this disease. PBOX
compounds used alone or in combination with other antineoplastic
agents may prove useful in the treatment of OSCC.
Acknowledgments
The authors of the present study are thankful to the
Dublin Dental University Hospital and Trinity College Dublin for
funding this study through the 1252 initiative. An abstract
describing this work was presented at the 23rd Biennial Congress of
the European Association for Cancer Research.
References
|
1
|
Zhang L, Zhou X, Yao X, Wu Y and Zhang Q:
Oral tongue cancer patients show a better overall survival than
base of tongue cancer patients. J Cancer Res Clin Oncol.
138:341–346. 2012. View Article : Google Scholar
|
|
2
|
Arosarena OA, Madsen M and Haug R: Special
considerations with floor of mouth and tongue cancer. Oral
Maxillofac Surg Clin North Am. 18:521–531. 2006. View Article : Google Scholar
|
|
3
|
Beenken SW and Urist MM: Head and Neck
Tumors. Current surgical diagnosis and treatment. Way LW and
Doherty GM: Lange Medical Books/McGraw-Hill; New York: pp. 282–297.
2003
|
|
4
|
World Health Organisation: World Cancer
Report. 2008
|
|
5
|
National Cancer Research Institute:
Surveillance, Epidemiology, and End Results (SEER) program.
2004
|
|
6
|
Andreadis C, Vahtsevanos K, Sidiras T,
Thomaidis I, Antoniadis K and Mouratidou D: 5-Fluorouracil and
cisplatin in the treatment of advanced oral cancer. Oral Oncol.
39:380–385. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galluzzi L, Senovilla L, Vitale I, et al:
Molecular mechanisms of cisplatin resistance. Oncogene.
31:1869–1883. 2012. View Article : Google Scholar
|
|
8
|
Zhang N, Yin Y, Xu SJ and Chen WS:
5-Fluorouracil: Mechanisms of resistance and reversal strategies.
Molecules. 13:1551–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nathwani SM, Butler S, Fayne D, et al:
Novel micro-tubule-targeting agents, pyrrolo-1,5-benzoxazepines,
induce apoptosis in multi-drug-resistant cancer cells. Cancer
Chemother Pharmacol. 66:585–596. 2010. View Article : Google Scholar
|
|
10
|
McElligott AM, Maginn EN, Greene LM, et
al: The novel tubulin-targeting agent pyrrolo-1,5-benzoxazepine-15
induces apoptosis in poor prognostic subgroups of chronic
lymphocytic leukemia. Cancer Res. 69:8366–8375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bright SA, McElligott AM, O'Connell JW, et
al: Novel pyrrolo-1,5-benzoxazepine compounds display significant
activity against resistant chronic myeloid leukaemia cells in
vitro, in ex vivo patient samples and in vivo. Br J Cancer.
102:1474–1482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maginn EN, Browne PV, Hayden P, et al:
PBOX-15, a novel microtubule targeting agent, induces apoptosis,
upregulates death receptors, and potentiates TRAIL-mediated
apoptosis in multiple myeloma cells. Br J Cancer. 104:281–289.
2011. View Article : Google Scholar :
|
|
13
|
Li YM and Broome JD: Arsenic targets
tubulins to induce apoptosis in myeloid leukemia cells. Cancer Res.
59:776–780. 1999.PubMed/NCBI
|
|
14
|
Woods CM, Zhu J, McQueney PA, Bollag D and
Lazarides E: Taxol-induced mitotic block triggers rapid onset of a
p53-independent apoptotic pathway. Mol Med. 1:506–526.
1995.PubMed/NCBI
|
|
15
|
Mulligan JM, Greene LM, Cloonan S, et al:
Identification of tubulin as the molecular target of
proapoptoticpyrrolo-1,5-ben-zoxazepines. Mol Pharmacol. 70:60–70.
2006.PubMed/NCBI
|
|
16
|
Greene LM, Fleeton M, Mulligan J, et al:
The pyrrolo-1,5-ben-zoxazepine, PBOX-6, inhibits the growth of
breast cancer cells in vitro independent of estrogen receptor
status and inhibits breast tumour growth in vivo. Oncol Rep.
14:1357–1363. 2005.PubMed/NCBI
|
|
17
|
Nathwani SM, Cloonan SM, Stronach M, et
al: Novel micro-tubule-targeting agents,
pyrrolo-1,5-benzoxazepines, induce cell cycle arrest and apoptosis
in prostate cancer cells. Oncol Rep. 24:1499–1507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mc Gee MM, Campiani G, Ramunno A, et al:
Activation of the c-Jun N-terminal kinase (JNK) signaling pathway
is essential during PBOX-6-induced apoptosis in chronic myelogenous
leukemia (CML) cells. J Biol Chem. 277:18383–18389. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaneda Y, Shimamoto H, Matsumura K, et al:
Role of caspase 8 as a determinant in chemosensitivity of
p53-mutated head and neck squamous cell carcinoma cell lines. J Med
Dent Sci. 53:57–66. 2006.PubMed/NCBI
|
|
20
|
Bunz F, Hwang PM, Torrance C, et al:
Disruption of p53 in human cancer cells alters the responses to
therapeutic agents. J Clin Invest. 104:263–269. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jackson JG, Pant V, Li Q, Chang LL,
Quintas-Cardama A, Garza D, Tavana O, Yang P, Manshouri T, Li Y,
El-Naggar AK and Lozano G: p53-mediated senescence impairs the
apoptotic response to chemotherapy and clinical outcome in breast
cancer. Cancer Cell. 21:793–806. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Verma NK, Dempsey E, Conroy J, et al: A
new micro-tubule-targeting compound PBOX-15 inhibits T-cell
migration via post-translational modifications of tubulin. J Mol
Med. 86:457–469. 2008. View Article : Google Scholar
|
|
23
|
Rudner AD and Murray AW: The spindle
assembly checkpoint. Curr Opin Cell Biol. 8:773–780. 1996.
View Article : Google Scholar : PubMed/NCBI
|