Introduction
Traumatic spinal cord injury (SCI) is a severe
pathological event with consequences which are sustained throughout
the life of the patient and eventually cause distress to the family
and society (1,2). SCI activates an inflammatory cascade
and during this, inflammatory cells from the circulation
deteriorate vital organs, including the liver, kidney and lungs
(3). Oxidative stress and
inflammation may result in post-SCI pathogenesis, including
cellular apoptosis, activation of free radicals and enhanced lipid
peroxidation (LPO) with subsequent depletion of anti-oxidant
agents, and have a pivotal role in SCI-induced secondary
organ/tissue damage (4–7). One of the imminent consequences of
SCI is a significant bone loss within a few months to a few years
of injury (8), leading to fracture
in 50% of patients with complete SCI. SCI-induced bone loss
generally affects the lower limbs of rats due to longer bones as
well as metaphysis, epiphysis and diaphysis of the femur and tibia
of rats (9,10). SCI-induced bone loss has
significant morbidity and is likely to aggravate already profound
disability. Treatment strategies to ameliorate bone loss after SCI
by physical methods include passive standing, minimal electrical
stimulation and body weight-supported treadmill training (11,12).
However, treatment using these strategies has no significant
effects. Effective interventions may require targeting of multiple
pathways to achieve significant clinical improvement after SCI, and
complications including bone loss are warranted. Coenzyme Q10
(CoQ10) is a key mediator of the electron transfer reaction in the
respiratory chain in mitochondria. A study suggested that CoQ10 has
a free-radical-quenching effect and also displays insulin-like
properties in diabetic patients (13). Previous studies provided evidence
that CoQ10 has potent anti-oxidant activity (14,15)
and protective efficacy in experimental SCI (16). Previous pre-clinical studies
demonstrated the anti-osteonecrotic effects (17) and the inhibitory effect on
osteoclast differentiation in bone-marrow-derived monocytes and RAW
264.7 cells of CoQ10, which are mediated by its
free-radical-scavenging mechanism (18). However, to the best of our
knowledge, the effect of CoQ10 in SCI-induced bone loss has not
been studied to date. Therefore, the present study was designed to
evaluate the therapeutic efficacy of CoQ10 against bone loss
induced by SCI in rats.
Materials and methods
Chemicals
Co-enzyme Q10, superoxide dismutase (SOD) and
malondialdehyde (MDA) diagnostic kits were obtained from
Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of the
highest available commercial grade.
Animals
Forty male Sprague-Dawley rats (8 weeks-old,
weighing 170–200 g) were obtained from the animal facility at
Nanchang University (Nanchang, China). The animals were maintained
under standard laboratory conditions of relative humidity (55±5%),
temperature (25±2°C), and light (12 h light/dark cycle). They were
fed standard diet pellets and water was provided ad libitum.
The study was approved by the ethics committee of The First
Affiliated Hospital of Nanchang University, (Nanchang, China).
Animal model of SCI
The rats were anesthetized by administration of
xylazine + ketamine [10 and 75 mg/kg, respectively;
intraperitoneally (i.p.)]. After the dorsum of the animals was
shaved and sterilized, an incision was made from the posterior to
the lower thoracic region. Laminectomy (thoracic T10–T12 vertebrae)
was performed to expose the spinal cord alone (sham group). After
the laminectomy, the lower thoracic cord was subsequently
completely transected with fine sterilized micro scissors (SCI
group) under a magnifier. Both stumps of the spinal cord were
gently lifted away to create a 1–2-mm gap, which was filled with
sponge gel. The muscle fascia and skin were sutured, and the rats
were returned to their cages. After completion of surgery, the
animals received a bolus of Lactate Ringers solution (5 ml; i.p.)
to compensate for blood loss, and antibiotic cover (systemic
gentamycin, 50 mg/kg intramuscularly; local treatment with
neosporin ointment) was provided. Furthermore, the SCI rats
received daily assistance in bladder emptying until spontaneous
miction recovered.
Evaluation of locomotor functions
The quality of locomotion was assessed using the
Basso, Beattie, and Bresnahan (BBB) locomotor rating score
(19).
Study design
Sprague-Dawley rats were divided into four groups
(n=10 each) and treated for 10 days as follows: i) Group I –
sham-operated rats (Sham); ii) Group II – SCI rats (SCI); iii)
Group III – sham-operated rats receiving CoQ10 (10 mg/kg)
intragastrically for 10 days (sham + CoQ10); iv) Group IV – SCI
rats receiving CoQ10 (10 mg/kg) for 10 days (SCI + CoQ10).
At the end of the experimental period, rats were
fasted overnight and sacrificed by decapitation. Blood was
collected in heparinized BD vacutainer (BD Biosciences, San Jose,
CA, USA) and serum samples were collected by centrifugation at 450
× g for 20 min. Femur and tibiae were immediately excised, rinsed
in ice-cold saline to remove the blood and stored at −80°C until
required.
Preparation of bone homogenate
Frozen bone samples (150 mg) were minced in a
phosphate buffer and homogenized in a MagNA Lyser instrument (Roche
Applied Science, Penzberg, Germany). Samples were centrifuged three
times at 530 × g for 20 sec with intermediate cooling 5 min in the
MagNA Lyser Cooling Block. The tissue homogenate was then
centrifuged at 10,000 × g at 4°C for 10 min. The supernatant was
separated, stored at −80°C and used for further biochemical
analysis.
Measurements of bone mineral content
(BMC) and bone mineral density (BMD)
At the end of experiment, one half of the
experimental rats (n=5) were anesthetized using xylazine and
ketamine (10 and 75 mg/kg, respectively i.p.; Sigma-Aldrich) and
the BMD and BMC of the left femur and tibia were estimated using
dual energy X-ray absorptiometry instrument (DEXA, Norland X46;
Norland, Fort Atkinson, WI, USA) equipped with dedicated software
(version 3.9.6) for small animal measurements. The BMC (expressed
in grams) was divided by the area of the site that was scanned to
obtain the BMD (expressed in g/cm2) (20).
Assessment of systemic oxidative
stress
SOD levels of femur and tibiae bone homogenate were
estimated by the method of Kakkar et al (21). The inhibition of reduction of nitro
blue tetrazolium to blue formazan in the presence of phenazine
methosulphate and nicotinamide adenine dinucleotide was measured at
560 nm using n-butanol as blank with a Shimadzu-1601 PC UV-Visible
scanning spectrophotometer (Shimadzu Corp., Kyoto, Japan). One unit
(U) of enzyme activity was defined as the amount of enzyme that
inhibits the reaction rate by 50% in 1 min under the defined assay
conditions and the results are expressed as U/mg protein.
MDA levels were determined by the method of Ohkawa
et al (22). Briefly, 0.5
ml femur and tibiae bone homogenate tissue was mixed with 2.5 ml
20% trichloroacetic acid in a 10-ml centrifuge tube. To the
mixture, 1 ml 0.67% thiobarbituric acid was added, followed by
mixing and heating for 30 min in a boiling water bath followed by
rapid cooling. Subsequently, 4 ml n-butyl-alcohol was added
followed by mixing. The mixture was centrifuged at 154 × g for 10
min. The resultant n-butyl-alcohol layer was separated and its MDA
content was determined from the absorbance at 535 nm. The results
are expressed as nM/mg protein.
Assay for inflammatory cytokine
levels
Concentrations of interleukin (IL)-6 and tumor
necrosis factor (TNF)-α were determined in serum using commercially
available TNF-α and IL-6 ELISA kits (USCN LIFE; Wuhan EIAab Science
Co., Ltd, Wuhan, China).
Bone gene expression analysis
The mRNA expression of genes relevant to
osteoclastogenesis [receptor activator of nuclear factor kappa-B
ligand (RANKL) and cathepsin K] and osteoblastogenesis
[core-binding factor alpha 1 (Cbfa1)] in the femur, was
investigated using reverse transcription quantitative polymerase
chain reaction (RT-qPCR) analysis. Briefly, the frozen samples of
distal femurs were finely ground into powder while immersed in
liquid nitrogen using a mortar and pestle free from RNase. The
frozen powder was transferred into a vial containing TRIzol
(Invitrogen Life Technologies Carlsbad, CA, USA) and extraction of
total RNA was performed using the QuantiTect™ nSYBR®
Green PCR kit (Shanghai Tiangen Chemical Co., Ltd., Shanghai,
China) in accordance with the manufacturer's instructions. 1
µg total RNA was digested with 10 µl DNase I
(Invitrogen Life Technologies) and from this 5 µl (500 ng)
was reverse transcribed with 200 U SuperScript II (Invitrogen Life
Technologies) using 500 ng oligo-dT and 250 ng random hexamers.
RT-PCR analysis was performed using QuantiTect™ nSYBR®
Green PCR (Tiangen, Shanghai, China) according to the
manufacturer's instructions. The RT-PCR data were based on SYBR
green amplification. The sequences of primers are listed in
Table I. The highly specific
measurement of mRNA was performed using the Light Cycler system
(Bio-Rad Laboratories, Hercules, CA, USA). PCR amplification was
performed in 96-well optical reaction plates for 40 cycles, with
cycles of 94°C for 30 sec, 58–63°C for 30 sec and 72°C for 60 sec.
Each sample was run and analyzed in duplicate. GAPDH mRNA as an
internal control was used to normalize the data to determine the
relative expression of the target genes. The fold changes relative
to values of the sham-operated group were obtained and used to
express the changes in gene expression.
 | Table ISequences of oligonucleotides used as
primers. |
Table I
Sequences of oligonucleotides used as
primers.
| Gene
(abbreviation) | Gene (full
name) | ID | Sequence
(5′→3′) | Tm (°C) | Size (bp) |
|---|
| RANKL | Receptor activator
of nuclear factor-κB ligand | 117516 | Forward:
TCAGGAGTTCCAGCTATGAT
Reverse: CCATCAGCTGAAGATAGTCC | 55 | 298 |
| Cathepsin K | Cathepsin K | 29175 | Forward:
GAGACATGACCAGCGAAGAA
Reverse: CACATATTGGAAGGCAGTGG | 56 | 332 |
| Cbfa1 | Core binding factor
alpha 1 | 367218 | Forward:
CGAAATGCCTCTGCTGTTAT
Reverse: TTCTGTCTGTGCCTTCTTGG | 53 | 194 |
| GAPDH | Glyceraldehyde
3-phosphate dehydrogenase | 2597 | Forward:
ACCACAGTCCATGCCATCAC
Reverse: TCCACCACCCTGTTGCTGTA | 60 | 452 |
Statistical analysis
Values are expressed as the mean ± standard
deviation. The results were statistically analyzed by one-way
analysis of variance followed by Dunnet's t-test using SPSS
software version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
CoQ10 alleviates decreased BMD and BMC in
rats following SCI
The BMD and BMC of femur and tibia were
significantly decreased in the SCI group as compared with that in
the sham group (P<0.05). Treatment with CoQ10 (10 mg/kg)
significantly alleviated the decreasing effect of SCI on BMC
(Fig. 1A) and BMD (Fig. 1B) (P<0.05). Treatment of
sham-operated rats with CoQ10 had no significant effect on these
parameters.
CoQ10 alleviates oxidative stress in
bones following SCI
Compared with the sham group, SOD levels in femur
and tibia were significantly depleted in SCI rats (P<0.05).
However, treatment with CoQ10 effectively restored the SOD status
to normal levels (Fig. 2A).
Furthermore, MDA, a toxic adduct formed during lipid peroxidation,
was significantly elevated in the femur and tibia of SCI rats
compared with that in the sham operated rats (P<0.05). However,
treatment with CoQ10 significantly attenuated the elevated MDA in
femur and tibia to normal levels (P<0.05) (Fig. 2B).
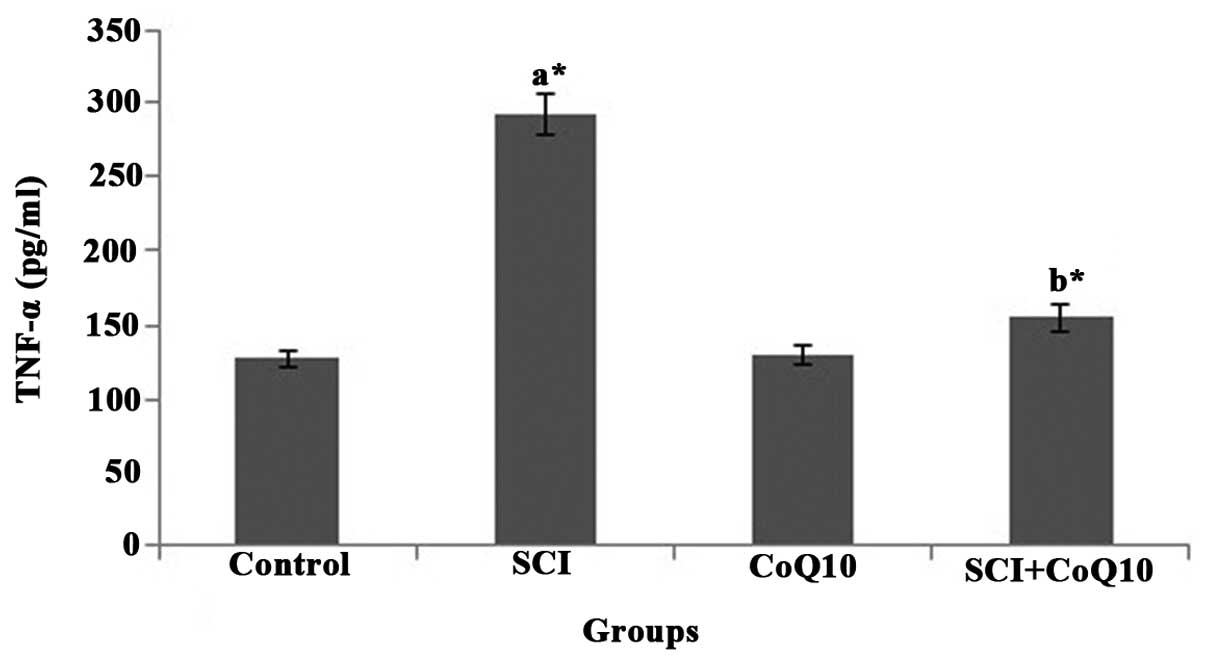
CoQ10 reduces serum inflammatory markers
in SCI rats
In the present study, SCI rats displayed a
significant elevation of serum inflammatory cytokines IL-6
(Fig. 3) and TNF-α (Fig. 4) (P<0.05). Oral administration
of CoQ10 effectively reduced the IL-6 and TNF-α levels in serum to
normal levels and thus minimized inflammation.
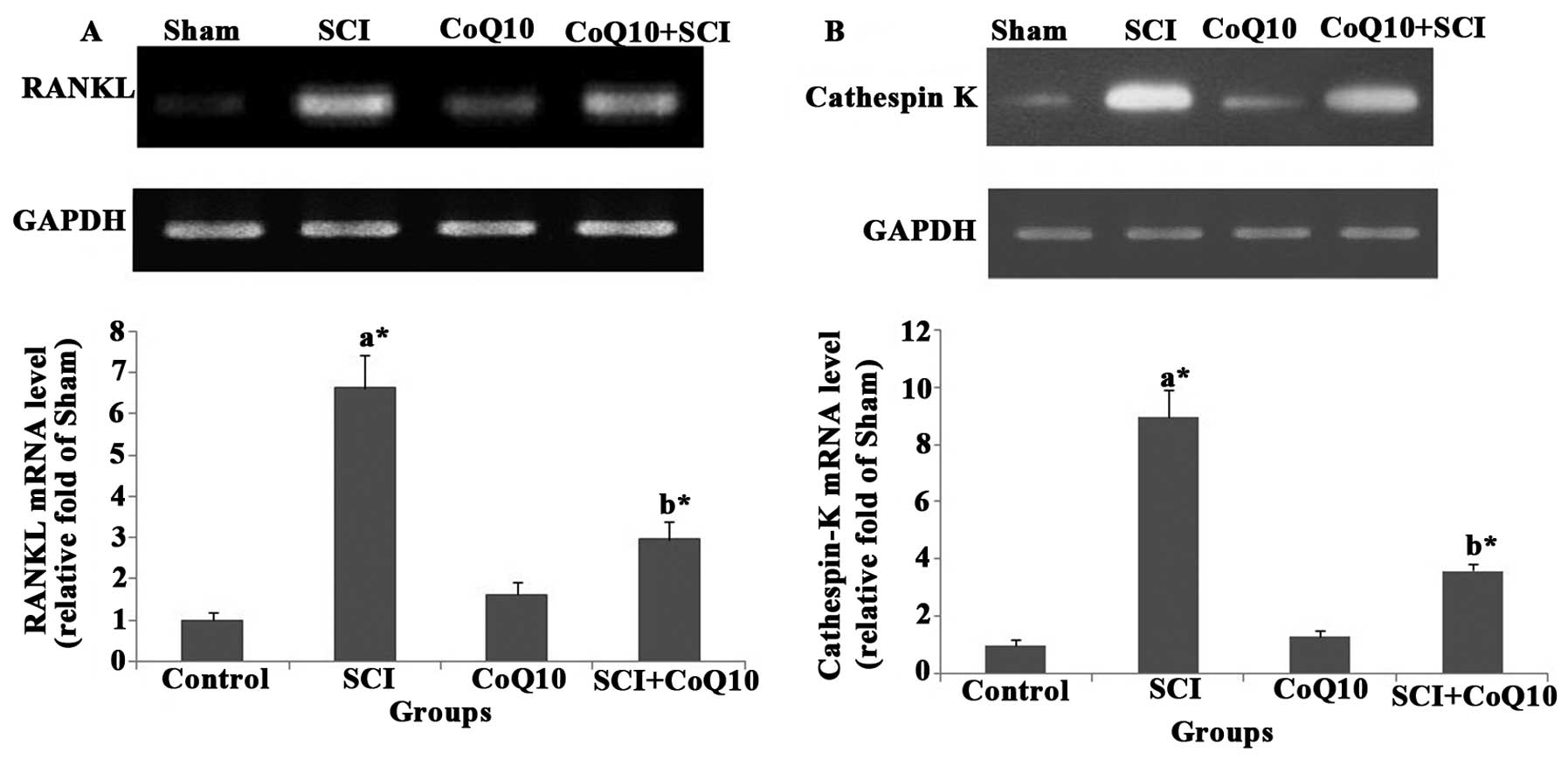
CoQ10 attenuates SCI-induced increases in
osteoclastogenesis gene expression in femurs of rats
The mRNA levels of osteoclast-specific genes RANKL
(Fig. 5A) and cathepsin K
(Fig. 5B) were increased in femurs
of SCI rats. Of note, treatment with CoQ10 significantly decreased
the RANKL and cathepsin K mRNA levels in femurs (P<0.05).
CoQ10 attenuates SCI-induced decreases in
osteoblastogenesis gene expression in femurs of rats
The mRNA levels of the osteoblast-specific gene
Cbfa1 (Fig. 6) were decreased in
femurs of SCI rats. However, administration of CoQ10 rescued the
mRNA levels of Cbfa1 in the femurs of rats following SCI
(P<0.05).
Discussion
Therapeutic agents including bisphosphonates,
estrogen and raloxifene have been implicated in the treatment of
bone diseases. However, despite their beneficial effects, they
exert certain adverse effects, including thromboembolism and
oesophageal irritation (23–25).
Therefore, the discovery of novel drugs to treat bone-associated
disorders is required. CoQ10 may be a candidate for the prevention
of osteoporosis, as it displays cytoprotective properties and acts
as an effective free radical scavenger.
The present study explored the efficacy of CoQ10 on
bone loss induced by SCI in a murine model. Complete SCI at the
lower segment of the thoracic cord of rats is routinely used in
pre-clinical studies on the pathogenesis of SCI-induced
osteoporosis (8,26,27).
It induces severe forms of osteoporosis and an array of factors
increasing the susceptibility to osteoporosis, including carrying
heavy objects, neural lesions and hormonal imbalance (28). Further direct denervation of bone
and indirect alteration of vasoregulation are attributed to
SCI-mediated osteoporosis (29).
Furthermore, negative imbalance of Ca2+ mediated by
altered hormone levels is involved in the post-SCI osteoporosis
(30). Previous studies on rodents
and humans suggested that following SCI, bone resorption is
increased due to osteoclast activation (31,32).
Morse et al (33) showed
that ten days post-SCI, the bone formation rate in rats at the
distal femur was significantly lower than that in sham-operated
rats. Furthermore, a significant reduction in the number of
osteoclasts was observed in the distal femur five days
post-SCI.
It is essential to asses and monitor post-SCI
osteoporosis to minimize the prevalence of fracture. BMD and BMC
are reliable markers in the assessment of osteoporosis and clearly
show the fracture risk in the majority of patients (34). In the present study, rats displayed
a significant decrease in BMD and BMC of femur and tibia following
SCI as compared with those in the sham group, indicating that
tibial and bone loss had occurred. Treatment with CoQ10 restored
the BMC and BMD to normal, which may be due to the re-establishment
of bone balance through preventing an increase in the number of
osteoclasts by inhibiting osteoclast maturation (18).
Reactive oxygen species have a pivotal role in the
development of secondary complications following SCI (35). In the present study, SCI rats
displayed elevated levels of femoral and tibial MDA with a
concomitant decrease in the levels of the antioxidant enzyme SOD.
These depleted levels of SOD may have been due to the involvement
of SOD in scavenging the free radicals generated by SCI. Thus,
these results demonstrated the role of oxidative stress in the
progression of osteoporosis following SCI, which is in
corroboration with the results of a previous study (36). Treatment with CoQ10 mitigated the
oxidative stress and restored the levels of MDA and SOD to normal
levels. Thus, the preventive effect of CoQ10 against bone loss may
be due to its free radical scavenging properties (37).
Elevated cytokine levels within the bone have been
involved in SCI-induced osteoporosis. Demulder et al
(38) reported that levels of IL-6
were increased in serum and bone samples of the sternum and iliac
crest from patients with SCI. Thus, the elevated cytokines may lead
to the recruitment of osteoclasts from marrow precursors and
enhance osteoclast activity in bones. In the present study,
elevated serum levels of IL-6 were significantly diminished by
CoQ10 treatment, which is in corroboration with previous study
(39). Furthermore, osteoclasts
ware highly activated and osteoblasts are suppressed by the
inflammatory cytokine TNF-α via the RANKL and osteoprotegerin (OPG)
system. In the present study, the elevated serum levels of TNF-α in
rats following SCIs were significantly reduced by CoQ10 treatment.
Previous studies reported the potential of CoQ10 to decrease TNF-α
(40,41). Thus, the anti-osteoporotic effect
rendered by CoQ10 may be mediated through an anti-cytokine
mechanism.
Previous studies reported increased bone resorption
activity post-SCI. RANKL, which is expressed on the surface of bone
marrow stromal/osteoblast precursor cells, T cells and B cells, is
the prime molecule involved in osteoclast synthesis. RANKLs bind to
their cognate receptors, RANK, on osteoclast lineage cells, and are
neutralized by the soluble, decoy receptor, OPG, which is also
produced by osteoblastic lineage cells (42–44).
A previous study showed that RANKL mRNA and protein expression in
cultured osteoblast-like cells from SCI rats was significantly
increased and resulted in increased osteoclastogenesis, thus
leading to osteoporosis after SCI (10). In the present study, rats displayed
increased mRNA expression of RANKL in the femur following SCI and
CoQ10 treatment significantly reversed the altered mRNA expression
to normal levels (45).
Cathepsin K is primarily expressed in osteoclasts
(46) and has a pivotal role in
the degradation of the collagen matrix components of bone
(predominantly type-I collagen) at acidic pH. Based on human
genetics (47,48), experimental genetics in mice
(49), substrate preference and
cellular distribution (50), the
pivotal role of cathepsin K in osteoclastic bone resorption has
been demonstrated. In the present study, rats displayed increased
mRNA expression of Cathepsin K in the femur post-SCI. Of note,
CoQ10 treatment downregu-lated cathepsin K in the femurs of rats
following SCI and thus prevented the degradation of the bone
matrix.
The bone-specific Cbfa1 gene is a
runt-domain-containing transcription factor essential for
osteoblastic differentiation and bone formation during
embryogenesis and post-natal life (51). Cbfa1 has two vital functions –
promotion of the initial phase of differentiation from mesenchymal
stem cells to pre-osteoblasts and inhibition of pre-osteoblast
differentiation to mature osteoblasts (52). As expected, in the present study,
rats displayed decreased mRNA expression of Cbfa1 in the femur
post-SCI. However, CoQ10 rescued Cbfa1 mRNA expression and promoted
bone matrix production.
In conclusion, treatment with CoQ10 mitigated the
bone loss in rats following SCI by increasing BMD and BMC levels,
attenuating oxidative stress, debilitating the cytokine levels,
depressing RANKL and cathepsin K expression, as well as restoring
Cbfa1 mRNA levels. Thus, CoQ10 may be an effective nutraceutical to
ameliorate osteoporosis after SCI. However, further molecular
studies are warranted to explore the feasibility of using CoQ10 as
an anti-osteoporotic agent.
References
|
1
|
Carlson GD and Gorden C: Current
developments in spinal cord injury research. Spine J. 2:116–128.
2002. View Article : Google Scholar
|
|
2
|
Becker D, Sadowsky CL and McDonald JW:
Restoring function after spinal cord injury. Neurologist. 9:1–15.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao F, Brown A, Dekaban GA, Omana V and
Weaver LC: CD11d integrin blockade reduces the systemic
inflammatory response syndrome after spinal cord injury. Exp
Neurol. 231:272–283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balentine JD: Pathology of experimental
spinal cord trauma I. The necrotic lesion as a function of vascular
injury. Lab Invest. 39:236–253. 1978.PubMed/NCBI
|
|
5
|
Wrathall JR, Teng YD and Choiniere D:
Amelioration of functional deficits from spinal cord trauma with
systemically administered NBQX, an antagonist of
non-N-methyl-D-aspartate receptors. Exp Neurol. 137:119–126. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bao F, John SM, Chen Y, Mathison RD and
Weaver LC: The tripeptide phenylalanine-(d) glutamate-(d) glycine
modulates leukocyte infiltration and oxidative damage in rat
injured spinal cord. Neuroscience. 140:1011–1022. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu NK, Zhang YP, Titsworth, et al: A
novel role of phospho-lipase A2 in mediating spinal cord secondary
injury. Ann Neurol. 59:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang SD, Jiang LS and Dai LY: Mechanisms
of osteoporosis in spinal cord injury. Clin Endocrinol (Oxf).
65:555–565. 2006. View Article : Google Scholar
|
|
9
|
Morse L, Teng YD, Pham L, et al: Spinal
cord injury causes rapid osteoclastic resorption and growth plate
abnormalities in growing rats (SCI-induced bone loss in growing
rats). Osteoporos Int. 19:645–652. 2008. View Article : Google Scholar
|
|
10
|
Jiang SD, Jiang LS and Dai LY: Spinal cord
injury causes more damage to bone mass, bone structure,
biomechanical properties and bone metabolism than sciatic
neurectomy in young rats. Osteoporos Int. 17:1552–1561. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Needham-Shropshire BM, Broton JG, Klose
KJ, Lebwohl N, Guest RS and Jacobs PL: Evaluation of a training
program for persons with SCI paraplegia using the Parastep 1
ambulation system, part 3: lack of effect on bone mineral density.
Arch Phys Med Rehabil. 78:799–803. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giangregorio LM, Hicks AL, Webber CE, et
al: Body weight supported treadmill training in acute spinal cord
injury: impact on muscle and bone. Spinal Cord. 43:649–657. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsumura T, Saji S, Nakamura R and
Folkers K: Evidence for enhanced treatment of periodontal disease
by therapy with coenzyme Q. Intl J Vitamin Nutr Res. 43:537–548.
1973.
|
|
14
|
Deichmann R, Lavie C and Andrews S:
Coenzyme q10 and statin-induced mitochondrial dysfunction. Ochsner
J. 10:16–21. 2010.
|
|
15
|
Kalayci M, Unal MM, Gul S, et al: Effect
of coenzyme Q10 on ischemia and neuronal damage in an experimental
traumatic brain-injury model in rats. BMC Neurosci. 12:752011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kerimoğlu A, Paşaoğlu O, Kanbak G, Hanci
V, Ozdemir F and Atasoy MA: Efficiency of coenzyme Q (10) at
experimental spinal cord injury. Ulus Travma Acil Cerrahi Derg.
13:85–93. 2007.In Turkish.
|
|
17
|
Kömürcü E, Oktay M, Kaymaz B, Hatay Gölge
U, Göksel F and Nusran G: Preventive effects of coenzyme Q10
(CoQ10) on steroid-induced osteonecrosis in rats. Acta Orthop
Traumatol Turc. 48:217–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moon HJ, Ko WK, Han SW, Kim DS, Hwang YS,
Park HK and Kwon IK: Antioxidants, like coenzyme Q10, selenite and
curcumin, inhibited osteoclast differentiation by suppressing
reactive oxygen species generation. Biochem Biophys Res Commun.
418:247–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Basso DM, Beattie MS and Bresnahan JC:
Graded histological and locomotor outcomes after spinal cord
contusion using the NYU weight-drop device versus transection. Exp
Neurol. 139:244–256. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jo HJ and Choi M: Effects of isoflavone
supplementation on the bone mineral density of growing female rats.
Nutr Res Pract. 2:68–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kakkar P, Das B and Viswanathan PN: A
modified spectrophoto-metric assay of superoxide dismutase. Indian
J Biochem Biophys. 21:130–132. 1984.PubMed/NCBI
|
|
22
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxidation in animal tissues by thiobarbituric acid
reaction. Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lloyd M: Treatment of postmenopausal
osteoporosis. N Engl J Med. 338:739–746. 1998.
|
|
24
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang SD, Jiang LS and Dai LY: Changes in
bone mass, bone structure, bone biomechanical properties and bone
metabolism after spinal cord injury: a 6-month longitudinal study
in growing rats. Calcif Tissue Int. 80:167–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu D, Zhao CQ, Li H, Jiang SD, Jiang LS
and Dai LY: Effects of spinal cord injury and hindlimb
immobilization on sublesional and supralesional bones in young
growing rats. Bone. 43:119–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giangregorio LM, Craven BC and Webber CE:
Musculoskeletal changes in women with spinal cord injury: a twin
study. J Clin Densitom. 8:347–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmid A, Huonker M, Stahl F, et al: Free
plasma catecholamines in spinal cord injured persons with different
injury levels at rest and during exercise. J Auton Nerv Syst.
68:96–100. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vaziri ND, Pandian MR, Segal JL, Winer RL,
Eltorai I and Brunnemann S: Vitamin D, parathormone and calcitonin
profiles in persons with long-standing spinal cord injury. Arch
Phys Med Rehabil. 75:766–769. 1994.PubMed/NCBI
|
|
31
|
Morse LR, Xu Y, Solomon B, et al: Severe
spinal cord injury causes immediate multi-cellular dysfunction at
the chondro-osseous junction. Transl Stroke Res. 2:643–650. 2011.
View Article : Google Scholar
|
|
32
|
Pietschmann P, Pils P, Woloszczuk W, Maerk
R, Lessan D and Stipicic J: Increased serum osteocalcin levels in
patients with paraplegia. Paraplegia. 30:204–209. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morse L, Teng YD, Pham L, et al: Spinal
cord injury causes rapid osteoclastic resorption and growth plate
abnormalities in growing rats (SCI-induced bone loss in growing
rats). Osteoporos Int. 19:645–652. 2008. View Article : Google Scholar
|
|
34
|
Majumdar S, Kothari M, Augat P, et al:
High-resolution magnetic resonance imaging: three-dimensional
trabecular bone architecture and biomechanical properties. Bone.
22:445–454. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jia Z, Zhu H, Li J, Wang X, Misra H and Li
Y: Oxidative stress in spinal cord injury and antioxidant-based
intervention. Spinal Cord. 50:264–274. 2012. View Article : Google Scholar
|
|
36
|
Sun Y, Shuang F, Chen DM and Zhou RB:
Treatment of hydrogen molecule abates oxidative stress and
alleviates bone loss induced by modeled microgravity in rats.
Osteoporos Int. 24:969–978. 2013. View Article : Google Scholar
|
|
37
|
Fouad AA, Al-Sultan AI, Refaie SM and
Yacoubi MT: Coenzyme Q10 treatment ameliorates acute cisplatin
nephrotoxicity in mice. Toxicology. 274:49–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Demulder A, Guns M, Ismail A, Wilmet E,
Fondu P and Bergmann P: Increased osteoclast-like cells formation
in long-term bone marrow cultures from patients with a spinal cord
injury. Calcif Tissue Int. 63:396–400. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee J, Hong YS, Jeong JH, et al: Coenzyme
Q10 ameliorates pain and cartilage degradation in a rat model of
osteoarthritis by regulating nitric oxide and inflammatory
cytokines. PLoS One. 8:e693622013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jin HJ, Xue Y, Chen G and Wu ZY: Effect of
coenzyme Q10 on the expression of tumor necrosis factor-α and
interleukin-10 in gingival tissue of experimental periodontitis in
rats. Zhonghua Kou Qiang Yi Xue Za Zhi. 48:660–663. 2013.In
Chinese.
|
|
41
|
Fouad AA and Jresat I: Hepatoprotective
effect of coenzyme Q10 in rats with acetaminophen toxicity. Environ
Toxicol Pharmacol. 33:158–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lacey DL, Timms E, Tan HL, et al:
Osteoprotegerin ligand is a cytokine that regulates osteoclast
differentiation and activation. Cell. 93:165–176. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hsu H, Lacey DL, Dunstan CR, et al: Tumor
necrosis factor receptor family member RANK mediates osteoclast
differentiation and activation induced by osteoprotegerin ligand.
Proc Natl Acad Sci USA. 96:3540–3545. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Simonet WS, Lacey DL, Dunstan CR, et al:
Osteoprotegerin: a novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Moon HJ, Ko WK, Jung MS, et al: Coenzyme
q10 regulates osteoclast and osteoblast differentiation. J Food
Sci. 78:H785–H891. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brömme D and Okamoto K: Human cathepsin
O2, a novel cysteine protease highly expressed in
osteoclastomas and ovary molecular cloning, sequencing and tissue
distribution. Biol Chem Hoppe Seyler. 376:379–384. 1995. View Article : Google Scholar
|
|
47
|
Ho N, Punturieri A, Wilkin D, et al:
Mutations of CTSK result in pycnodysostosis via a reduction in
cathepsin K protein. J Bone Miner Res. 14:1649–1653. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Johnson MR, Polymeropoulos MH, Vos HL,
Oetiz De Luna RI and Francomano CA: A nonsense mutation in the
cathepsin K gene observed in a family with pycnodysostosis. Genome
Res. 6:1050–1055. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Saftig P, Hunziker E, Wehmeyer O, et al:
Impaired osteoclastic bone resorption leads to osteopetrosis in
cathepsin-K-deficient mice. Proc Natl Acad Sci USA. 95:13453–13458.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pennypacker BL, Oballa RM, Levesque S,
Kimmel DB and Duong le T: Cathepsin K inhibitors increase distal
femoral bone mineral density in rapidly growing rabbits. BMC
Musculoskelet Disord. 14:3442013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xiao G, Jiang D, Ge C, et al: Cooperative
interactions between activating transcription factor 4 and
Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene
expression. J Biol Chem. 280:30689–30696. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu W, Toyosawa S, Furuichi T, et al:
Overexpression of Cbfa1 in osteoblasts inhibits osteoblast
maturation and causes osteopenia with multiple fractures. J Cell
Biol. 155:157–166. 2001. View Article : Google Scholar : PubMed/NCBI
|