Introduction
Periodontitis is defined as a bacteria-induced
disease that gradually destroys periodontal tissues, including the
gums, cementum, periodontal ligaments and supporting alveolar bone
(1,2). Data from 2009–2010 showed that almost
half of the US population over 30 years of age (47.2%) suffered
from a certain degree of periodontitis, including 8.7% with mild
disease, 30.0% with moderate disease and 8.5% with severe
periodontitis (3). Due to the high
prevalence of periodontitis and accompanying loss of teeth or the
edentulous jaw, most elderly people regard oral health as an
important aspect of life quality for various physical, social and
psychological reasons (4).
Osteoporosis is a common disease characterized by
systemic bone loss and impaired bone microarchitecture.
Post-menopausal women are usually more vulnerable to osteoporosis
due to their decreased oestrogen levels that affect bone metabolism
(5,6). Guiglia et al (7) described a possible association
between osteoporosis and periodontal disease, suggesting that
osteoporosis may facilitate the alveolar bone resorption caused by
periodontitis (7). Specifically,
osteoporosis results in an increase in certain inflammatory
factors, a number of which also participate in the progression of
periodontitis.
In recent decades, numerous studies have focused on
the association between osteoporosis and periodontitis at the bone
level. Several studies have reported that osteoporosis promotes the
loss of periodontal attachment, loss of alveolar bone height and
even tooth loss (8–10). Tezal et al (11) reported that low skeletal bone
mineral density (BMD) is associated with loss of proximal alveolar
bone and clinical attachment. By contrast, several studies have
suggested that this association was weak (12), and Brennan-Calanan et al
(13) reported that systemic bone
density and oral infection independently influence oral bone loss
in post-menopausal women. However, previous studies have also shown
a great diversity in sample sizes and measurement methodologies,
and most have been retrospective clinical studies (14–17).
Thus, whether oestrogen deficiency-induced systemic bone loss
jeopardizes alveolar bone remains controversial.
A small number of well-controlled experimental
animal studies have investigated the association between
osteoporosis and periodontitis. Through histometric analyses and
assessment of serum alkaline phosphatase and calcium, Duarte et
al (18) demonstrated that
oestrogen-deficiency may significantly increase bone loss resulting
from ligature-induced periodontitis in rats, and that there was a
synergistic effect between oestrogen deficiency and plaque
accumulation. Amadei et al (19) observed a significant increase in
bone loss, morphometrically evaluated using photo documentation,
when ligation occurred 90 days after rats underwent ovariectomy,
suggesting that long-term oestrogen deficiency affects
ligature-induced alveolar bone loss. However, other studies were
unable to correlate the absence of ovarian hormones with
periodontal alterations in rats using radiographic analyses with
digital dental X-ray equipment (20). In the present study, using
micro-computed tomography (micro-CT) analysis, the effect of
oestrogen deficiency-induced osteoporosis on the alveolar bone of
rats with experimental periodontitis (EP) was explored. It was
hypothesized that oestrogen deficiency-induced osteoporosis not
only facilitates alveolar bone loss, but also jeopardizes bone
microarchitecture in local alveolar bone.
Materials and methods
Animals, treatments and experimental
design
Forty-four female, six-month-old Sprague-Dawley rats
with a mean weight of 400±30 g were obtained from the Department of
Laboratory Animal Science (Ninth People's Hospital, Shanghai Jiao
Tong University School of Medicine, Shanghai, China). The rats were
housed in a 21°C room with a 12-h light/dark cycle. The general
condition of the animals was monitored daily and body weight was
recorded weekly. The Ethics Committee and the Animal Care and Use
Committee of Shanghai Jiao Tong University School of Medicine
(Shanghai, China) approved the experimental protocol and the
procedures performed.
After two weeks of adaptation, the rats were
randomly divided into four groups, with 11 rats in each group: The
control, ligature, OVX and OVX + ligature groups. Rats in the OVX
and OVX + ligature groups underwent bilateral ovariectomy, whereas
the other animals underwent a sham surgery (21,22).
Four weeks later, EP was induced by placing 3/0 silk sutures
(Johnson & Johnson Medical, Shanghai, China) subgingivally
around the bilateral first and second maxillary molars (M1 and M2,
respectively) for four weeks. Ligature placement initiated local
inflammation and alveolar crest bone resorption (23,24).
To ensure the establishment of EP, the ligatures were checked twice
weekly and replaced when necessary. All treatments were conducted
under general anaesthesia (10% chloral hydrate; Sigma-Aldrich, St.
Louis, MO, USA; 4 ml/kg via intraperitoneal injection).
Each rat was injected intraperitoneally with
tetracycline (TE; 25 mg/g), alizarin red (AL; 20 mg/kg), and
calcein (CA, 10 mg/kg) (all from Sigma-Aldrich) at 25, 15, and 5
days, respectively, prior to sacrification. At the designated
end-point, blood samples were collected by cardiac puncture under
anaesthesia and centrifuged (6,500–7,000 × g, 15–20 min) to recover
the serum, which was stored at −80°C until analysis. Subsequently,
the rats were sacrificed with an overdose of anaesthesia. Bilateral
maxillary bone specimens were harvested, fixed in 4%
paraformaldehyde (Sigma-Aldrich) for 48 h and transferred to 70%
ethanol prior to further testing.
Micro-CT scanning and assessment of the
alveolar crest height (ACH)
A cone-beam micro-CT system (Skyscan1176; Skyscan,
Kontich, Belgium) at Soochow University Orthopaedic Institute
(Suzhou, China) was used to scan maxillary bone specimens. The
X-ray generator was set at a voltage of 50 KV, a current of 500
µA and a fixed shutter speed of 900 msec. The images were
re-constructed using NRecon (version 1.5.1.4; Skyscan, Kontich,
Belgium). Fig. 1A illustrates the
region of interest (ROI) for analysis of tooth-supporting alveolar
bone in the maxillae. On the basis of a selected Hounsfield Unit
(HU) grayscale threshold value, microstructural indicators of BMD,
bone volume/tissue volume ratio (BV/TV), trabecular thickness
(Tb.Th), trabecular separation (Tb.Sp), trabecular number (Tb.N),
structure-model index (SMI) and connectivity density (Conn.Dn) were
calculated using CTAn (version 1.10; Skyscan). These measures were
used to quantify the bone (BV/TV), determine the average width of
the bone structure (Tb.Th), determine the number of traversals
across the bone trabeculae per unit length (Tb.N), determine the
distance between trabeculae that corresponds to the bone marrow
measurement (Tb.Sp), determine the number of trabecular elements
that can be removed without changing the bone network and provide
an estimate for the number of trabecular connections per
mm3 (Conn.Dn), and indicate the relative prevalence of
rod-like or plate-like trabecular bone (SMI). SMI was defined as an
interval between 0 and 3, where 0 is an ideal plate-like structure
and 3 is a cylinder.
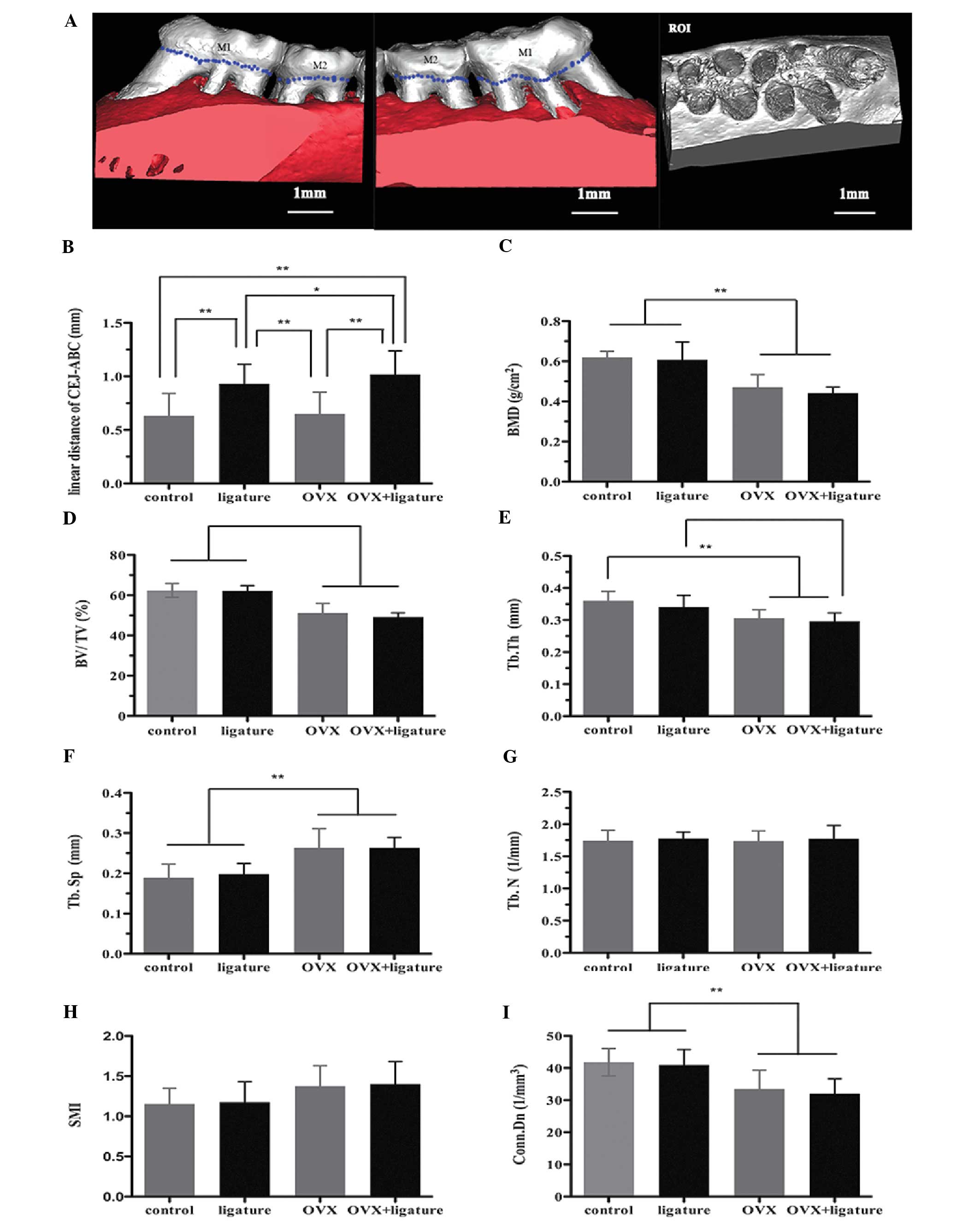 | Figure 1Effects of ovariectomy on ACH and
alveolar bone microarchitecture of rats with experimental
periodontitis by micro-computed tomography analysis. (A) Buccal and
palatal sides of the maxillary alveolar bone, where the ACH was
measured between the CEJ and the ABC in the mesiolingual,
mesiobuccal, distolingual and distobuccal regions of the M1 and M2.
The ROI is a cuboidal bone body that encompasses the M1 and M2
roots. (B) Analysis of the CEJ-ABC linear distance after 1 month of
ligation. (C-I) Analysis of micro-computed tomography volumetric
parameters: (C) BMD, (D) BV/TV ratio, (E) Tb.Th, (F) Tb.Sp, (G)
Tb.N, (H) SMI and (I) Conn.Dn. Values are expressed as the mean ±
standard deviation (*P<0.05; **P<0.01).
OVX, ovariectomy; ACH, alveolar crest height; CEJ, cement-enamel
junction; ABC, alveolar bone crest; ROI, region of interest; M1,
first maxillary molar; M2, second maxillary molar; BMD, bone
mineral density; BV/TV, bone volume/tissue volume; Tb.Th,
trabecular thickness; Tb.Sp, trabecular separation; Tb.N,
trabecular number; SMI, structure-model index; Conn.Dn,
connectivity density. |
According to a previous study (25), the linear distance between the
cement-enamel junction (CEJ) and alveolar bone crest (ABC) was
measured to reflect the decreased volume of alveolar crest height
(ACH). The decreased ACH was obtained by averaging the CEJ-ABC
distances measured at the mesio-lingual, mesiobuccal, distolingual
and distobuccal parts of the specimen. Clinically, a decreased ACH
illustrates the integrity of tooth-supporting alveolar bone, with
lower CEJ-ABC values reflecting better-quality alveolar bone
(26).
Bone histomorphometry
The left maxillae were sequentially de-hydrated,
de-calcified, embedded in methyl methacrylate (Sigma-Aldrich).
Sections were prepared from the occlusal surface of the tooth crown
to the alveolar bone mesiodistally along the plane parallel to the
long axis of the tooth and then cut to 100–200 µm using the
Leica SP1600 Microtome (Leica Microsystems, Heidelberg, Germany)
and polished to a final thickness of approximately 20 µm by
sequential usage of P300, P800 and P1200 sandpaper. To obtain the
mineral apposition rate (MAR), fluorescent labeling, which was
performed in the live rats as described above, was visualized. A
confocal laser-scanning microscope (TCS Sp2 AOBS; Leica
Microsystems, Wetzlar, Germany) was used to capture images of the
fluorescent labeling line (27).
The excitation/emission wavelengths for each of the fluorochromes
were 405/580 nm (TE; yellow), 543/617 nm (AL; red) and 488/517 nm
(CA; green). Sections were also stained with Van Gieson's fuchsin
(Sigma-Aldrich) for histological observation. The tabecular bone
dynamic parameters were measured from a 1 mm2 box
positioned at the interradicular region of M1 (magnification,
×100), using tetracycline and calcein labels. MAR was measured
using a Bioquant image analysis system (Bioquant OSTEO II Version
8.12.20, Bioquant Image Analysis Corporation, Nashville, TN,
USA).
The right maxillae were de-calcified in 10% EDTA
(Sigma-Aldrich) for 2 months and then embedded in paraffin. Serial
sagittal sections (5 µm) were stained with
osteoclast-specific, tartrate-resistant acid phosphatase (TRAP;
Sigma-Aldrich), observed microscopically (Eclipse 90i; Nikon,
Tokyo, Japan) and micrographed. ImageJ 1.46r software (National
Institutes of Health, Bethesda, MD, USA) was used to analyze the
TRAP- and Van Gieson's fuchsin-stained sections. The number of
TRAP-positive, multinucleated osteoclasts was counted in each
sample.
Serum bone resorption biomarkers
The serum bone-specific resorption markers,
tartrate-resistant acid phosphatase 5 b (TRACP5b) and C-terminal
telopeptide of type I collagen (CTX-1), were quantified in all
serum samples using rat ELISA kits (SB-TR102 and AC-06F1; IDS,
Fountain Hills, AZ, USA).
Statistical analysis
All values are expressed as the mean ± standard
deviation. One-way analysis of variance with Bonferroni's post hoc
test was performed to compare the between-group means for all
outcomes. The Statistical Package for the Social Sciences, version
17.0 (SPSS, Inc., Chicago, IL, USA) was used for all analyses.
P<0.05 or P<0.01 was considered to indicate a statistically
significant difference between groups.
Results
Effect of ovariectomy on ACH
To obtain ACH values, the linear CEJ-ABC distances
at four sites for each specimen were measured and averaged
(Fig. 1). The ACH decreased by an
average of 0.2979 mm in the ligature group and by 0.3858 mm in the
OVX + ligature group, compared with that in the control animals
(both P<0.001; Fig. 1B).
Compared to that in the OVX group, the ACH decreased by 0.2814 mm
in the ligature group and by 0.3693 mm in the OVX + ligature group
(P<0.001; Fig. 1B). Statistical
analysis of the comparison between the control and OVX groups
indicated that ovariectomy did not have any effect on the ACH of
non-ligature rats. However, ovariectomy promoted alveolar bone
resorption, affecting the ACH in the rats in the OVX + ligature
group, as indicated by the lower ACH in the OVX + ligature group
compared with that in the ligature group (P=0.042; Fig. 1B). Furthermore, an obvious ACH
decrease in the ligature group and in the OVX + ligature group was
observed between M1 and M2 in the Van Gieson's fuchsin-stained
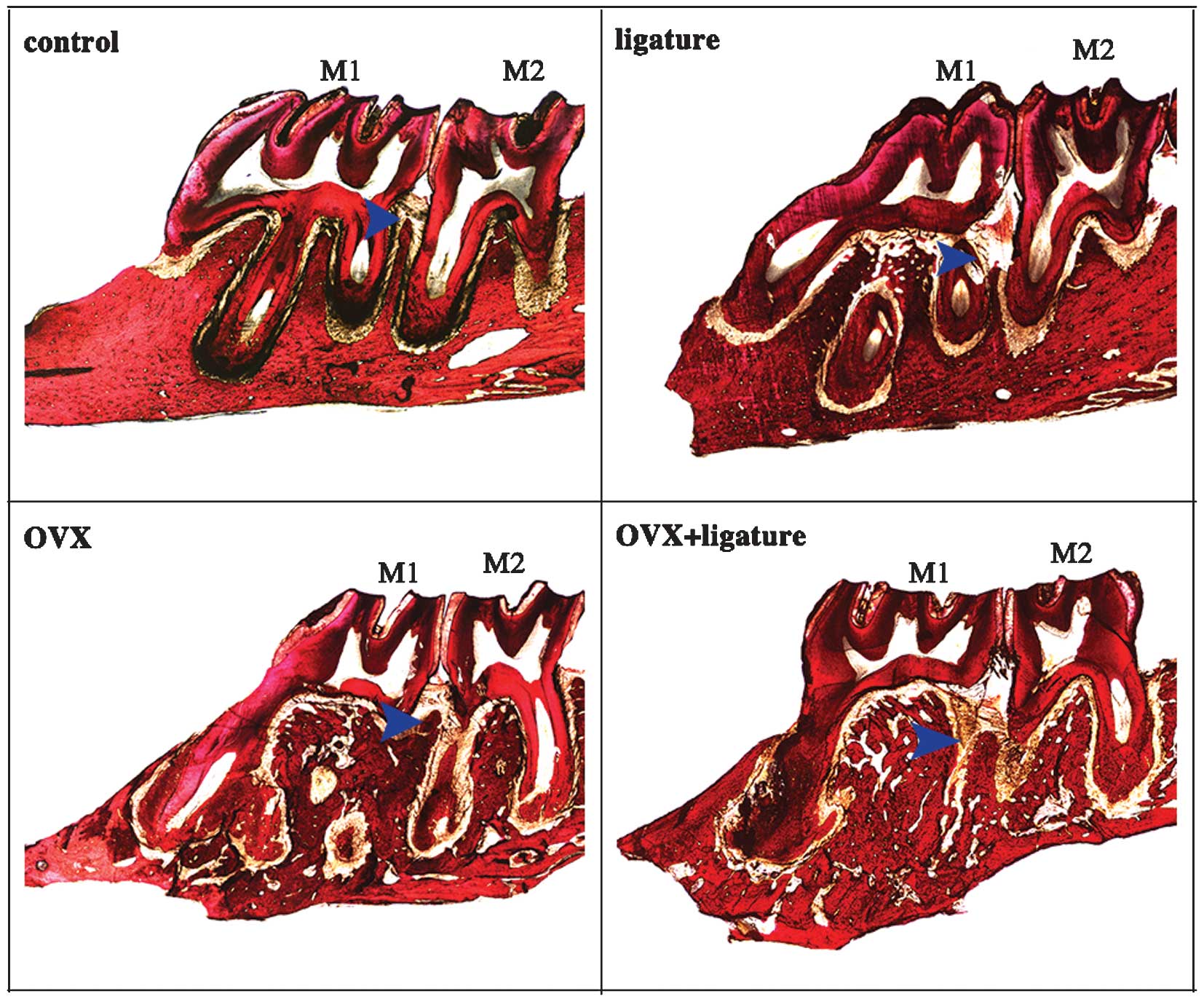
sections (Fig. 2; blue arrow).
Effect of ovariectomy on alveolar bone
microarchitecture
Based on the analysis of the ROI in the alveolar
bone, OVX rats exhibited lower BMDs than non-OVX rats (P<0.001;
Fig. 1C). A decreased BV/TV ratio
was associated with a reduced Tb.Th as well as an increased Tb.Sp
in the OVX and OVX + ligature groups compared with those in the
control and ligature groups (P<0.001; Fig. 1D-F). There was also a significant
decrease in the Conn.Dn in the OVX and OVX + ligature groups,
whereas the Tb.N and SMI remained relatively consistent among the
groups (Fig. 1G-I). Fig. 2 shows the bone profile in a
longitudinal section. Obvious osteolysis was detected in the OVX
rats, which was not present in the ligature-only group.
Effect of ovariectomy on alveolar bone
MAR and the number of active osteoclasts
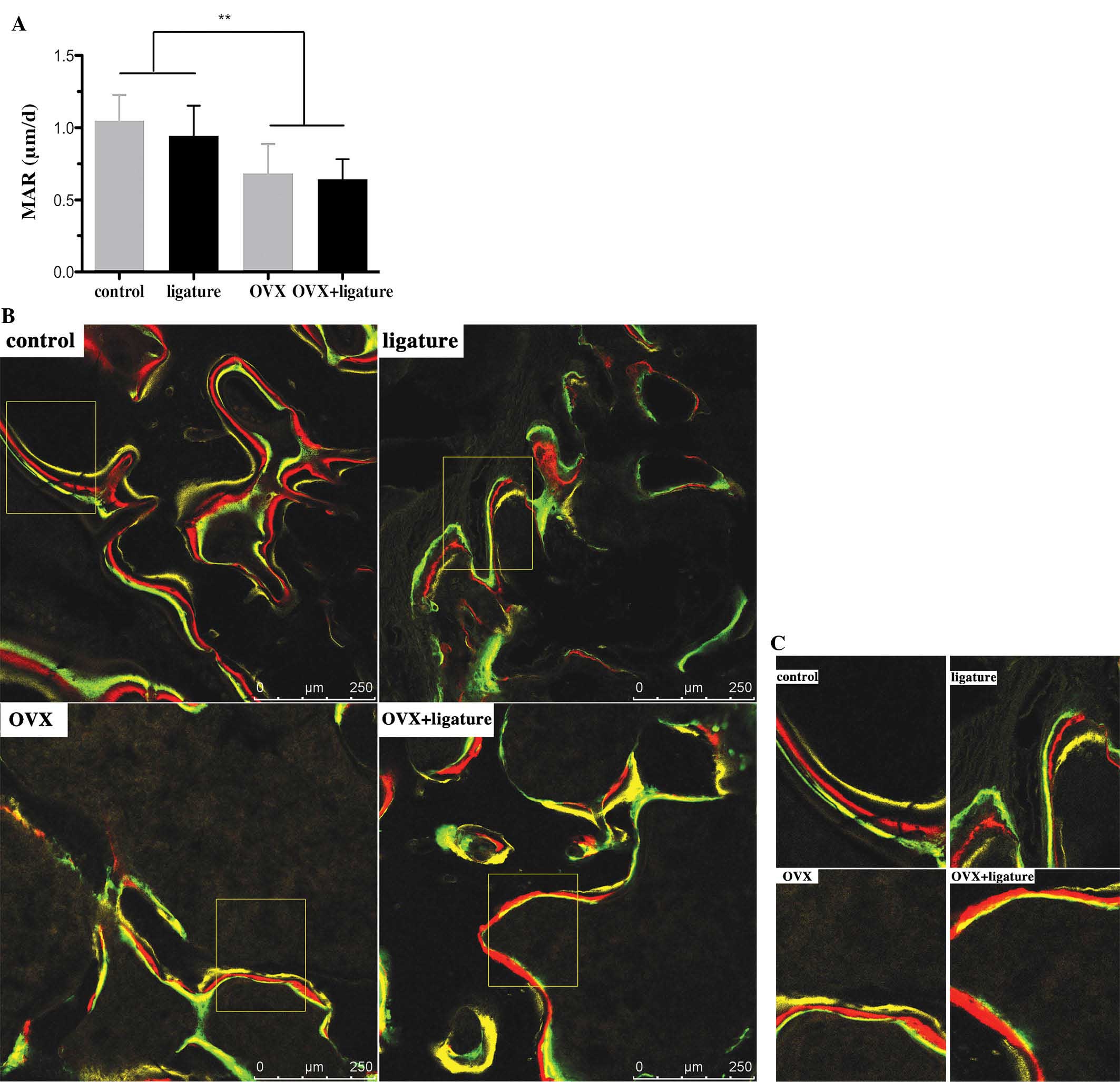
Due to the oestrogen deficiency resulting from
ovariectomy, significantly lower MAR values were observed in the
OVX rats compared with those in the non-OVX rats (P<0.001;
Fig. 3A). EP mildly aggravated the
trend, as indicated by the slightly lower MAR value in the OVX +
ligature animals than that in the OVX-only group. The yellow, red
and green fluorescent labelling lines were intertwined in the OVX +
ligature group, in contrast to the obvious separation between the
lines in the control group (Fig. 3B
and C).
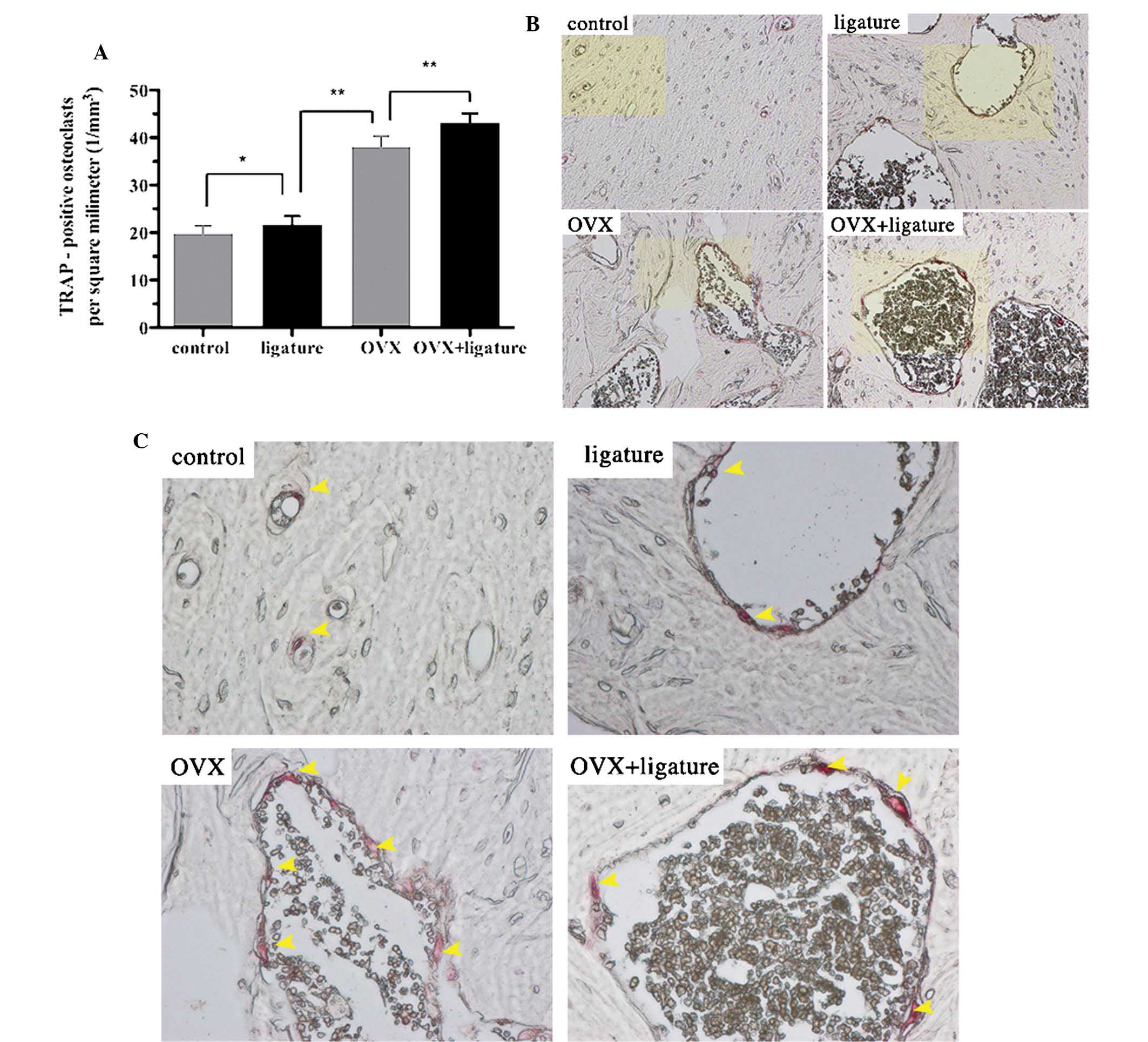
Analysis of the TRAP-stained sections showed that
the number of osteoclasts was increased in the ligature, OVX and
OVX + ligature groups by 9.59, 92.42 and 117.42%, respectively,
compared to that in the control group (Fig. 4A). In addition, variations in the
shape and location of the osteoclasts were also found among the
four groups. In contrast to the control and ligature groups, the
OVX group and particularly the OVX + ligature group exhibited
larger, irregularly shaped osteoclasts and more eroded bone
surfaces (Fig. 4B and C;
arrows).
Effect of ovariectomy on serum levels of
TRACP5b and CTX-1
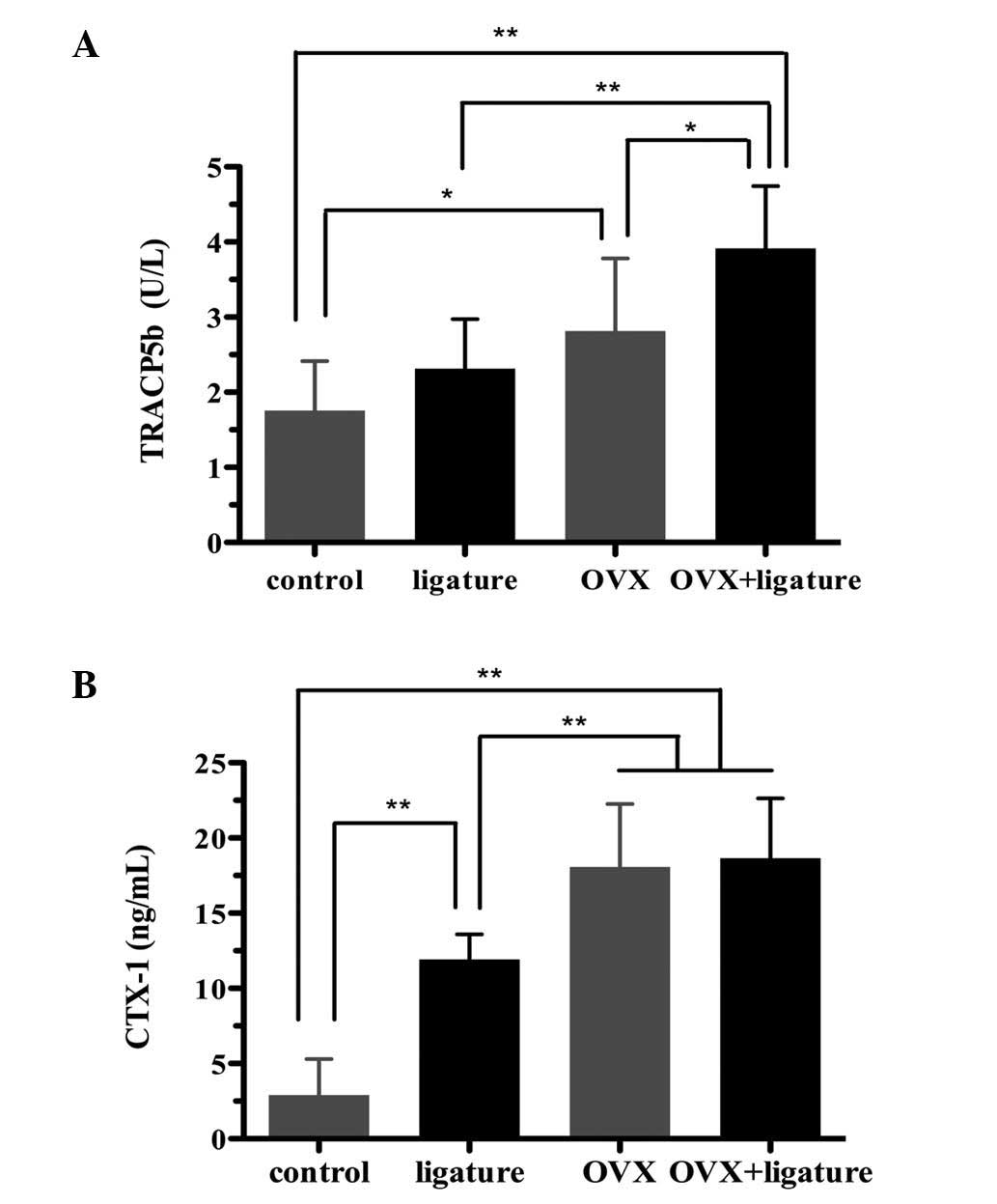
The OVX + ligature group showed elevated serum
TRACP5b levels as compared with those in the OVX (P=0.017),
ligature (P<0.001) and control (P<0.001) groups, as well as
elevated serum CTX-1 levels compared to those in the ligature
(P=0.001) and control (P<0.001) groups. In addition, the OVX
group showed increased serum TRACP5b levels compared to those in
the ligature (P=0.921) and control (P=0.030) groups, as well as
increased serum CTX-1 levels compared with those in the ligature
(P=0.001) and control (P<0.001) groups. Furthermore, the
ligature group showed significantly higher serum CTX-1 levels
(P<0.001) and slightly higher TRACP5b levels (P=0.839) compared
with those in the control group (Fig.
5).
Discussion
Extensive epidemiologic and experimental studies
have proven the existence of systemic risk factors pertaining to
the initiation, progression and severity of periodontitis (28,29).
A novel concept also recognizes that systemic risk factors may
determine the rate of progression, age at onset and severity of
periodontal disease (30). Among
these risk factors, osteoporosis is one of the six main factors
(30). In addition, periodontitis
and osteoporosis are major health problems among the elderly
(31). Therefore, an explicit
understanding of the correlation between the two diseases is
critical to the prevention of the morbidity and mortality
associated with the disorders, particularly in the elderly.
However, additional clarification regarding the association between
osteoporosis and periodontitis is still required, in addition to an
understanding of the extent to which osteoporosis contributes to
the overall risk of periodontitis. To better understand these
associations, experimental animal models are valuable for initial
investigations. Sex steroid deficiency, particularly that of
oestrogen, is considered to be the main cause of osteoporosis, and
OVX rats are widely used as animal models of osteoporosis (18,20,32).
In addition, the periodontal tissue of rats resembles that of
humans (33).
Alveolar bone is an important component of dental
treatments, including prosthodontic, implant and orthodontic
treatments (31,34). When analyzing bone mass and
volumetric bone parameters, the present study identified that
ovariectomy had a significant influence on all parameters except
Tb.N and SMI, whereas local simulation of periodontitis failed to
significantly change these parameters. This observation was in
accordance with a previous study (19), which showed that ligation at 90
days after ovariectomy induced a significant increase in bone loss
compared with that in the ligature only group. However, ligatures
concurrent with ovariectomy only slightly aggravated the alveolar
bone loss, compared with that following ovariectomy alone. The ACH
is a measure for the evaluation of periodontitis (9,13).
In the present study, ovariectomy alone did not reduce the ACH, but
aggravated the reduction in ACH in combination with ligatures. Low
alveolar BMD and impaired alveolar bone structure may lead to
increased bone resorption of the alveolar crest when periodontitis
occurs concurrently with ovariectomy. In addition,
ovariectomy-induced oestrogen deficiency disturbs the cytokines
that may increase host susceptibility to infection, which also
facilitates the progression of periodontitis (31,35–37).
The coupling of bone resorption and bone formation
is of great significance to bone metabolism, which is affected by
hormones and cytokines (38). In
the present study, the bone formation rate was lower than the bone
resorption rate in OVX rats, in contrast to the balanced parameters
in non-OVX rats, which could be observed from the MAR rate. In
contrast to the control and ligature groups, the MAR rate decreased
in the OVX and OVX + ligature groups, while there was no
statistical difference between the control group and ligature
group. The mixed fluorescent labelling line and the reduced MAR of
the OVX rats indicated that oestrogen deficiency-induced
osteoporosis influenced the balance of alveolar bone formation and
resorption. However, no significant MAR reduction was observed in
the ligature rats, which may be due the time required for bacterial
accumulation prior to impacting bone metabolism after ligation. The
local accumulation of bacteria and bacteria-derived factors may
stimulate a local inflammatory reaction and activation of the
innate immune system. The immune cells secrete cytokines that
promote osteoclast maturation, leading to an imbalance in bone
metabolism (1). However, these
processes take time to occur. Therefore, rats in the OVX + ligature
group showed the lowest MAR among the four groups due to the
synergic effect. In the present study, the number of TRAP-positive
osteoclasts demonstrated an increasing trend in the ligature, OVX
and OVX + ligature groups. However, ligature-induced periodontitis
led to a significantly increased number of osteoclasts.
Furthermore, the levels of two specific serum bone resorption
markers, TRACP5b and CTX-1, increased in the ligature rats, but not
in the OVX rats. Interleukin (IL)-6 and IL-1 in bone marrow cells
are known to stimulate osteoclastic bone resorption under
conditions of oestrogen deficiency (39).
Periodontitis is a disease normally caused by
bacterial infection, which produces factors or antigens that
stimulate local inflammatory reactions and activities of the innate
immune system (1). Previous
studies have suggested that cyto-kines, including IL-17 or tumour
necrosis factor-α (TNF-α), have a key role in periodontal alveolar
bone resorption (40–43). IL-17 exerts its osteoclastogenic
activity by enhancing receptor activator of nuclear factor kappa-B
ligand expression in osteo-blasts and CD4+ T cells, and
promotes alveolar resorption when released in excessive amounts
(44–46). TNF-α directly contributes to
periodontal damage through its effect on osteoclastogenesis,
through amplification of inflammatory immune reactions and through
the inhibition of differentiation and bone nodule formation
(47–49). Thus, oestrogen deficiency,
associated with increased systemic levels of IL-17 or TNF-α, may
augment the local levels of these factors in the alveolar bone and
facilitate alveolar bone resorption. This concept is in accord with
previously presented results that suggested low oestrogen induced
T- and B-cell abnormalities, increased local production of the
bone-active cytokines, and finally resulted in periodontitis
progression (50).
In conclusion, the present study demonstrated that
ovariectomy resulted in the deterioration of the alveolar bone
microarchitecture, ACH reduction, decline in the bone formation
rate and increased osteoclast activity. These observations
indicated that post-menopausal osteoporosis impacts the progression
of periodontitis.
Acknowledgments
Grant support for this project was provided by
Shanghai Leading Academic Discipline Project (nos. T0202 and
S30206) and the National College Students' Innovative
Entrepreneurial Training Plan (no. 2013053). The authors would like
to thank Dr. Tingting Tang, who provided advice on osteoporosis, as
well as Drs Qiming Fan, Shuhong Zhang and Shuangyan Zhang,
(Department of Orthopaedics, Shanghai Ninth People's Hospital,
Shanghai Jiao Tong University School of Medicine, Shanghai, China),
who provided experimental guidance, and Dr Junjie Lu, who provided
great assistance with the animal surgeries (Department of SPF
laboratory, Shanghai Ninth People's Hospital, Shanghai Jiao Tong
University School of Medicine).
References
|
1
|
Di Benedetto A, Gigante I, Colucci S and
Grano M: Periodontal disease: linking the primary inflammation to
bone loss. Clin Dev Immunol. 2013:5037542013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eke PI, Dye BA, Wei L, Thornton-Evans GO
and Genco RJ: CDC Periodontal Disease Surveillance workgroup: James
Beck GDRP: Prevalence of periodontitis in adults in the United
States: 2009 and 2010. J Dent Res. 91:914–920. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McGrath C and Bedi R: The importance of
oral health to older people's quality of life. Gerodontology.
16:59–63. 1999. View Article : Google Scholar
|
|
5
|
Nordin BE, Wishart JM, Clifton PM, et al:
A longitudinal study of bone-related biochemical changes at the
menopause. Clin Endocrinol (Oxf). 61:123–130. 2004. View Article : Google Scholar
|
|
6
|
NIH Consensus Development Panel on
Osteoporosis Prevention, Diagnosis, and Therapy: Osteoporosis
prevention, diagnosis, and therapy. JAMA. 285:785–795. 2001.
View Article : Google Scholar
|
|
7
|
Guiglia R, Di Fede O, Lo Russo L, Sprini
D, Rini GB and Campisi G: Osteoporosis, jawbones and periodontal
disease. Med Oral Patol Oral Cir Bucal. 18:e93–e99. 2013.
View Article : Google Scholar :
|
|
8
|
Yoshihara A, Seida Y, Hanada N and
Miyazaki H: A longitudinal study of the relationship between
periodontal disease and bone mineral density in community-dwelling
older adults. J Clin Periodontol. 31:680–684. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wactawski-Wende J, Hausmann E, Hovey K,
Trevisan M, Grossi S and Genco RJ: The association between
osteoporosis and alveolar crestal height in postmenopausal women. J
Periodontol. 76(11 Suppl): 2116–2124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ronderos M, Jacobs DR, Himes JH and
Pihlstrom BL: Associations of periodontal disease with femoral bone
mineral density and estrogen replacement therapy: cross-sectional
evaluation of US adults from NHANES III. J Clin Periodontol.
27:778–786. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tezal M, Wactawski-Wende J, Grossi SG, Ho
AW, Dunford R and Genco RJ: The relationship between bone mineral
density and periodontitis in postmenopausal women. J Periodontol.
71:1492–1498. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lundström A, Jendle J, Stenström B, Toss G
and Ravald N: Periodontal conditions in 70-year-old women with
osteoporosis. Swed Dent J. 25:89–96. 2001.
|
|
13
|
Brennan-Calanan RM, Genco RJ, Wilding GE,
Hovey KM, Trevisan M and Wactawski-Wende J: Osteoporosis and oral
infection: independent risk factors for oral bone loss. J Dent Res.
87:323–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aspalli SS, Shetty VS, Parab PG, Nagappa
G, Devnoorkar A and Devarathnamma MV: Osteoporosis and
periodontitis: Is there a possible link? Indian J Dent Res.
25:316–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin TH, Lung CC, Su HP, et al: Association
between periodontal disease and osteoporosis by gender: A
nationwide population-based cohort study. Medicine (Baltimore).
94:e5532015. View Article : Google Scholar
|
|
16
|
Moeintaghavi A, Pourjavad M, Dadgar S and
Tabbakh NS: Evaluation of the association between periodontal
parameters, osteoporosis and osteopenia in post menopausal women. J
Dent (Tehran). 10:443–448. 2013.
|
|
17
|
Hattatoglu-Sonmez E, Ozcakar L,
Gokce-Kutsal Y, Karaagaoglu E, Demiralp B and Nazliel-Erverdi H: No
alteration in bone mineral density in patients with periodontitis.
J Dent Res. 87:79–83. 2008. View Article : Google Scholar
|
|
18
|
Duarte PM, Gonçalves PF, Sallum AW, Sallum
EA, Casati MZ and Humberto Nociti F Jr: Effect of an
estrogen-deficient state and its therapy on bone loss resulting
from an experimental periodontitis in rats. J Periodontal Res.
39:107–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amadei SU, Souza DM, Brandão AA and Rocha
RF: Influence of different durations of estrogen deficiency on
alveolar bone loss in rats. Braz Oral Res. 25:538–543. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anbinder AL, Prado Mde A, Spalding M, et
al: Estrogen deficiency and periodontal condition in rats: a
radiographic and macroscopic study. Braz Dent J. 17:201–207. 2006.
View Article : Google Scholar
|
|
21
|
Thompson DD, Simmons HA, Pirie CM and Ke
HZ: FDA guidelines and animal models for osteoporosis. Bone.
17:125S–133S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu XL, Li CL, Lu WW, Cai WX and Zheng LW:
Skeletal site-specific response to ovariectomy in a rat model:
change in bone density and microarchitecture. Clin Oral Implants
Res. 26:392–398. 2015. View Article : Google Scholar
|
|
23
|
Cai X, Li C, Du G and Cao Z: Protective
effects of baicalin on ligature-induced periodontitis in rats. J
Periodontal Res. 43:14–21. 2008.PubMed/NCBI
|
|
24
|
Breivik T, Opstad PK, Gjermo P and Thrane
PS: Effects of hypothalamic-pituitary-adrenal axis reactivity on
periodontal tissue destruction in rats. Eur J Oral Sci.
108:115–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park CH, Abramson ZR, Taba M Jr, et al:
Three-dimensional micro-computed tomographic imaging of alveolar
bone in experimental bone loss or repair. J Periodontol.
78:273–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu KF, Ho YP, Ho KY, Wu YM, Wang WC and
Chou YH: Clinical case report on treatment of generalized
aggressive periodontitis: 5-year follow-up. Int J Periodontics
Restorative Dent. 35:395–400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang XQ, Wang SY, Zhao J, Zhang XL and
Zhang ZY: Sequential fluorescent labeling observation of maxillary
sinus augmentation by a tissue-engineered bone complex in canine
model. Int J Oral Sci. 1:39–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kornman KS: Mapping the pathogenesis of
periodontitis: A new look. J Periodontol. 79:1560–1568. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Borrell LN and Papapanou PN: Analytical
epidemiology of peri-odontitis. J Clin Periodontol. 32(Suppl 6):
132–158. 2005. View Article : Google Scholar
|
|
30
|
Genco RJ and Borgnakke WS: Risk factors
for periodontal 2000 disease. Periodontol. 62:59–94. 2013.
View Article : Google Scholar
|
|
31
|
Wactawski-Wende J, Grossi SG, Trevisan M,
et al: The role of osteopenia in oral bone loss and periodontal
disease. J Periodontol. 67(10 Suppl): 1076–1084. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sultan N and Rao J: Association between
periodontal disease and bone mineral density in postmenopausal
women: a cross sectional study. Med Oral Patol Oral Cir Bucal.
16:e440–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Page RC, Offenbacher S, Schroeder HE,
Seymour GJ and Kornman KS: Advances in the pathogenesis of
periodontitis: summary of developments, clinical implications and
future directions. Periodontol 2000. 14:216–248. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hirai T, Ishijima T, Hashikawa Y and
Yajima T: Osteoporosis and reduction of residual ridge in
edentulous patients. J Prosthet Dent. 69:49–56. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Golden SH, Robinson KA, Saldanha I, Anton
B and Ladenson PW: Clinical review: Prevalence and incidence of
endocrine and metabolic disorders in the United States: A
comprehensive review. J Clin Endocrinol Metab. 94:1853–1878. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hildebolt CF, Pilgram TK,
Yokoyama-Crothers N, et al: The pattern of alveolar crest height
change in healthy postmenopausal women after 3 years of
hormone/estrogen replacement therapy. J Periodontol. 73:1279–1284.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Payne JB, Reinhardt RA, Nummikoski PV and
Patil KD: Longitudinal alveolar bone loss in postmenopausal
osteo-porotic/osteopenic women. Osteoporos Int. 10:34–40. 1999.
View Article : Google Scholar
|
|
38
|
Wronski TJ, Dann LM, Scott KS and Cintron
M: Long-term effects of ovariectomy and aging on the rat skeleton.
Calcif Tissue Int. 45:360–366. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miyaura C, Kusano K, Masuzawa T, et al:
Endogenous bone-resorbing factors in estrogen deficiency:
cooperative effects of IL-1 and IL-6. J Bone Miner Res.
10:1365–1373. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cardoso CR, Garlet GP, Crippa GE, et al:
Evidence of the presence of T helper type 17 cells in chronic
lesions of human periodontal disease. Oral Microbiol Immunol.
24:1–6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vernal R, Dutzan N, Chaparro A, Puente J,
Antonieta Valenzuela M and Gamonal J: Levels of interleukin-17 in
gingival crevicular fluid and in supernatants of cellular cultures
of gingival tissue from patients with chronic periodontitis. J Clin
Periodontol. 32:383–389. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Graves D: Cytokines that promote
periodontal tissue destruction. J Periodontol. 79(8 Suppl):
1585–1591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Garlet GP, Martins W Jr, Fonseca BA,
Ferreira BR and Silva JS: Matrix metalloproteinases, their
physiological inhibitors and osteoclast factors are differentially
regulated by the cytokine profile in human periodontal disease. J
Clin Periodontol. 31:671–679. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu JJ, Ruddy MJ, Wong GC, et al: An
essential role for IL-17 in preventing pathogen-initiated bone
destruction: recruitment of neutrophils to inflamed bone requires
IL-17 receptor-dependent signals. Blood. 109:3794–3802. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Weaver CT, Harrington LE, Mangan PR,
Gavrieli M and Murphy KM: Th17: an effector CD4 T cell lineage with
regulatory T cell ties. Immunity. 24:677–688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tjoa ST, de Vries TJ, Schoenmaker T,
Kelder A, Loos BG and Everts V: Formation of osteoclast-like cells
from peripheral blood of periodontitis patients occurs without
supplementation of macrophage colony-stimulating factor. J Clin
Periodontol. 35:568–575. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yarilina A, Xu K, Chen J and Ivashkiv LB:
TNF activates calcium-nuclear factor of activated T cells (NFAT)c1
signaling pathways in human macrophages. Proc Natl Acad Sci USA.
108:1573–1578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang H, Zhao N, Xu X, et al:
Dose-specific effects of tumor necrosis factor alpha on osteogenic
differentiation of mesenchymal stem cells. Cell Prolif. 44:420–427.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Inagaki K, Kurosu Y, Sakano M, et al:
Osteoporosis and periodontal disease in postmenopausal women:
Association and mechanisms. Clin Calcium. 16:269–277. 2006.In
Japanese. PubMed/NCBI
|