Introduction
Chronic myeloid leukemia (CML) is a hematopoietic
stem cell disorder; which has serious implications on health and
life expectancy (1). It is
diagnosed by the presence of a specific abnormality karyotype
Philadelphia (Ph) chromosome, harboring the BCR-ABL oncogene
(2). CML follows progression from
a chronic phase to an accelerated phase or to a rapidly fatal blast
crisis within 3–5-years in patients (3). It has been reported that imatinib,
nilotinib and dasatinib can be used in the treatment of patients
with CML (4). At present,
treatment strategies for leukemia remain unsatisfactory, thus it is
imperative to fully elucidate the molecular mechanisms of leukemia.
It will be undoubtedly a general trend that molecular therapy
combined with anti-leukemia natural compound are employed in
leukemia treatment (5).
High mobility group box 1 (HMGB1), a DNA-binding
nuclear protein predominantly involved in the inflammatory
response, has a close association with the growth of tumor cells,
invasion and metastasis (6,7). It
is the prototypic damage-associated molecular pattern (DAMP)
molecule and has been implicated in several inflammatory disorders
(8). High levels of HMGB1 have
been previously detected in leukemia cells (9). HMGB1 is known to be a type of
anti-apoptotic binding protein and its over-expression is able to
inhibit cell apoptosis, leading to the occurrence of leukemic
growth (10). The upregulation of
HMGB1 mRNA expression has been identified in a number of tumor
types (11–13). In addition, HMGB1 has been reported
to reduce the sensitivity of K562 human myeloid leukemia cells to
anticancer drugs (14). Therefore,
targeting the HMGB1 ligand or its receptor represents an important
potential application in leukemia therapy. Cordycepin
(3′-deoxyadenosine) is a key bioactive component isolated from
C. militaris. It has been reported to possess numerous
pharmacological activities, including immunological stimulation,
anticancer and antileukemic effects (15–17).
Previous studies have indicated that cordycepin has a range of
molecular targets and influences numerous biochemical and molecular
processes (18–20). However, the molecular mechanisms of
cordycepin on leukemia remain to be fully elucidated.
In the present study, HMGB1 knockdown cells were
established using the lentiviral infection method. The effects of
HMGB1 knockdown in combination with cordycepin treatment on
proliferation, apoptosis, reactive oxygen species (ROS) and
adhesion of the K562 leukemia cell line were evaluated. In
addition, whether cordycepin exhibited synergistic action with the
HMGB1 knockout was investigated, which aimed to provide insight
into CML treatment.
Materials and methods
Cell culture, transfection and
treatment
The K562 and 293T human CML cell lines were
purchased from the American Type Culture Collection (Manassas, VA,
USA) and cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen Life Technologies, Carlsbad, CA, USA) containing 10%
(v/v) heat-inactivated fetal bovine serum (FBS; Gibco Life
Technologies- Carlsbad, CA, USA), 100 mg/ml streptomycin and 100
U/ml penicillin (Beyotime Institute of Biotechnology, Haimen,
China) at 37°C in a humidified 5% CO2 atmosphere. Cells
with HMGB1 high-expression were screened for transfection by means
of western blotting. The small interfering RNA (siRNA)-HMGB1
plasmid was provided by JRDUN Biotechnogy (Shanghai) Co., Ltd.
(Shanghai, China). The HMGB1 plasmid (3 mg) or mock-vehicle were
transfected into K562 cell lines in 6-well plates (1 mg/ml) using
the pCMV-G-NR-U6-shRNA lentiviral vector (Shanghai Genechem Co.,
Ltd., Shanghai, China), according to the manufacturer's
instructions. Cells were treated with cordycepin (30 μmol/l)
for 48 h, and then were subjected to cell assays.
Cell viability assay
For the cell viability assay, cells
(5×105/ml) were seeded into 96-well plates and cultured
for 0, 24, 48 and 72 h at 37°C. Subsequently, 100 μl serum
free DMEM containing 10% Cell Counting kit-8 (CCK-8; Beyotime
Institute of Biotechnology) reagent (v/v) was added into each well
and the cells were cultured for 1 h in 5% CO2 at 37°C.
Finally, optical density values (OD) were read at 450 nm using a
SpectraMax® i3× microplate reader (Molecular Devices,
Sunnyvale, CA, USA).
Detection of cell cycle and apoptosis by
flow cytometric analysis
The cell cycle was assessed according to the
percentage of cells with DNA using the propidium iodide (PI)
staining technique. With or without cordycepin treatment for 48 h,
cells (5~10×104) were harvested and stained using an
Annexin V-fluorescein isothiocyanate/PI kit (BD Biosciences,
Franklin Lakes, NJ, USA). Staining was performed according to the
manufacturer's instructions. The apoptosis of K562 cells was
determined by flow cytometric analysis using a FACSCaliber flow
cytometer (BD Biosciences).
Detection of ROS
The generation of ROS was assessed by flow
cytometry. In brief, cells (5×104 cells/well) were
cultured and washed with phosphate-buffered saline (PBS) and
resuspended in complete medium followed by incubation with 50
μM dihydrorhodamine (DHE; Vigorous Biotechnology, Beijing,
China) for 30 min at 37°C. ROS fluorescence intensity was
determined by flow cytometry with excitation at 490 nm and emission
at 520 nm.
Cell adhesion assay
The cell adhesion assay was conducted in 12-well
plates according to the method described previously (21). The wells were precoated with
fibronectin (Sigma-Aldrich, St. Louis, MO, USA) overnight at room
temperature. K562 cells were harvested and re-suspended in DMEM
containing 10% FBS; then, cells were added (2×105/well)
to each well and incubated at 37°C for 1 h. The wells were washed
twice with warm PBS to remove the unattached cells, and the
attached cells were fixed with methanol for 15 min and stained with
crystal violet (Sigma-Aldrich) for 20 min. Subsequent to staining,
the cells were observed using an optical BX51 microscope (Olympus,
Tokyo, Japan).
Western blot analysis
Following the above treatments, cells were harvested
and lysed in radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology) and protease inhibitor cocktail
(Sigma-Aldrich) for 10 min at 4°C. The protein concentration was
determined using Bicinchoninic Acid Protein Assay Reagent (Thermo
Fisher Scientific, Waltham, MA, USA). Subsequently, equal
quantities of denatured protein (20 μg) were separated on
10% SDS-PAGE gels (Beyotime Institute of Biotechnology), then
transferred to nitrocellulose membranes (Pall Corporation,
Pensacola, FL, USA) and incubated overnight at 4°C with a
cyclooxygenase 2 (COX-2, Abcam, Cambridge, MA, USA, cat. no.
Ab62331, 1:500), receptor for advanced glycation end products
(RAGE, Abcam, cat. no. Ab54741, 1:1,000), Bax (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA, cat. no. Sc-493, 1:150) and
Bcl-2 (Santa Cruz Biotechnology, Inc., cat. no. Sc-492, 1:100)
monoclonal primary antibodies, followed by incubation with a goat
anti-mouse/horseradish peroxidase conjugated secondary antibody and
chemiluminescence detection (ECL; Millipore, Billerica, MA, USA).
To ensure equivalent protein loading, antibodies targeted against
GAPDH were used, and the protein expression levels were normalized
to GAPDH. The western blots presented in the figures are
representative of three independent experiments unless otherwise
indicated.
Statistical analysis
All data are presented as the mean ± standard
deviation of three determinations. For statistical analysis, Prism,
version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used.
P<0.05 was considered to indicate a statistically significant
difference.
Results
HMGB1 knockdown and cordycepin inhibit
the proliferation of K562 cells
In order to evaluate the effects of HMGB1 knockdown
with/without cordycepin treatment on the proliferation of K562
cells, the OD (450 nm) value of K562 cells was detected using the
CCK-8 assay. As presented in Table
I, the proliferation of K562 cells in the mock with cordycepin,
siRNA-HMGB1 and siRNA-HMGB1 with cordycepin groups was
significantly inhibited at 24, 48 and 72 h (P<0.01), when
compared with the mock group. The results of the present study
indicate that the knockdown of HMGB1 may significantly inhibit the
proliferation of K562 cells.
 | Table IEffect of HMGB1 knockdown on
proliferation of the K562 cell line. |
Table I
Effect of HMGB1 knockdown on
proliferation of the K562 cell line.
| Group | 0 h | 24 h | 48 h | 72 h |
|---|
| Mock | 0.317±0.0025 | 0.531±0.0062 | 0.839±0.0070 | 1.054±0.0269 |
| Mock +
cordycepin | 0.317±0.0055 |
0.476±0.0045a |
0.671±0.0060a |
0.838±0.0096a |
| siRNA-HMGB1 | 0.316±0.0055 |
0.460±0.0032a |
0.638±0.0096a |
0.771±0.0060a |
| siRNA-HMGB1 +
cordycepin | 0.315±0.0059 |
0.417±0.0053a |
0.561±0.0064a |
0.661±0.0064a |
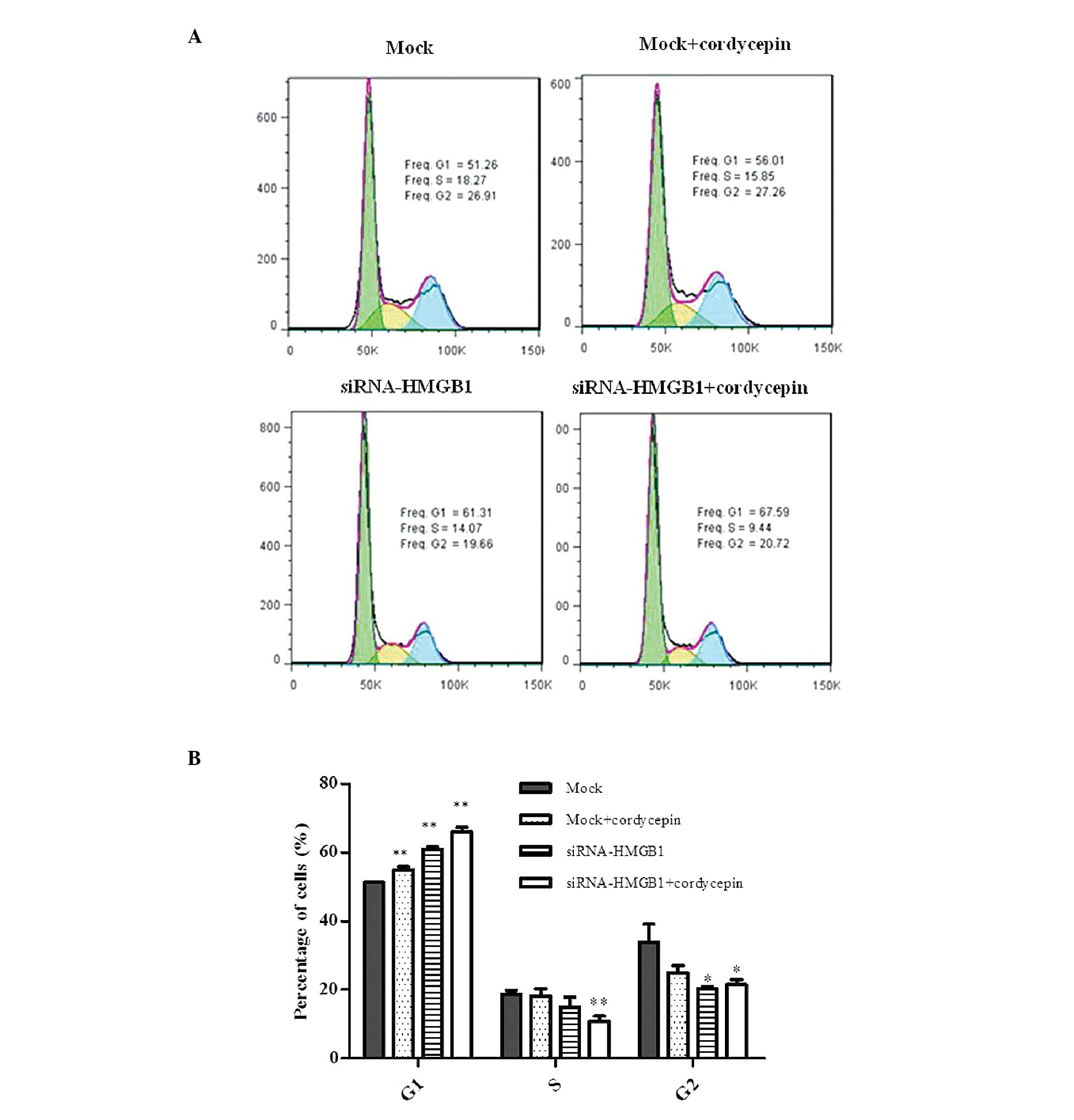
Cell cycle analysis demonstrated that the mock cells
treated with cordycepin, HMGB1 knockdown cells and HMGB1 knockdown
cells treated with cordycepin exhibited G1 arrest prior
to the appearance of apoptosis in K562 cells. The cells in the S
and G2 phases were additionally reduced compared with
the mock (Fig. 1A and B).
HMGB1 knockdown and cordycepin encourage
the apoptosis of K562 cells
To estimate the anti-proliferative effect of HMGB1
knockdown, cordycepin treatment and the combined action of HMGB1
knockdown + cordycepin in contributing to the inhibition of K562
cell apoptosis, flow cytometric analysis was performed. From the
results obtained, the mock cells treated with cordycepin, HMGB1
knockdown cells and HMGB1 knockdown cells treated with cordycepin
exhibited significantly increased apoptosis rates of K562 cells
compared with the mock (Fig.
2).
ROS generation was identified following
HMGB1 and cordycepin treatment
Excessive generation of ROS in the cell is also
shown to induce apoptosis (22).
The fluorescent probe DHE was used to determine the levels of ROS
production in K562 cells. As presented in Fig. 3, rapid generation of ROS was
detected following HMGB1 knockdown and cordycepin treatment.
HMGB1 knockdown and cordycepin inhibit
the adhesion of BGC-823 cells by downregulating RAGE levels
As presented in Fig. 4A
and B, cell adhesion was significantly inhibited, compared with
that in the mock group. The present study indicated that the
knockdown of HMGB1 was able to significantly suppress adhesion of
K562 cells. RAGE is a receptor for DAMP molecules and reacts in
particular with HMGB1 (23). RAGE
acts as an adhesion molecule and the expression level was observed
to be significantly downregulated; thus, suggesting that HMGB1
knockdown and treatment with cordycepin are able to inhibit
adhesion by downregulating RAGE expression (Fig. 4C and D).
HMGB1 knockdown and cordycepin inhibit
proliferation of K562 cells by downregulating COX-2 levels
COX-2 is a key enzyme in arachidonic acid
metabolism, which serves an essential role in cell proliferation
and apoptosis (24). As presented
in Fig. 5, the levels of COX-2
expression in normal cells were higher in cordycepin-treated cells,
HMGB1 knockdown cells and cordycepin-treated knockdown cells. The
result may indicate that HMGB1 and cordycepin inhibit proliferation
of K562 cells by downregulating COX-2 expression.
HMGB1 knockdown and cordycepin promote
K562 cell apoptosis by regulating apoptotic factors
In order to elucidate the mechanism of K562 cell
apoptosis induced by HMGB1, the expression levels of the
apoptosis-associated proteins, Bax and Bcl-2, were detected by
western blot analysis. Bax was identified to exhibit a positive
role on apoptosis, while Bcl-2 is an anti-apoptotic protein. Bax
was upregulated in cordycepin-treated cells, siRNA-HMGB1
plasmid-transfected cells and transfected cells seeded with
cordycepin, compared with mock cells. Conversely, Bcl-2 expression
was observed to be lower than that in the mock cells. The relative
value of Bax/Bcl-2 is presented in Fig. 6. The Bax/Bcl-2 value of knockdown
cells seeded with cordycepin was notably higher than that in the
mock group. These results indicated that HMGB1 knockdown and
cordycepin treatment promoted K562 cell apoptosis by regulating
apoptotic factors.
Discussion
Leukemia is a malignant hematopoietic disorder
characterized by the clonal proliferation of hematopoietic stem
cells and immature myeloid precursors. Accompanied with complex
symptoms, difficulty in treatment and a poor prognosis, leukemia is
a great threat to a patients' health and survival chances.
Treatment of leukemia predominantly consists of chemotherapy,
radiotherapy, bone marrow transplantation or stem cell
transplantation and targeted therapy (25). Molecular therapy and treatment
using natural products for CML are currently areas of research
focus. HMGB1, a protein expressed abundantly in CML cells, has been
reported to serve a critical role in CML development and
progression (14), while
cordycepin has been demonstrated to exhibit antileukemia properties
(26). The current study
investigated the effects on proliferation, apoptosis, ROS levels
and adhesion following HMGB1 knockdown, cordycepin treatment and a
combination of the two in order to provide a theoretical basis for
early clinical diagnosis and intervention.
In the current study, the combination of HMGB1
knockdown and cordycepin treatment was observed to effectively
suppress the proliferation of K562 human CML cells. The impact of
HMGB1 knockdown with cordycepin on the cell cycle demonstrated that
HMGB1, cordycepin and their combination arrested the cell cycle at
G1 phase. Previous studies have indicated that COX-2
expression activates certain signaling pathways that control
proliferation and apoptosis (27–29).
The expression of COX-2 in the experimental group was downregulated
compared with the mock group, which suggested that HMGB1 knockdown
and cordycepin inhibited cell proliferation via the downregulation
of COX-2 expression.
In addition, the present study indicated that
knockdown of HMGB1 significantly inhibited the adhesion of K562
cells. The combination of HMGB1 knockdown and cordycepin was
observed to exhibit improved action compared with either treatment
alone. RAGE is a predominant receptor in mediating the effects of
HMGB1 (30). It has been reported
that RAGE evolved from a cell adhesion molecule family and acts as
an adhesion molecule in mammalian cells (31). The HMGB1 knockdown and
cordycepin-treated groups exhibited downregulated expression of
RAGE compared with the mock group. The interaction of HMGB1 with
RAGE can influence adhesion in HMGB1 knockdown and
cordycepin-treated cells.
Apoptosis, the process of programmed cell death, is
the common mechanism that chemotherapies, cytotoxic agents or
radiation therapies target to induce cancer cell death. HMGB1
knockdown, cordycepin and their combination increase K562 cellular
apoptosis. The combination of HMGB1 knockdown and cordycepin
induced apoptosis according to the flow cytometric analysis, with a
greater significance than either treatment alone. Bax and Bcl-2 are
proteins that serve important roles in cell apoptosis (32,33).
Evaluation of the expression levels of apoptotic proteins Bax and
Bcl-2 further confirmed the conclusion that the combination of
HMGB1 knockdown and cordycepin synergistically induced cellar
apoptosis. The levels of ROS were additionally observed to increase
in the treatment groups. ROS are free radicals, for example
O2−, OH, H2O2 and
1O2, which have numerous effects on signal
transduction. ROS production and consequential oxidative stress
have been previously implicated in cellular apoptosis (34). In the current study, the ROS levels
were observed to be significantly increased in the HMGB1 knockdown
and cordycepin groups, which may be the cause of the increased
apoptosis. The above results indicated that HMGB1 knockdown
promoted the apoptosis of K562 cells by regulating apoptotic
factors and the ROS levels, and that combination therapy with
cordycepin resulted in increased apoptosis.
In conclusion, the current study identified that
HMGB1 knockdown in combination with cordycepin resulted in increase
apoptosis, decreased proliferation, and reduced adhesion of human
CML K562 cells, as well as increased ROS, and the corresponding
mechanisms were investigated. These observations may provide
insight towards fully elucidating the molecular mechanism of CML
progression and pathogenesis, thus benefiting the development of
therapeutic strategies for the disease.
References
|
1
|
Druker BJ: STI571 (Gleevec) as a paradigm
for cancer therapy. Trends Mol Med. 8(Suppl 4): S14–S18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Melo JV, Hughes TP and Apperley JF:
Chronic myeloid leukemia. Hematology (Am Soc Hematol Educ Program).
2003:132–152. 2003. View Article : Google Scholar
|
|
3
|
Baran Y, Salas A, Senkal CE, Gunduz U,
Bielawski J, Obeid LM and Ogretmen B: Alterations of
ceramide/sphingosine 1-phosphate rheostat involved in the
regulation of resistance to imatinib-induced apoptosis in K562
human chronic myeloid leukemia cells. J Biol Chem. 282:10922–10934.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cagnetta A, Garuti A, Marani C, et al:
Evaluating treatment response of chronic myeloid leukemia: Emerging
science and technology. Curr Cancer Drug Targets. 13:779–790. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji J, Wang HS, Gao YY, Sang LM and Zhang
L: Synergistic antitumor effect of KLF4 and curcumin in human
gastric carcinoma cell line. Asian Pac J Cancer Prev. 15:7747–7752.
2014. View Article : Google Scholar
|
|
6
|
Süren D, Yıldırım M, Demirpençe Ö, Kaya V,
Alikanoğlu AS, Bülbüller N, Yıldız M and Sezer C: The role of high
mobility group box 1 (HMGB1) in colorectal cancer. Med Sci Monit.
20:530–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueda M, Takahashi Y, Shinden Y, Sakimura
S, Hirata H, Uchi R, Takano Y, Kurashige J, Iguchi T, Eguchi H, et
al: Prognostic significance of high mobility group box 1 (HMGB1)
expression in patients with colorectal cancer. Anticancer Res.
34:5357–5362. 2014.PubMed/NCBI
|
|
8
|
Sims GP, Rowe DC, Rietdijk ST, Herbst R
and Coyle AJ: HMGB1 and RAGE in inflammation and cancer. Annu Rev
Immunol. 28:367–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang D, Kang R, Zeh HJ III and Lotze MT:
High-mobility group box 1 and cancer. Biochim Biophys Acta.
1799:131–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu Y, Xie M, He YL, Xu WQ, Zhu S and Cao
LZ: Role of high mobility group box 1 in adriamycin-induced
apoptosis in leukemia K562 cells. Ai Zheng. 27:929–933. 2008.In
Chinese. PubMed/NCBI
|
|
11
|
Wild CA, Brandau S, Lotfi R, Mattheis S,
Gu X, Lang S and Bergmann C: HMGB1 is overexpressed in tumor cells
and promotes activity of regulatory T cells in patients with head
and neck cancer. Oral Oncol. 48:409–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Curtin JF, Liu N, Candolfi M, Xiong W,
Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, et
al: HMGB1 mediates endogenous TLR2 activation and brain tumor
regression. PLoS Med. 6:e102009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Tian J and Hao Q: HMGB1 combining
with tumor-associated macrophages enhanced lymphangiogenesis in
human epithelial ovarian cancer. Tumour Biol. 35:2175–2186. 2014.
View Article : Google Scholar
|
|
14
|
Zhao M, Yang M, Yang L, et al: HMGB1
regulates autophagy through increasing transcriptional activities
of JNK and ERK in human myeloid leukemia cells. BMB Rep.
44:601–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura K, Yoshikawa N, Yamaguchi Y,
Kagota S, Shinozuka K and Kunitomo M: Antitumor effect of
cordycepin (3′-deoxyadenosine) on mouse melanoma and lung carcinoma
cells involves adenosine A3 receptor stimulation. Anticancer Res.
26:43–47. 2006.PubMed/NCBI
|
|
16
|
Zhou X, Meyer CU, Schmidtke P and Zepp F:
Effect of cordycepin on interleukin-10 production of human
peripheral blood mononuclear cells. Eur J Pharmacol. 453:309–317.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koç Y, Urbano AG, Sweeney EB and McCaffrey
R: Induction of apoptosis by cordycepin in ADA-inhibited
TdT-positive leukemia cells. Leukemia. 10:1019–1024.
1996.PubMed/NCBI
|
|
18
|
Chen YH, Wang JY, Pan BS, Mu YF, Lai MS,
So EC, Wong TS and Huang BM: Cordycepin enhances cisplatin
apoptotic effect through caspase/MAPK pathways in human head and
neck tumor cells. Onco Targets Ther. 6:983–998. 2013.PubMed/NCBI
|
|
19
|
Jeong MH, Lee CM, Lee SW, Seo SY, Seo MJ,
Kang BW, Jeong YK, Choi YJ, Yang KM and Jo WS: Cordycepin-enriched
Cordyceps militaris induces immunomodulation and tumor growth delay
in mouse-derived breast cancer. Oncol Rep. 30:1996–2002.
2013.PubMed/NCBI
|
|
20
|
Zhang P, Huang C, Fu C, Tian Y, Hu Y, Wang
B, Strasner A, Song Y and Song E: Cordycepin (3′-deoxyadenosine)
suppressed HMGA2, Twist1 and ZEB1-dependent melanoma invasion and
metastasis by targeting miR-33b. Oncotarget. 6:9834–9853.
2015.PubMed/NCBI
|
|
21
|
Yao Z and Shulan Z: Inhibition effect of
Guizhi-Fuling-decoction on the invasion of human cervical cancer. J
Ethnopharmacol. 120:25–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar
|
|
23
|
Herold K, Moser B, Chen Y, et al: Receptor
for advanced glycation end products (RAGE) in a dash to the rescue:
Inflammatory signals gone awry in the primal response to stress. J
Leukoc Biol. 82:204–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grösch S, Tegeder I, Niederberger E,
Bräutigam L and Geisslinger G: COX-2 independent induction of cell
cycle arrest and apoptosis in colon cancer cells by the selective
COX-2 inhibitor celecoxib. FASEB J. 15:2742–2744. 2001.PubMed/NCBI
|
|
25
|
Hirji I, Gupta S, Goren A, et al: Chronic
myeloid leukemia (CML): Association of treatment satisfaction,
negative medication experience and treatment restrictions with
health outcomes, from the patient's perspective. Health Qual Life
Outcomes. 11:1672013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong JW, Jin CY, Park C, et al: Induction
of apoptosis by cordycepin via reactive oxygen species generation
in human leukemia cells. Toxicol In Vitro. 25:817–824. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan XL, Chen L, Li MX, et al: Elevated
expression of Foxp3 in tumor-infiltrating Treg cells suppresses
T-cell proliferation and contributes to gastric cancer progression
in a COX-2-dependent manner. Clin Immunol. 134:277–288. 2010.
View Article : Google Scholar
|
|
28
|
Möbius C, Stein HJ, Spiess C, Becker I,
Feith M, Theisen J, Gais P, Jütting U and Siewert JR: COX2
expression, angiogenesis, proliferation and survival in Barrett's
cancer. Eur J Surg Oncol. 31:755–759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sánchez-Fidalgo S, Martín-Lacave I,
Illanes M and Motilva V: Angiogenesis, cell proliferation and
apoptosis in gastric ulcer healing. Effect of a selective cox-2
inhibitor. Eur J Pharmacol. 505:187–194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okuma Y, Liu K, Wake H, et al: Anti-high
mobility group box-1 antibody therapy for traumatic brain injury.
Ann Neurol. 72:373–384. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sessa L, Gatti E, Zeni F, et al: The
receptor for advanced glycation end-products (RAGE) is only present
in mammals and belongs to a family of cell adhesion molecules
(CAMs). PLoS One. 9:e869032014. View Article : Google Scholar
|
|
32
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hoshyar R, Bathaie SZ and Sadeghizadeh M:
Crocin triggers the apoptosis through increasing the Bax/Bcl-2
ratio and caspase activation in human gastric adenocarcinoma, AGS,
cells. DNA Cell Biol. 32:50–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang L, Wang P, Wang H, et al: Fucoidan
derived from Undaria pinnatifida induces apoptosis in human
hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated
mitochondrial pathway. Mar Drugs. 11:1961–1976. 2013. View Article : Google Scholar : PubMed/NCBI
|