Introduction
Bone is the most common organ for advanced-stage
solid tumor metastasis, particularly those arising from the breast,
prostate and multiple myeloma (1).
Bone metastases disrupts skeletal metabolism and results in
potentially debilitating or life-limiting skeletal-associated
events, including intractable, chronic bone pain (2). Several approaches have been suggested
to treat advanced-stage solid tumors and skeletal complications,
including radiation therapy, chemotherapy, bisphosphonates and
analgesia (3). Using these
strategies, previous studies have demonstrated that zoledronic
acid, a third-generation bisphosphonate, improves the control of
bone pain in patients with cancer (4,5).
Paclitaxel, a chemotherapeutic drug, reduces the growth of
osteolytic lesions and exhibits anti-tumor and anti-resorptive
effects in bone metastases in rat models (6). These effects may also reduce bone
pain in patients with cancer.
At present, the anti-nociceptive effect of
paclitaxel of bone metastases remains to be fully elucidated. In
addition, due to the combined use of opioid analgesics or
non-steroidal anti-inflammatory drugs, the anti-nociceptive effect
of zoledronic acid and paclitaxel for metastatic bone pain is often
masked and, therefore, remains unclear. Therefore, it is of
interest to investigate the respective analgesic effects of
zoledronic acid and paclitaxel in bone metastases.
Previous studies have demonstrated that the Type I
collagen-crosslinked N telopeptide (NTx) is a bone absorption
marker for the occurrence and progression of bone metastases, which
can be used to predict the prognosis of bone disease (7,8).
Therefore, the present study aimed to investigate the correlation
between urinary levels of NTx and the anti-nociceptive responses of
zoledronic acid and paclitaxel in bone metastases using a rat
model, and to examine the potential underlying mechanisms.
Materials and methods
Preparation of cells
Walker 256 rat mammary gland carcinoma cells were
provided by the Laboratory of the Department of Anesthesiology in
Tongji Hospital (Huazhong University of Science and Technology,
Wuhan, China). The cells (1×106/ml) were cultured in
RPMI 1640 medium (Gibco Life Technologies, Carlsbad, CA, USA),
supplemented with 10% fetal bovine serum and 2%
penicillin/streptavidin in a humidified atmosphere of 5%
CO2. The cells were collected by centrifugation for 3
min at 240 × g, rinsed with calcium-and magnesium-free Hank's
solution, counted with a hemocytometer (DHC-N01; Incyto, Cheonan,
Korea) and re-centrifuged under the same conditions. The cells were
diluted to a final concentration for femoral inoculation of
105 cells in 10 µl Hank's solution, and were
maintained on ice prior to surgery.
Animals and induction of bone
metastases
Female Wistar rats 7 weeks-old, weighing 220-250 g
(n=8/group; Experimental Animal Research Center, Wuhan, China;
certificate no. SCXK E 2008-0005) were housed in a specific
pathogen-free room (22±0.5°C; 12-h light/dark cycle; food and water
ad libitum). All experiments were performed in accordance
with the ethical guidelines of the National Institutes of Health
(9), and the present study was
approved by the ethics committee of Soochow University (Suzhou,
China).
The surgical procedure for the induction of bone
metastases was the same as that reported previously by Gui et
al. Briefly, the rats were fully anesthetized with 6% chloral
hydrate (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and
laid in the prone position. Subsequent to shaving and disinfection
of the left leg, a 1 cm-long incision was made over the third
trochanter and rectus femoris muscle along the medical edge, to
expose the shaft of the femur. A needle was then inserted
vertically into the shaft of femur to reach the intramedullary
canal of the femur. The needle was then replaced with a
micro-injection syringe containing 10 µl Walker 256 cells
(105 cells) or Hank's solution (sham group). Following
slow injection and a 2 min delay to allow cell dispersion within
the bone marrow, the syringe was removed and the drill hole was
sealed using bone wax (Friends of Shanghai Medical Devices Co.,
Ltd., Shanghai, China). The site was thoroughly washed with sterile
de-ionized water. The muscle and skin were finally stitched and
disinfected.
Drug administration
The rats were randomly divided into six groups (8
rats/group). The first two groups were the healthy control group
(naive) and surgical procedure control group (sham). In the
remaining four groups, the animals were allowed to develop bone
metastasis for 10 days following the implantation of Walker 256
cells. The groups were defined, as follows: Cancer control
(no-therapy); cancer with NS (intraperitoneal injection of normal
saline on day 10); cancer with ZOL, involving subcutaneous
administration of 0.1 mg/kg zoledronic acid (Zometa®;
Novartis Pharma Schweiz AG, Rotkreuz, Switzerland), on day 10; and
cancer with TA, involving intra-peritoneal injection of 5 ml, 30 mg
and 2 mg/kg paclitaxel (Taxol®; Bristol-Myers Squibb
S.R.L., Rome, Italy), on day 10.
Mechanical allodynia
Mechanical allodynia was assessed using a dynamic
plantar aesthesiometer (21025; Ugo Basile S.R.L., Comerio, Italy)
as described previously (10),
which is an automated von Frey-type system. Briefly, the rats were
placed individually into elevated wire mesh bottomed enclosures,
and a straight metal filament was pushed against the hind paw with
increasing force. When the animal withdrew its hind paw or the
preset cut-off was reached (50 g), the force was automatically
registered in grams and the filament was automatically removed.
Measurements of urinary Type I
collagen-crosslinked NTx
Bone resorption was assessed by measuring the levels
of urinary NTx, associated with creatinine (nmol/mmol/creatinine).
The rats were placed in metabolic cages each week to collect 24 h
urine. Freshly collected urine was centrifuged at 670 × g for 5
min. The supernatant was frozen at −80°C until required for
analysis of the NTx levels using a commercially available
enzyme-linked immunosorbent assay (ELISA) kit (Rat cross linked
N-telopeptide of type I collegan, NTX ELISA kit; cat. no.
CSB-E09243r; Cusabio Biotech Co., Ltd., Wuhan, China), according to
the manufacturer's instructions. Each sample was assayed in
duplicate.
Histological analysis
The mice were sacrificed by CO2
inhalation, and the femoral bones were dissected and the specimens
were fixed in 4% paraformaldehyde for 2 days and decalcified in 10%
EDTA for 3 weeks. Subsequent to embedding in paraffin, the sections
were cut into 4 µm-sections and stained with Harris'
hematoxylin and eosin (H&E; Beyotime Institute of
Biotechnology, Haimen, China). The femoral sections were then
subjected to staining for tartrate-resistant acid phosphatase
(TRAP; Nanjing Jiancheng Bioengineering Institute, Nanjing, China),
according to the manufacturer's instructions. TRAP-positive cells
were observed under an inverted microscope (IX53; Olympus
Corporation, Tokyo, Japan) and five randomly selected fields were
analyzed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The total RNA was extracted from the L4–6 spinal
cord and dorsal root ganglion (DRG) using TRIzol reagent (Takara
Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's instructions. Total RNA (2 µg) was used for
RT to synthesize cDNA using SuperScriptase III (Thermo Fisher
Scientific, Burlington, ON, Canada) with random primers. To detect
the mRNA expression levels of c-fos, TRPV1 and ASIC3, a SYBR Green
method (SYBR® Green PCR Master Mix; Thermo Fisher
Scientific) was used with the following primers: C-fos, forward TGC
CAA TCT ACT GAA AGA GA and reverse TCCAGGGAGGTCACAGAC; TRPV1,
forward GGAAGACAGACAGCCTGA and reverse ATCTGCTCCATTCTCCAC; and
ASIC3, forward AATACCGCATCTTTGGAT and reverse CACATAGCGAGACTCACAG.
RT-qPCR analysis of gene expression was performed using an ABI 7900
Sequence Detector (Applied Biosystems Life Technologies, Foster
City, CA, USA). The thermal cycling conditions included 40 cycles
of 95°C for 15 sec and 55°C for 1 min. The relative value of target
mRNA expression was normalized to the expression of GAPDH,
calculated using the 2ΔΔCT method and compared with the
control group.
Western blot analysis
L4–6 spinal cord tissues were collected from each
group of animals. The total protein of the ipsilateral spinal cords
was extracted using radioimmunoprecipitation assay protein lysis
buffer (Beyotime Institute of Biotechnology). Protein concentration
was determined using a bicinchoninic acid assay kit (Beyotime
Institute of Biotechnology). Equal quantities of protein (200
µg) were fractionated using SDS-PAGE (10%; Beyotime
Institute of Biotechnology), transferred onto polyvinylidene
difluoride membranes (Merck Millipore, Darmstadt, Germany) and then
blocked with 10% non-fat dry milk for 3 h at room temperature.
Primary antibodies for c-fos (cat. no. sc-52), TRPV1 (cat. no.
sc-28759; polyclonal rabbit anti-rat; 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and GAPDH (monoclonal rabbit
anti-rat; 1:10,000; Epitomics, Burlingame, CA, USA) were incubated
overnight at 4°C, followed by probing for 2 h at room temperature
with an anti-rabbit horseradish peroxidase-conjugated secondary
antibody (1:5,000; Santa Cruz Biotechnology, Inc.). Finally, the
immunoreactive bands were visualized in enhanced chemiluminescence
solution (Pierce Biotechnology, Inc., Rockford, IL, USA) and
exposed onto X-ray films.
Statistical analysis
SPSS software, version 12.0 (SPSS, Inc., Chicago,
IL, USA) was used to perform statistical analysis. One-way analysis
of variance, followed by Bonferroni's post-hoc test, was used for
statistical comparison among the various groups. P<0.05 was
considered to indicate a statistically significant difference
(Eastman Kodak, Rochester, NY, USA).
Results
Zoledronic acid and paclitaxel attenuate
mechanical allodynia
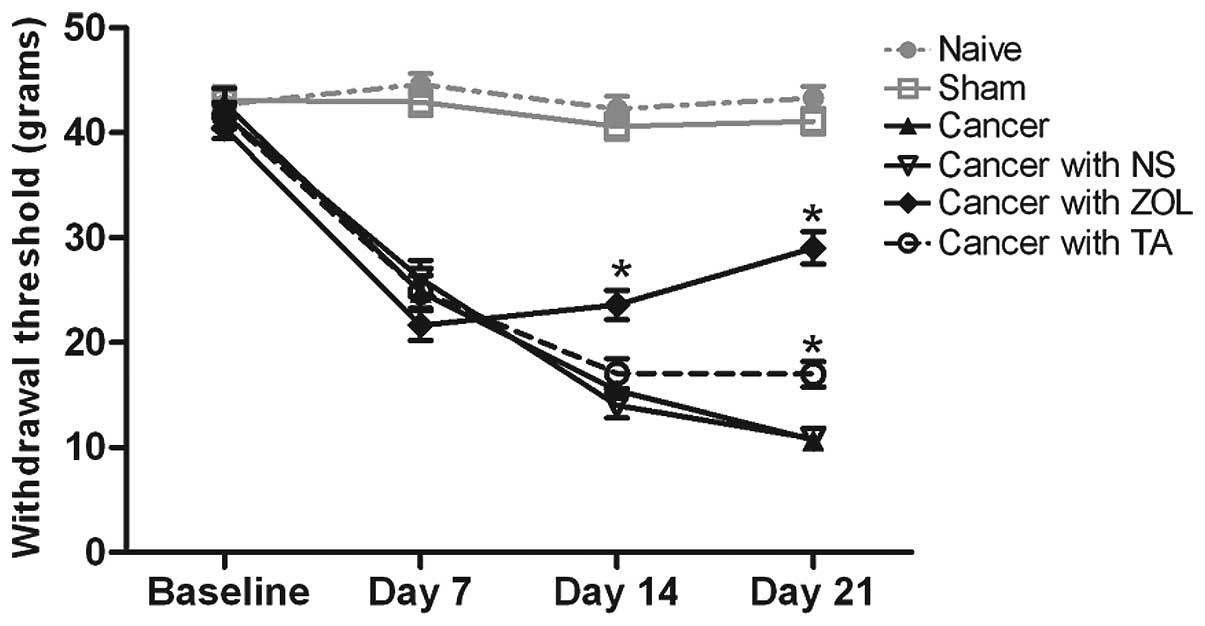
Significant reductions in the withdrawal thresholds
of the dynamic von Frey test (indicating allodynia) were observed
by day 7 following intrafemur inoculation of the Walker 256 rat
mammary gland carcinoma cells (P<0.05; Fig. 1). Compared with the cancer group
and cancer with NS group following the administration of zoledronic
acid on day 10 post-implantation, the mechanical allodynia of the
rats were significantly attenuated from day 14 onwards (P<0.05).
In the cancer with TA group, the rats exhibited a reversal of the
altered withdrawal response on day 21, whereas a significant
reduction in allodynia was observed in the cancer with ZOL group
(P<0.05).
Correlation between the urinary levels of
NTx and the anti-nociceptive responses of zoledronic acid and
paclitaxel
Analyses of the urinary levels of NTx from each
group of rats is shown in Fig. 2.
At baseline and day 7, no significant difference were observed in
the urinary levels of NTx among each group (P=0.824). At day 14
post-inoculation of cancer cells (day 4 post-administration), the
urinary levels of NTx were significantly increased in the cancer
group, cancer with NS group and cancer with TA group, compared with
the naive and sham groups (P<0.05), whereas no significant
differences were observed between the urinary levels of NTx in the
cancer with ZOL group and the naive or sham groups. In the cancer
with TA group, the urinary level of NTx was significantly reduced,
compared with the cancer group and cancer with NS group on day 21
post-implantation (day 11 post-administration). Collectively, these
results suggested that the reduced effect of zoledronic acid on
urinary levels of NTx occurred earlier than that of paclitaxel.
Furthermore, a positive correlation was observed between the
anti-nociceptive responses of zoledronic acid and paclitaxel and
the urinary levels of NTx, which was statistically significant
(correlation coefficient=0.619; P<0.001).
Histological alterations in tumor growth
following zoledronic acid and paclitaxel treatment
The bones in the naive and sham control groups
exhibited no infiltration of the bone marrow space by malignant
tumors. On day 14 post-tumor cell inoculation (day 4
post-administration of saline, zoledronic acid or paclitaxel), all
the rats, which were injected with Walker 256 cells developed
tumors, which were visible in the cancer, cancer with NS, cancer
with ZOL and cancer with TA groups (Fig. 3). The extent of bone marrow
replacement by the tumor in each cancer group was similar (red
dotted line). However, in the cancer with ZOL group, more residual
trabecular bones within the tumor (yellow arrow) were observed,
compared with the cancer, cancer with NS and cancer with TA
groups.
Zoledronic acid treatment significantly
reduces osteoclast numbers
The femur sections on day 14 post-tumor cell
inoculation (day 4 post-administration of saline, zoledronic acid
or paclitaxel), were stained for TRAP to identify osteoclasts
(Fig. 4). Few TRAP-positive cells
were observed in the naïve and sham groups, compared with which,
the number of osteoclasts in each cancer group was increased
significantly (P<0.05). ZOL treatment resulted in significantly
fewer osteoclasts, compared with the cancer group, cancer with NS
group and cancer with TA group (P<0.05).
Zoledronic acid and paclitaxel
significantly reduce the relative mRNA expression levels of c-fos
in the spinal cord and ASIC3 in the DRG
In order to evaluate the effects of zoledronic acid
and paclitaxel on spinal nerve activity, the mRNA levels of c-fos
and TRPV1 in the ipsilateral spinal cord, and ASIC3 in the
ipsilateral DRG were assessed on days 14 and 21 post-tumor cell
inoculation. As shown in Fig. 5,
the relative mRNA levels of c-fos and TRPV1 in the ipsilateral
spinal cord and of ASIC3 in the ipsilateral DRG in the cancer,
cancer with NS, cancer with ZOL and cancer with TA groups were
significantly increased, compared with the naive and sham groups
(P<0.05). Compared with the cancer group and cancer with NS
group, the mRNA levels of c-fos in the spinal cord and of ASIC3 in
the DRG in the cancer with ZOL group were significantly reduced on
days 14 and 21 post-inoculation (P<0.05). This reduction in the
expression of c-fos was observed in the cancer with TA group on day
21 post-inoculation. The mRNA expression of TRPV1 in the spinal
cord was not significantly different among the cancer groups.
Zoledronic acid and paclitaxel
significantly reduce the protein expression of c-fos in the spinal
cord
Western blot analysis (Fig. 6) was performed using tissues of rat
ipsilateral spinal cord on days 14 and 21 post-tumor cell
inoculation, which expressed c-fos and TRPV1 mRNA, as described
above. The protein expression of GAPDH was used as an internal
control. The results demonstrated that the protein expression
levels of c-fos and TRPV1 in the spinal cord were significantly
upregulated following tumor cell inoculation, compared with the
naive and sham groups (P<0.05). The increase in the protein
expression of c-fos was attenuated following treatment with
zoledronic acid and paclitaxel on day 11 post-administration (day
21 post-tumor cell inoculation). The protein expression of TRPV1 in
spinal cord did not differ amongst the cancer groups.
Discussion
The chemotherapy-induced peripheral neuropathic pain
of paclitaxel has been extensively discussed (11–13).
The results of the present study indicated for the first time, to
the best of our knowledge, that paclitaxel alleviated the pain
induced by bone cancer in a rat model. The results may be due to
the fact that the paclitaxel dose (2 mg/kg, once on day 14) was
lower than those previously used in chemotherapy-induced peripheral
neuropathy investigations (2 mg/kg, every day or every other day
for 4–5 injections in total) (11,14–16).
Previous studies have demonstrated that the incidence and severity
of chemotherapy-induced peripheral neuropathy of the majority of
chemotherapeutic agents, including paclitaxel, are dependent on the
cumulative dose of the drug (17,18).
In the present study, the anti-nociceptive effects in the
paclitaxel group were weaker and of later-onset than those in the
zoledronic acid group.
NTx is a type I collagen, which is the only collagen
present in bone tissue and accounts for 90% of the bone matrix
(19). Previous studies have
demonstrated that the NTx originated separately from type I
collagen and formed a new epitope in the stage of bone resorption,
exhibiting high specificity (19,20).
NTx is a direct product of osteoclasts (21). In the present study, the
anti-nociceptive responses of zoledronic acid and paclitaxel were
positively correlated with urinary levels of NTx in the rats. In
order to analyze the possible mechanism of this correlation,
H&E and TRAP staining of the femoral sections were performed in
each group. The extent of infiltration of the bone marrow space by
the tumor in the cancer with ZOL group and cancer with TA group did
not differ significantly from the cancer control group in the
H&E-stained sections. However, in the cancer with ZOL group,
more residual trabecular structural elements were observed than in
the cancer group. In addition, TRAP staining revealed that the
number of osteoclasts present in the cancer with ZOL group was
significantly reduced compared with the cancer, cancer with NS and
cancer with TA groups. In the cancer with TA group, the number of
osteoclasts was similar to that in the cancer group. The results of
the present study suggested that alterations in the urinary levels
of NTx and the bone pain responses may have been due to the
activity of the osteoclasts in the cancer with ZOL group. However,
the causes of the changes in the urinary levels of NTx and the bone
pain responses in the cancer with TA group cannot be determined
from these data. A possible explanation may be a secondary
anti-tumor response, although the anti-tumor effects did not result
in differences in tumor spaces between the cancer with TA group and
the cancer control group.
The present study also analyzed the mRNA expression
levels of c-fos and TRPV1 in the spinal cord and ASIC3 in the DRG.
The c-fos protein is the product of the immediate-early gene, which
is rapidly expressed in neurons following various types of noxious
stimuli (22). In addition,
upregulation of the c-fos protein has been previously used as a
marker for the activation of nociceptive pathways in the spinal
cord following peripheral nerve injury (23). In the present study, it was
demonstrated that the spinal mRNA expression of c-fos in the ZOL
group was reduced more rapidly (4 days post-treatment), compared
with that of the cancer with TA group (11 days post-treatment).
This suggested that zoledronic acids rapidly relieved peripheral
nociceptive injury in bone metastasis, which may have been a
consequence of the direct interaction between zoledronic acid and
osteoclasts. In addition, the effects of paclitaxel on the
remission of noxious stimuli were of later onset, which may have
resulted from a secondary anti-tumor response.
ASIC3 is activated by reductions in pH and is
important in musculoskeletal pain (24). The present study demonstrated that
the spinal mRNA expression of ASIC3 in the ZOL group was reduced
more rapidly (4 days post-treatment), compared with the control,
suggesting that zoledronic acid may also alter the local acid
environment of bone, which can reduce the activation of neurons and
further relieve pain.
The TRPV1 channel is voltage sensitive and is
activated by acidic pH, the exogenous irritant, capsaicin, and
endogenous molecules (25). TRPV1
is also sensitized by a wide range of pro-inflammatory agents,
including bradykinin, nerve growth factor, adenosine triphosphate
and the chemokines (26). There
possibility of cross-talk between the various activators of TRPV1
requires consideration. The results of the present study revealed
no significant differences in the mRNA and protein TRPV1 expression
levels in the ZOL and TA groups, compared with the control group.
This suggested that the treatment outcome of zoledronic acid or
paclitaxel on bone cancer pain is not the result of osteoclasts, an
acid environment or tumor progression alone, and that various
factors are involved in bone cancer pain, which may interact and
offset each other.
In conclusion, the present study demonstrated that a
low dose paclitaxel was observed led to a weaker and delayed
anti-nociceptive effect on bone cancer pain, compared with
zoledronic acid. Zoledronic acid may directly interact with
osteoclasts and alter the local acidic environment of bone, which
results in rapid relief from peripheral nociceptive injury in bone
metastasis. In addition, urinary levels of NTx may predict the
anti-nociceptive responses of zoledronic acid and paclitaxel in rat
models of bone metastases.
References
|
1
|
Jimenez-Andrade JM, Mantyh WG, Bloom AP,
Ferng AS, Geffre CP and Mantyh PW: Bone cancer pain. Ann N Y Acad
Sci. 1198:173–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Middlemiss T, Laird BJ and Fallon MT:
Mechanisms of cancer-induced bone pain. Clin Oncol (R Coll Radiol).
23:387–392. 2011. View Article : Google Scholar
|
|
3
|
Fitch M, Maxwell C, Ryan C, Löthman H,
Drudge-Coates L and Costa L: Bone metastases from advanced cancers:
Clinical implications and treatment options. Clinical J Oncol Nurs.
13:701–710. 2009. View Article : Google Scholar
|
|
4
|
De Luca A, Lamura L, Gallo M, Daniele G,
D'Alessio A, Giordano P, et al: Pharmacokinetic evaluation of
zoledronic acid. Expert Opin Drug Metab Toxicol. 7:911–918. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoh K, Kubota K, Ohmatsu H, Goto K, Niho S
and Ohe Y: Feasibility study of zoledronic acid plus
cisplatin-docetaxel as first-line treatment for advanced non-small
cell lung cancer with bone metastases. Anticancer Res.
32:4131–4135. 2012.PubMed/NCBI
|
|
6
|
Merz M, Komljenovic D, Zwick S, Semmler W
and Bäuerle T: Sorafenib tosylate and paclitaxel induce
anti-angiogenic, anti-tumour and anti-resorptive effects in
experimental breast cancer bone metastases. Eur J Cancer.
47:277–286. 2011. View Article : Google Scholar
|
|
7
|
Coleman RE, Major P, Lipton A, et al:
Predictive value of bone resorption and formation markers in cancer
patients with bone metastases receiving the bisphosphonate
zoledronic acid. J Clin Oncol. 23:4925–4935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi S: Bone metabolic markers for
evaluation of bone metastases. Clin Calcium. 23:391–400. 2013.In
Japanese. PubMed/NCBI
|
|
9
|
Kobayashi S: Public health for scientific
study of society and health (11). Keypoints in ethical guidelines
for medical research. Nihon Koshu Eisei Zasshi. 58:909–912.
2011.
|
|
10
|
Doré-Savard L, Otis V, Belleville K, et
al: Behavioral, medical imaging and histopathological features of a
new rat model of bone cancer pain. PLoS One. 5:e137742010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nieto FR, Cendán CM, Sánchez-Fernández C,
Cobos EJ, Entrena JM, Tejada MA, Zamanillo D, Vela JM and Baeyens
JM: Role of sigma-1 receptors in paclitaxel-induced neuropathic
pain in mice. J Pain. 13:1107–1121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao M, Yan X and Weng HR: Inhibition of
glycogen synthase kinase 3β activity with lithium prevents and
attenuates paclitaxel-induced neuropathic pain. Neuroscience.
254:301–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reeves BN, Dakhil SR, Sloan JA, et al:
Further data supporting that paclitaxel-associated acute pain
syndrome is associated with development of peripheral neuropathy:
North Central Cancer Treatment Group trial N08C1. Cancer.
118:5171–5178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruiz-Medina J, Baulies A, Bura SA and
Valverde O: Paclitaxel-induced neuropathic pain is age dependent
and devolves on glial response. Eur J Pain. 17:75–85. 2013.
View Article : Google Scholar
|
|
15
|
Choi SS, Koh WU, Nam JS, Shin JW, Leem JG
and Suh JH: Effect of ethyl pyruvate on Paclitaxel-induced
neuropathic pain in rats. Korean J Pain. 26:135–141. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Boyette-Davis JA, Kosturakis AK,
Li Y, Yoon SY, Walters ET and Dougherty PM: Induction of monocyte
chemoattractant protein-1 (MCP-1) and its receptor CCR2 in primary
sensory neurons contributes to paclitaxel-induced peripheral
neuropathy. J Pain. 14:1031–1044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brzeziński K: Chemotherapy-induced
polyneuropathy. Part I. Pathophysiology Contemp Oncol (Pozn).
16:72–78. 2012.
|
|
18
|
Grisold W, Cavaletti G and Windebank AJ:
Peripheral neuropathies from chemotherapeutics and targeted agents:
diagnosis, treatment and prevention. Neuro Oncol. 14(Suppl 4):
iv45–iv54. 2012. View Article : Google Scholar :
|
|
19
|
Hanson DA, Weis MA, Bollen AM, Maslan SL,
Singer FR and Eyre DR: A specific immunoassay for monitoring human
bone resorption: Quantitation of type I collagen cross-linked
N-telopeptides in urine. J Bone Miner Res. 7:1251–1258. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jablonka F, Schindler F, Lajolo PP,
Pinczowski H, Fonseca FL, Barbieri A, Massonetto LH, Katto FT and
Del Giglio A: Serum cross-linked n-telopeptides of type 1 collagen
(NTx) in patients with solid tumors. Sao Paulo Med J. 127:19–22.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Joerger M and Huober J: Diagnostic and
prognostic use of bone turnover markers. Recent Results Cancer Res.
192:197–223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buritova J, Tarayre JP, Besson JM and
Colpaert F: The novel analgesic and high-efficacy 5-HT1A receptor
agonist, F 13640 induces c-Fos protein expression in spinal cord
dorsal horn neurons. Brain Res. 974:212–221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jergova S and Cizkova D: Long-term changes
of c-Fos expression in the rat spinal cord following chronic
constriction injury. Eur J Pain. 9:345–354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sluka KA, Rasmussen LA, Edgar MM,
O'Donnell JM, Walder RY, Kolker SJ, Boyle DL and Firestein GS:
Acid-sensing ion channel 3 deficiency increases inflammation but
decreases pain behavior in murine arthritis. Arthritis Rheum.
65:1194–1202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salazar H, Jara-Oseguera A and Rosenbaum
T: The TRPV1 channel as a target for the treatment of pain. Rev
Neurol. 48:357–364. 2009.PubMed/NCBI
|
|
26
|
Neelands TR, Zhang XF, McDonald H and
Puttfarcken P: Differential effects of temperature on
acid-activated currents mediated by TRPV1 and ASIC channels in rat
dorsal root ganglion neurons. Brain Res. 1329:55–66. 2010.
View Article : Google Scholar : PubMed/NCBI
|