Introduction
With the progress of tissue engineering, bone
mesenchymal stem cells (BMSCs) are frequently investigated and are
being increasingly accepted as useful tools for bone tissue
engineering due to their potential to differentiate into a variety
of lineages (1). For example,
BMSCs have been used successfully in reconstruction of the skull,
based on scientific evidence that BMSCs can be induced into
osteoblasts (2). In oral
implantation, it has been suggested that the osteoblasts adhering
at the implant surface originate from the bone marrow and migrate
to the location of the implant (3). At present, the osteogenic lineage
differentiation of BMSCs may be the most well described, and bone
morphogenetic proteins (BMPs) may be the best-characterized
cytokines driving osteogenic differentiation (4).
BMP is a member of the transforming growth factor-β
superfamily. It is a multifunctional acidic polypeptide, which is
predominantly synthesized and secreted by osteoblasts (5). At present, >20 subtypes of BMPs
have been identified (6). Among
these, BMP-2 is an important regulating factor in osteogenesis
(7). BMP-2 is a polypeptide growth
factor containing 396 amino acids, the function of which induces
undifferentiated mesenchymal cells into cartilage and bone tissues
(8). BMP-2 may provide a basis for
a tissue-engineered bone construct, which is compatible with the
growing craniofacial skeleton, but without the morbidities
associated with distant graft harvest (5). However, the induced effects of BMP-2
on osteogenesis remain to be elucidated and require further
investigations.
In the present study, BMP-2 was overexpressed in
BMSCs through lentivirus vectors to determine the effects of BMP-2
on BMSCs osteogenetic differentiation and to improvie understanding
of the molecular basis of BMP-2-mediated osteogenesis.
Materials and methods
BMSC primary culture and
identification
A total of 32 Sprague-Dawley (SD) rats, aged 18
weeks and weighing ~300 g, were provided by the Animal Experiments
Center, Zhongshan Hospital, Fudan University (Shanghai, China). The
femur and tibia were obtained and soft tissues were removed prior
to immersion in low-glucose Dulbecco's modified Eagle's medium
(L-DMEM, cat. no. 11885-092; Gibco Life Technologies, Carlsbad, CA,
USA). Rats were housed in groups of three under controlled
temperature (22±2°C), relative humidity (55±10%), 12-h light/dark
cycle (7:00 a.m. to 7:00 p.m.) and provided with food and water
ad libitum. Carbon dioxide (CO2) inhalation was
used as a method of euthanasia for rats. Rats in the euthanasia
chamber were exposed to 100% CO2 for 7 min. All animal
experiments were performed according to the EU directives of 2010
on the protection of animals used for scientific purposes. The
marrow cavities were washed with L-DMEM and the resulting solution,
containing the bone marrow, was collected. Following collection,
the BMSCs were cultured with fresh L-DMEM containing 10% fetal
bovine serum (FBS; cat. no. 10099-141; Gibco Life Technologies) in
the incubator (NAPCO 5410; NAPCO, Chicago, IL, USA) at 37°C and 5%
CO2. At 48 h post-seeding, the culture medium was
replaced for the first time, and these cells were termed the first
generation (P1) cells. The cell morphology and growing conditions
were visualized using an inverted microscope (BX-40; Olympus,
Hamburg, Germany).
Flow cytometric analysis
The fluorescein isothiocyanate (FITC)-conjugated
CD29 BMSC biomarker (Abcam, Cambridge, MA, USA) was analyzed using
a BD™ Flow Cytometer (BD Biosciences, Franklin Lakes, NJ, USA)
using BD CellQuest Pro software version 5.1 (BD Biosciences) to
identify the harvested cells. The cells were then incubated with 10
µl CD90 primary anti-rat polyclonal antibodies
(FITC-conjugated; 1:1,000 dilution; Vector Laboratories, Inc.,
Burlingame, CA, USA) and with a negative control of the same
type.
Cell counting kit-8 (CCK-8) analysis
The P1 cells of the BMSCs were seeded into a 96-well
plate at a density of 1×103 and six repeated wells were
arranged as a blank control. CCK-8 solution (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was added to each well (10
µl per well), according to the manufacturer's instructions.
The optical density (OD) value was measured at a wavelength of 450
nm, with the results presented as the mean.
Overexpression of BMP-2 in BMSCs
Lentivirus vectors containing the BMP-2 and enhanced
green fluorescent protein (EGFP) genes were constructed with the
assistance of Shanghai Genechem (Shanghai, China), and were
expanded by culture of HT1080. The HT1080 cell line was used for
lentivirus vector production (Shanghai BioHermes Bio-Pharmaceutical
Technology, Co., Ltd., Shanghai, China). The total cells were
divided into three groups. The BMP-2 group was composed of cells
transduced with Lenti-BMP-2. The cells in the mock group were
transduced with blank lentivirus vectors, while the cells in the
control group remained untransduced. The P3 BMSCs were infected at
a multiple of infection (MOI) of 25, transferred to fresh medium
and maintained in an incubator at 37°C and 5% CO2. The
effects of transduction and growing conditions were observed using
fluorescence microscopy (Olympus BX61; Olympus).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from each group using TRIzol
(Invitrogen Life Technologies, Carslbad, CA, USA) and purified,
according to the manufacture's instructions. RT of the mRNA was
performed using a Reverse Transcription kit (Takara, Bio., Inc.,
Shiga, Japan), according to the manufacturer's instructions. The
expression level of genes were normalized to β-actin. The primers
used for amplification were designed, according to the nucleic acid
sequences in the Gene bank and are listed in Table I. qPCR was performed using SYBR
Green I (Takara, Bio, Inc.), according to the manufacturer's
instructions. The amplification procedure used the following
program: Denaturation at 95°C for 5 min, 95°C for 30 sec, 55°C for
30 sec, 72°C for 1 min (repeated for 30 cycles) and extension at
72°C for 8 min. The total volume of 5 µl qPCR product was
separated onto 1.5% agarose gels for electrophoresis. The net index
(NI) of the stripes' gray scale was analyzed using ScanImage
software version 4.1 (BD Biosciences) and were compared with that
of β-actin, the internal reference. All experiments were performed
in triplicate.
 | Table IPrimers used for amplification. |
Table I
Primers used for amplification.
| Gene | Reverse (5′-3′) | Forward (5′-3′) |
|---|
| BMP-2 |
TTGGAGGAGAAACAAGGTG |
AACAATGGCATGATTAGTGG |
| Col-I |
CAGACGGGAGTTTCTCCTCGGACGT |
GACCAGGAGGACCAGGAAGTCCACGT |
| OPN |
TGGTTTGCCTTTGCCTGTTCG |
ATGGCTTTCATTGGAGTTGCTTG |
| OCN |
GGCGTCCTGGAAGCCAATGTG |
GACCAGGAGGACCAGGAAGTCCACGT |
| β-actin |
AGCAGAGAATGGAAAGTCAAA |
ATGCTGCTTACATGTCTCGAT |
Western blot analysis
The cells from each group were washed with
phosphate-buffered saline (PBS) and lysis buffer (Qiagen, Valencia,
CA, USA) pre-cooled on ice, and eight samples were selected from
each group. Proteins (3 µg/µl) were separated onto
10% SDS-PAGE polyacrylamide gels (Invitrogen Life Technologies) for
electrophoresis. The nitrocellulose membranes (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), used as the transference
medium, were blocked in non-fat milk in Tris-buffered saline with
Tween 20 (TBST) for 3 h, and hybridized with primary antibodies for
BMP-2 (rabbit anti-rat BMP-2 polyclonal antibody; 1:200; EMD
Millipore, Billerica, MA, USA; cat. no. P12643), osteopontin (mouse
anti-rat OPN monoclonal antibody; 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; cat. no. sc-21742),
osteocalcin (mouse anti-rat OCN monoclonal antibody; 1:1,000, Santa
Cruz Biotechnology, Inc.; cat. no. sc-365797) and collagen type I
(goat anti-rat COL1 polyclonal antibody; 1:500; Santa Cruz
Biotechnology, Inc.; cat. no. sc-166865). overnight at 4°C. The
membranes were washed with PBS three times and were incubated with
secondary antibodies (Jackson Immunoresearch Laboratories, Inc.,
West Grove, PA, USA) for 2 h at room temperature. The protein bands
were visualized by enhanced chemiluminescence detection reagents
(Applygen Technologies Inc., Beijing, China) subsequent to washing
in PBS three times. The NI of the gray scale of the bands were
assessed, with GAPDH used as the internal reference. All
experiments were performed in triplicate.
Alkaline phosphatase (ALP) activity
To determine whether the early osteogenic
differentiation of BMSCs was induced by BMP-2, ALP staining was
performed, as previously reported (9). Following 3, 7, 14 and 21 days of
culture, the cells were fixed in 10% formalin, washed with PBS and
then incubated in staining solution, containing a mixture of 0.02%
5-bromo-4-chloro-3-indolyl phosphate (BCIP; Santa Cruz
Biotechnology, Inc.) and 0.03% nitro blue tetrazolium (NBT; Santa
Cruz Biotechnology, Inc.) in 0.1 M TBS, which was added into 5 ml
AP buffer (100 mM Tris-HCl, 100 mM NaCl, 5 mM MgCl2 and
0.05% Tween 20, pH 9.5) and incubated for 1 h at room temperature.
Furthermore, the activity of ALP and protein content of the three
groups were measured on days 3, 7, 14 and 21. The cells lysates
were prepared, and the activity of ALP in the lysates was
determined using a Lab-Assay-ALP colorimetric assay kit (Wako Pure
Chemicals, Osaka, Japan), according to the manufacturer's
instructions. The total protein concentrations were determined
using a commercial BCA Protein Assay kit (Beyotime Institute of
Biotechnology, Shanghai, China). The activity of ALP was calculated
as nmol/h phosphorylated Nitrophenol (p-NP) release and was further
normalized to the cell protein content.
Alizarin red staining
The BMSCs with or without BMP-2 were dissociated and
passaged using 0.25% trypsin (Sigma-Aldrich) to produce adherent
cell slices. When the cells reached confluence at 80% on the cover
slips, the medium was substituted with osteogenetic induction
solution (Shanghai Sangon Biotech, Co., Ltd., Shanghai, China)
containing 0.1 µmol/l dexamethasone, 50 mg/l ascorbic acid
and 10 mmol/l β-glycerophosphate, and cultured at 37°C and 5%
CO2. The medium was replaced every 3 days. On the 21st
day, the cells were fixed with 95% ethanol for 10 min, and
incubated in 2% Alizarin red staining solution (Sigma-Aldrich) for
5 min. The staining reactions were terminated by washing the cells
with PBS. Calcification deposits were identified in the matrix,
which appeared bright red under light microscopy (Olympus CX23;
Olympus) and images of the cells were captured. The calcification
was quantified by determining the densities and areas of Alizarin
red staining using an image analysis program (Multi Gauge V3.0
software; Fujifilm, Tokyo, Japan).
Adherence assay
The cells were routinely passaged and counted, and
then diluted in serum-free medium to a density of
2×105/ml. Subsequently, 100 µl of the cell
suspensions were seeded into each well in a 96-well plate, which
had been previously wrapped with fibronectin. After 3 h incubation
at room temperature, the medium was removed and the non-adherent
cells were removed by washing in PBS. The adherent cells in each
well were fixed using 50 µl 4% paraformaldehyde for 10 min
and stained with 50 µl gentian violet staining solution
(Invitrogen Life Technologies) for 15 min at room temperature.
Following staining, the cells were counted under a microscope
(Olympus). After staining with 100 µl gentian violet in each
well, the cells were maintained at 37°C and, after 20, 40 and 60
min, the OD value was measured using microplate spectrophotometer
(Shimadzu UV-2450; Shimadzu Corp., Kyoto Japan) at a wavelength of
585 nm.
Subcellular fractionation
Cellular fractionation was performed using NE-PER
Nuclear and Cytoplasmic Extraction reagents (Pierce Biotechnology,
Inc., Rockford, IL, USA), according to the manufacturer's
instructions. Subsequently, fractions were processed, as described
above for western blotting. The membranes were incubated with
either rabbit anti-Runt-related transcription factor (Runx)-2
polyclonal antibody (1:500; Santa Cruz Biotechnology, Inc.; cat.
no. sc-10758), or rabbit anti-p-small mothers against
decapentaplegicp (p-Smad) 1/5/8 antibody (1:1,000; Cell Signaling
Technology, Inc.; cat. no. 9516) overnight at 4°C. The subsequent
steps were performed as described above. To normalize the bands,
the filters were removed and re-probed with anti-paxillin (1:1,000;
BioLegend, Inc., San Diego, CA, USA) and anti-B23/nucleophosmin
(Santa Cruz Biotechnology, Inc.) antibodies. The density of the
bands were quantified densitometrically. Densitometric
quantification of the protein bands was analyzed by TINA software
version 2.1 (Raytest Isotopenmessgeräte GmbH, Straubenhardt,
Germany).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Comparisons between groups were analyzed using
analysis of variance using SPSS 11.0 software (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference. The P-values were corrected for multiple
testing procedures and to control for type I error rates.
Results
Morphological characteristics and
identification of BMSCs
The BMSCs were spherical in shape shortly following
seeding, the majority of which were suspended in the medium. After
48 h, the majority became adherent and exhibited a different
morphology, predominantly spindle-shaped or polygonal. The nuclei
were usually large and located in the middle or margin of the
cells. Scattered adherent fibroblast-like cells were observed on
the 3rd day of culture, following which the passaged cells became
completely adherent within 24 h. The shape of the spindle in the P3
cells was consistent. Following subculture for >20 generations,
the cells maintained vigorous growth and amplification, and no
obvious difference in the duration required to reach confluence
were observed (Fig. 1). In
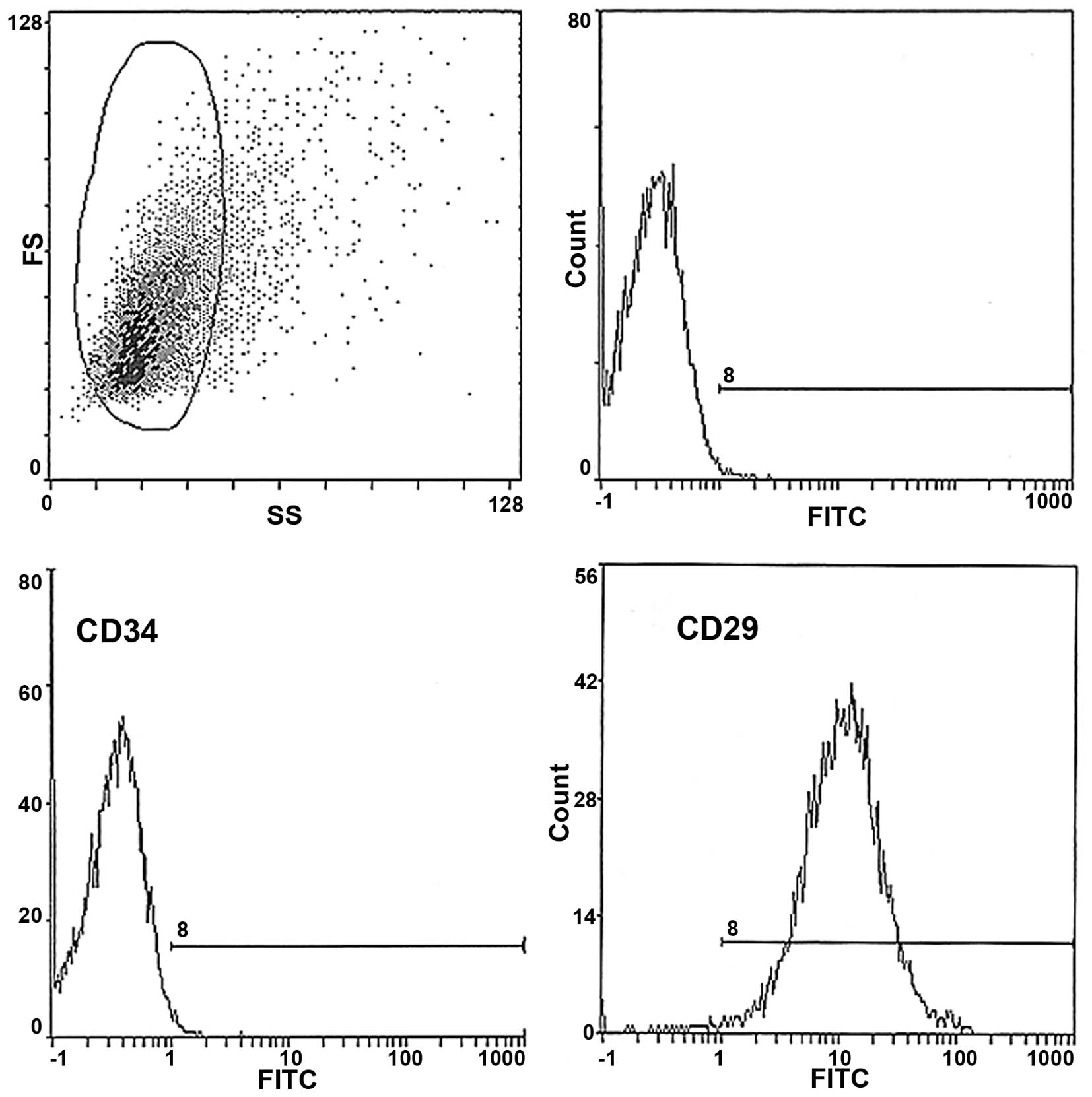
addition, the biomarkers of BMSCs were characterized using flow
cytometry (Fig. 2), which revealed
>85% of the expanded P3 BMSCs were positive for the surface
biomarker of CD29, characteristic of BMSCs. In addition few
hematopoietic cells were observed, as indicated by the low level of
CD34-expressing cells. These results indicated that the P3 cells
were highly purified BMSCs.
Growth pattern of the BMSCs
The growth curve, which was produced according to
the OD values of each well (Fig.
3), demonstrated that the BMSCs of regular seeding density had
approximately the same growth circle. In the first 2–3 days
following seeding, the cells grew relatively slowly, exhibiting a
delayed growth phrase. Consequently, the cells then grew rapidly to
reach the logarithmic growth phrase. On days 7–8, the total number
of cells reached the highest value. Subsequent to the following
growth plateau, the rate of cell growth slowed.
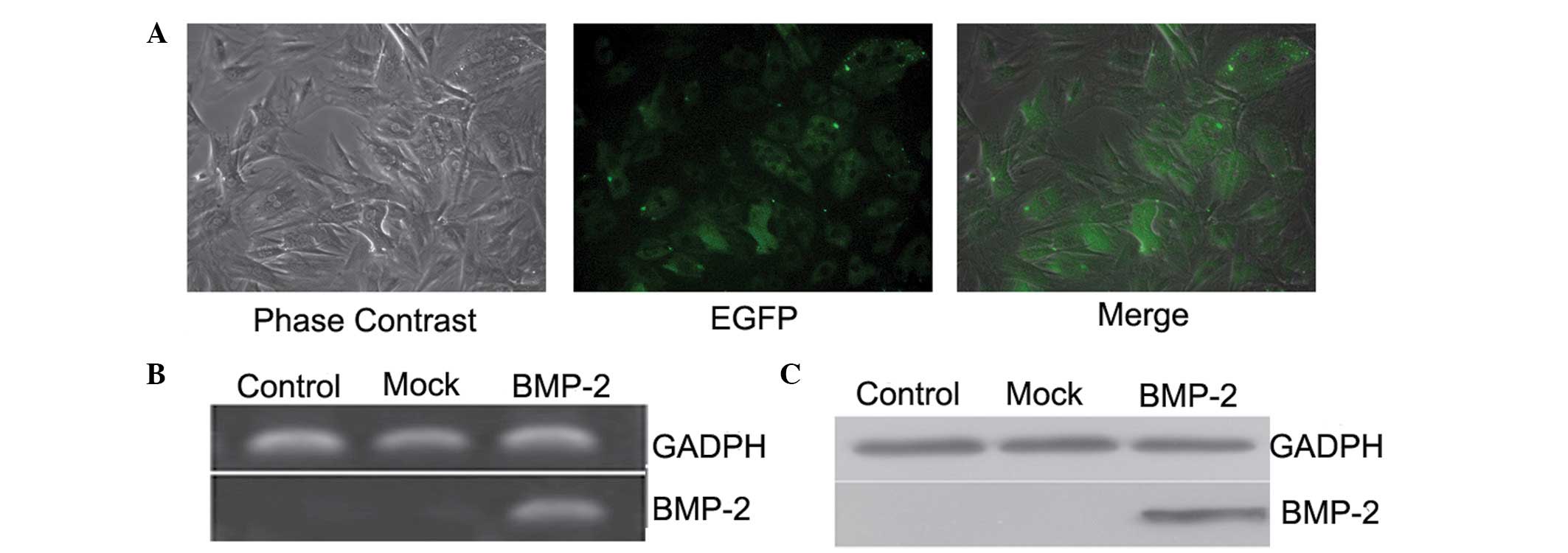
Overexpression of BMP-2 in the BMSCs
Overexpression of BMP-2 in the BMSCs was induced
using lentiviral particles containing a construct, which encoded
EGFP. At 72 h post-transduction, green fluorescence was detected
throughout the cytoplasm, in which the transduction efficiency was
>90% (Fig. 4A). The RT-qPCR
analysis demonstrated that, compared with the mock and control cell
groups, the mRNA (Fig. 4B) and
protein (Fig. 4C) levels of BMP-2
were significantly increased in the BMP-2-transduced BMSCs, which
further confirmed that BMP-2, the target gene, had been
successfully transduced into the BMSCs and was expressed
efficiently in the cells.
ALP activity in the BMP-2-induced
BMSCs
The effects of overexpression of BMP-2 on the
osteogenic differentiation of BMSCs were examined by examining the
expression of ALP, an early osteogenic marker, at indicated
time-points (Fig. 5A). Compared
with that detected in control BMSCs, the expression of ALP in the
BMP-2-transduced BMSCs increased gradually between 2.4-and 4.8-fold
between day 3 and 21 (Fig.
5B).
Extracellular matrix mineralization
The osteogenic effects of BMP-2 were further
characterized by examining the mineralization of the extracellular
matrix through Alizarin red staining. As shown in Fig. 6, the extent of mineralization,
indicated by the quantity of orange mineralized nodules and
calcification areas, was increased 2.3-fold in the BMSCs transduced
with the BMP-2 lentiviral particles, compared with the control
BMSCs, after 21 days of culture.
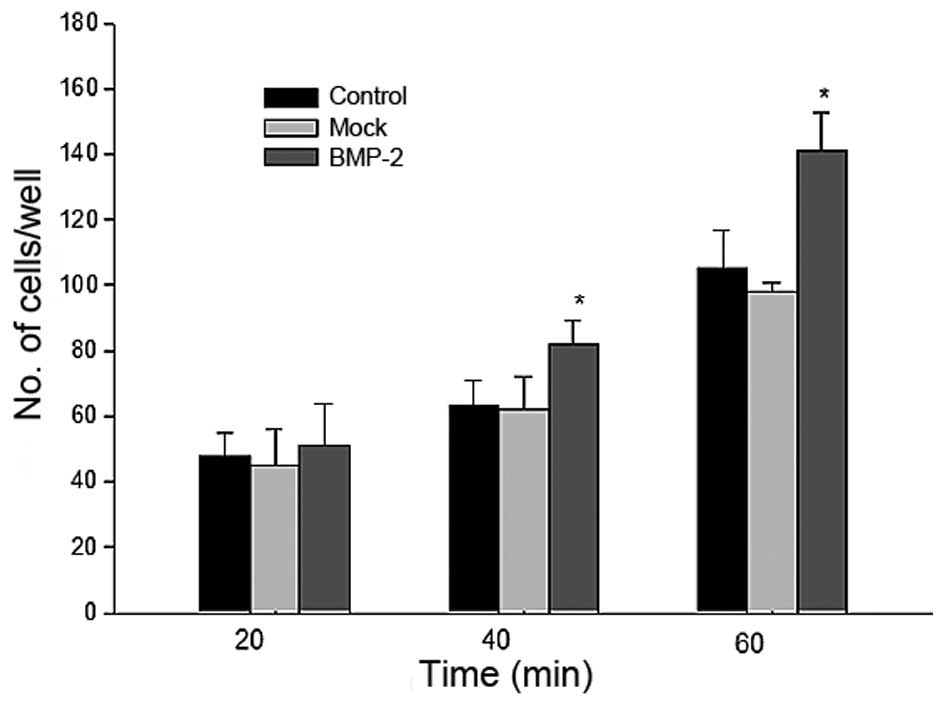
Adherence improvement by BMP-2 in
BMSCs
As it is understood that the growth and
differentiation of several types of cell, particularly stem cells,
is regulated by cell-cell and cell-matrix interactions (10), the adhesive ability of the
BMP-2-infected BMSCs was evaluated. The result of the adherence
assay revealed that the adhesive ability of the BMSCs significantly
improved with time following BMP-2 transduction (Fig. 7).
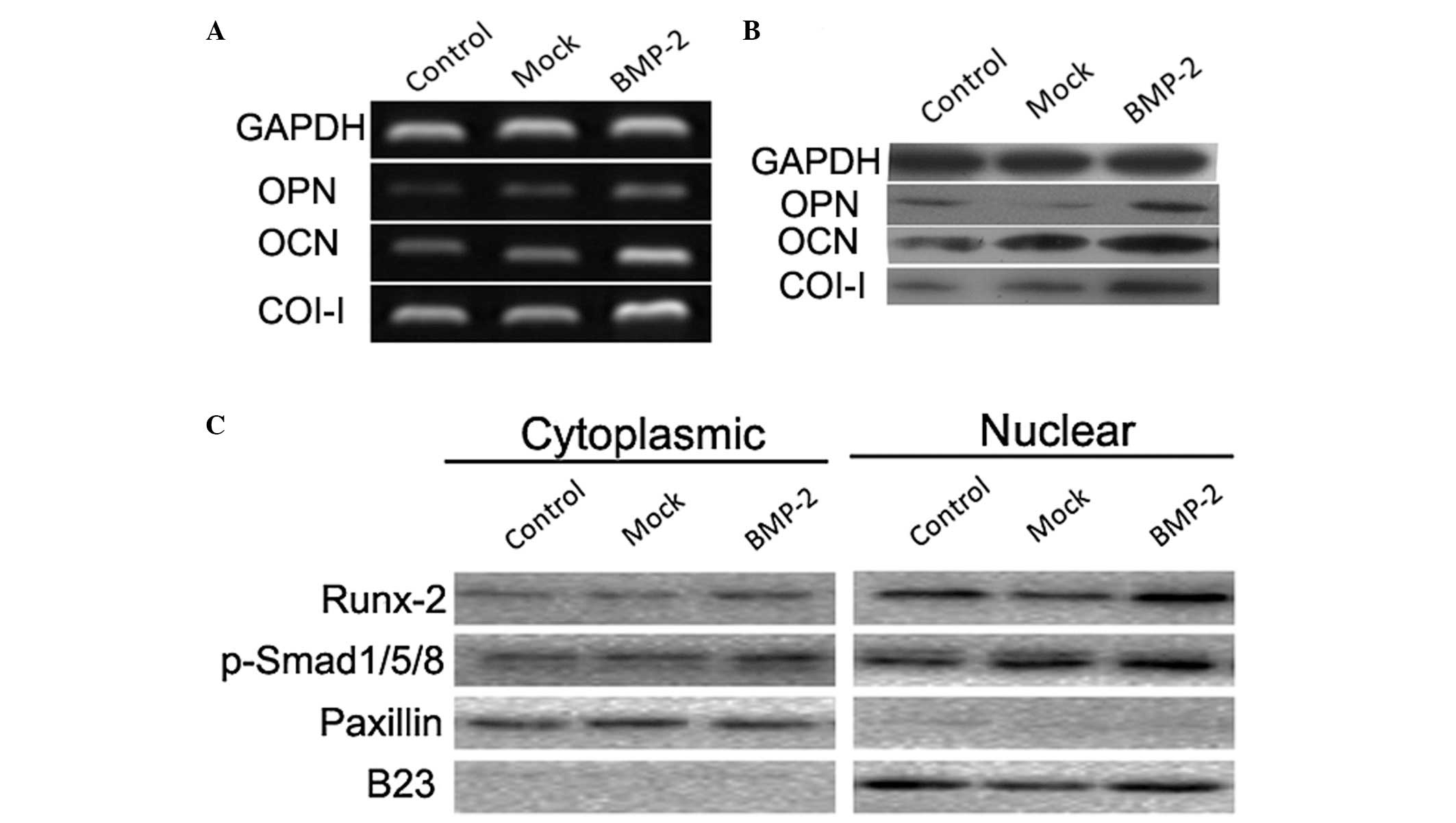
Expression and nuclear accumulation of
osteogenic markers promoted by BMP-2
The mRNA and protein levels of OPN, OCN and Col-I in
the BMSCs were significantly higher in the BMP-2 group, compared
with those in the mock and control groups (Fig. 8A and B). In addition, western blot
analysis of the cytoplasmic and nuclear fractions demonstrated that
the nuclear accumulations of Runx-2 and p-Smads1/5/8 were
significantly increased following BMP-2 transduction into the
BMSCs, compared with the control and mock cells (Fig. 8C).
Discussion
Advances in molecular biology have coalesced with an
improved understanding of craniofacial biology to enable what has
been termed generative craniofacial surgery (11) Due to various advantages, the use of
BMSCs as seeding cells is widespread in investigations of gene
therapy, cell substitution therapy and tissue engineering (12–14).
In the present study, BMSCs were successfully isolated and purified
from an SD rat, based on the combination of morphological
characteristics and flow cytometeric analysis using a BMSC-specific
marker. This provided an experimental basis for the subsequent
experiments.
During the differentiation of BMSCs towards an
osteoblastic lineage, several hormones and cytokines are involved
and regulate osteogenesis (15).
Among these molecules, BMPs are important cytokines in modulating
osteogenesis (16,17). Reports have revealed that the
procedure of BMP undergoing osteogenetic induction can be divided
into four steps: The tendency period, differentiation period, bone
formation period and remodeling period. During the whole procedure,
BMP-2 is considered to be one of the most active induction factors
(13). At present, BMP-2 has
demonstrated significant promise as a clinically useful
osteo-inductive agent, however, key questions require answering
prior to the use of this protein on a large scale as an adjunct to
craniofacial surgery (18).
Whether BMSC differentiation towards osteoblasts is induced
directly by BMP-2 remains to be fully elucidated; and previous
findings have demonstrated that BMP-2 either promotes or inhibits
osteogenesis (19). As BMP-2 may
facilitate a 'paradigm shift', in which molecular techniques reduce
the morbidity and mortality rates of craniofacial interventions,
while simultaneously enhancing the effectiveness of these
procedures, the present study transduced BMP-2 into BMSCs. This was
performed using lentiviral vectors, based on evidence that
lentiviral vectors are superior to other vectors, including
adenoviral vectors, and are considered the ideal vector of gene
therapy (20,21). BMP-2 is also critically involved in
mediating the condensation of mesenchymal cells and directly
differentiating along an osteoblastic phenotype (22). Owing to local stimulation of BMP-2,
BMSCs gather through chemotaxis and differentiated into cartilage
and bone tissues (23–25). In the present study, forced
expression of BMP-2 in the BMSCs not only induced ALP activity and
promoted the formation of calcium nodules, but also improved the
adherence of BMSCs. BMP-2 also mediated the osteogenic markers,
ALP, OCN and Col-I. These results indicated that the
BMP-2-transduced BMSCs were in a functional status in the process
of osteogenetic differentiation (26,27),
as Col-I is secreted by mature osteoblasts (28). In addition, the present study
demonstrated that the nuclear accumulation of Runx-2 and
hosphor-Smads1/5/8 was significantly increased following
BMP-2-transduction of the BMSCs. In the process of osteogenic
induction, Runx2 activates and translocates into the nucleus, and
acts with Smads through direct binding in a transcriptional
activator complex. Runx2 recruits R-Smads to the complex to
initiate BMP-responsive gene transcription (29–31).
In conclusion, the results of the present study
demonstrated that BMP-2 facilitated the osteogenic differentiation
of BMSCs via inducing ALP activity, promoting mineralization,
enhancing adherence and mediating the expression and activation of
certain associated osteogenic markers. These results indicate the
importance of BMP-2 in osteogenesis and, despite several questions
remaining unanswered, these results provide experimental evidence
supporting advances in craniofacial surgery.
Acknowledgments
The study was supported by a grant from the Shanghai
Natural Science Foundation (grant. no. 10JC1402600).
References
|
1
|
Lee HS, Huang GT, Chiang H, Chiou LL, Chen
MH, Hsieh CH and Jiang CC: Multipotential mesenchymal stem cells
from femoral bone marrow near the site of osteonecrosis. Stem Cell.
21:190–199. 2003. View Article : Google Scholar
|
|
2
|
Derubeis AR and Cancedda R: Bone marrow
stromal cells (BMSCs) in bone engineering: limitations and recent
advances. Ann Biomed Eng. 32:160–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dimitriou R and Babis GC: Biomaterial
osseointegration enhancement with biophysical stimulation. J
Musculoskelet Neuronal Interact. 7:253–265. 2007.PubMed/NCBI
|
|
4
|
van Hout WM, Mink van der Molen AB,
Breugem CC, Koole R and Van Cann EM: Reconstruction of the alveolar
cleft: can growth factor-aided tissue engineering replace
autologous bone grafting? A literature review and systematic review
of results obtained with bone morphogenetic protein-2. Clin Oral
Investig. 15:297–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith DM, Cooper GM, Mooney MP, Marra KG
and Losee JE: Bone morphogenetic protein 2 therapy for craniofacial
surgery. J Craniofac Surg. 19:1244–1259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wozney JM, Rosen V, et al: Novel
regulations of bone formation molecular clones and activities.
Science. 242:1528–1534. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Date T, Doiguchi Y, Nobuta M and Shindo H:
Bone morphogenetic protein-2 induces differentiation of multipotent
C3H10T1/2 cells into osteoblasts, chondrocytes and adipocytes in
vivo and in vitro. J Orthop Sci. 9:503–508. 2004. View Article : Google Scholar
|
|
8
|
Khosla S, Westendorf JJ and Oursler MJ:
Building bone to reverse osteoporosis and repair fractures. J Clin
Invest. 118:421–428. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
James AW, Theologis AA, et al:
Estrogen/estrogen receptor alpha signaling in mouse posterofrontal
cranial suture fusion. PLoS One. 4:e71202009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shur I, Zilberman M, Benayahu D and Einav
S: Adhesion molecule expression by osteogenic cells cultured on
various biodegradable scaffolds. J Biomed Mater Res A. 75:870–876.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McCarthy JG, Stelnicki EJ, Mehrara BJ and
Longaker MT: Distraction osteogenesis of the craniofacial skeleton.
Plast Reconstr Surg. 107:1812–1827. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou W, Edelman GM and Mauro VP:
Transcript leader regions of two Saccharomyces cerevisiae mRNAs
contain internal ribosome entry sites that function in living
cells. Proc Natl Acad Sci USA. 98:1531–1536. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim CS, Kim JI, Kim J, Choi SH, Chai JK,
Kim CK and Cho KS: Ectopic bone formation associated with
recombinant human bone morphogenetic proteins-2 using collagen
sponge and beta tricalcium phosphate as carriers. Biomaterials.
26:2501–2507. 2005. View Article : Google Scholar
|
|
14
|
Yamashita M, Otsuka F, Mukai T, et al:
Simvastatin antagonizes tumor necrosis factor-alpha inhibition of
bone morphogenetic proteins-2-induced osteoblast differentiation by
regulating Smad signaling and Ras/Rho-mitogen-activated protein
kinase pathway. J Endocrinol. 196:601–613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hermes M, Osswald H, Mattar J and Kloor J:
Influence of an altered methylation potential on mRNA methylation
and gene expression in HepG2 cells. Exp Cell Res. 294:325–334.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kirker-Head CA, Gerhart TN, Schelling SH,
Hennig GE, Wang E and Holtrop ME: Long-term healing of bone using
recombinant human bone morphogenetic protein 2. Clin Orthop Relat
Res. 222–230. 1995.PubMed/NCBI
|
|
17
|
Guo J and Wu G: The signaling and
functions of heterodimeric bone morphogenetic proteins. Cytokine
Growth Factor Rev. 23:61–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chin M, Ng T, Tom WK and Carstens M:
Repair of alveolar clefts with recombinant human bone morphogenetic
protein (rhBMP-2) in patients with clefts. J Craniofac Surg.
16:778–789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Onishi M, Fujita Y, Yoshikawa H and
Yamashita T: Inhibition of Rac1 promotes BMP-2-induced osteoblastic
differentiation. Cell Death Dis. 4:e6982013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Englund U, Ericson C, Rosenblad C, Mandel
RJ, Trono D, Wictorin K and Lundberrg C: The use of a recombinant
lentiviral vector for ex vivo gene transfer into the rat CNS.
Neuroreport. 11:3973–3977. 2000. View Article : Google Scholar
|
|
21
|
Wilson DR: Viral-mediated gene transfer
for cancer treatment. Curr Pharm Biotechnol. 3:151–164. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chung UI, Kawaguchi H, Takato T and
Nakamura K: Distinct osteogenic mechanisms of bones of distinct
origins. J Orthop Sci. 9:410–414. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kugimiya F, Kawguchi H and Chung UI: BMP
and bone formation. Clin Calcium. 14:173–179. 2004.In Japanese.
PubMed/NCBI
|
|
24
|
Wozney JM and Seeherman HJ: Protein-based
tissue engineering in bone and cartilage repair. Curr Opin
Biotechnol. 15:392–398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boden SD: The ABCs of BMPs. Orthop Nurs.
24:49–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kao PC, Riggs BL and Schryver PG:
Development and evaluation of an osteocalcin
chemiluminoimmunoassay. Clin Chem. 39:1369–1374. 1993.PubMed/NCBI
|
|
27
|
Rawadi G, Vayssière B, Dunn F, Baron R and
Roman-Roman S: BMP-2 controls alkaline phosphatase expression and
osteoblast mineralization by a Wnt autocrine loop. J Bone Miner
Res. 18:1842–1853. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hosoi T: Bone and bone related biochemical
examinations: Bone and collagen related metabolities. Structure and
metabolisms of collagen. Clin Calcium. 16:971–976. 2006.In
Japanese. PubMed/NCBI
|
|
29
|
Choi JY, Pratap J, Javed A, et al:
Subnuclear targeting of Runx/Cbfa/AML factors is essential for
tissue-specific differentiation during embryonic development. Proc
Natl Acad Sci USA. 98:8650–8655. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Afzal F, Pratap J, Ito K, et al: Smad
function and intranuclear targeting share a Runx2 motif required
for osteogenic lineage induction and BMP2 responsive transcription.
J Cell Physiol. 204:63–72. 2005. View Article : Google Scholar
|
|
31
|
Kugimiya F, Yano F, Ohba S, Igawa K,
Nakamura K, Kawaguchi H and Chung UI: Mechanism of osteogenic
induction by FK506 via BMP/Smad pathways. Biochem Biophys Res
Commun. 338:872–879. 2005. View Article : Google Scholar : PubMed/NCBI
|