Introduction
Esophageal squamous cell carcinoma (ESCC) is a
common malignant tumor of the digestive tract and is associated
with a poor prognosis (1,2). Patients with ESCC often have a poor
outcome as it is a difficult disease to diagnose at an early stage
(3,4). Advanced esophageal cancer carries a
particularly unfavorable prognosis due to the rapid spread of the
tumor beyond the esophageal wall (5,6). A
treatment strategy for early ESCC has been established based around
endoscopic procedures, and standard curative surgery is now
performed in advanced ESCC. In Japan, the total 5-year survival
rate of patients with ESCC is ~50%; that of patients with early
ESCC is >70%, while in advanced cases it is ~20%, due to the
high incidence of recurrence, metastasis and invasion of adjacent
organs (3,7). The prognosis of ESCC remains poor
despite recent therapeutic advances. Therefore, further
clinicopathological studies are required in order to analyze the
mechanisms underlying local invasion and metastasis in ESCC.
Podoplanin, a 38 kDa transmembrane protein, was
identified as an independent platelet aggregation-inducing factor,
and is known to be a specific marker for lymphatic vessels
(8). A recent study reported that
podoplanin promoted epithelial-mesenchymal transition (EMT)
(9). It has also been shown to
stimulate collective cell invasion and migration without EMT, by
inducing a rearrangement of the actin cytoskeleton of MCF7 breast
cancer cells (10). A number of
studies have reported that podoplanin expression is correlated with
lymph node metastasis, disease stage, lymphatic and vascular
invasion, recurrence and a poor prognosis in ESCC (11,12).
Vimentin is an important protein constituent of cellular
intermediate filaments in normal and tumor mesenchymal cells
(13,14). Therefore, vimentin expression is
one of the primary indicators of the development of EMT in
carcinomas, which suggests a tumor with an aggressive phenotype
with invasive and/or metastatic potential (15). Vimentin expression in ESCC has also
been shown to be an independent predictor of lymph node metastasis
(16,17). However, to the best of our
knowledge, there have been no clinicopathological studies
investigating podoplanin and vimentin expression together in ESCC.
The present study focused on the expression of podoplanin in the
membrane, and its clinicopathological significance in the
progression of ESCC.
Materials and methods
Patients
In total, 139 patients with ESCC who had undergone
surgical resection of their tumor at Tokai University Hospital
(Kanagawa, Japan) between January 2003 and December 2005, were
enrolled into the study. The study was approved by the ethics
committee of the Institutional Review Board of Tokai University
Hospital (IRB no. 13R-33; Isehara, Japan). All participants
provided informed consent, according to the Institutional Review
Board of Tokai University Hospital. The 139 patients (128 males and
11 females; age range, 42–82 years; mean age, 63.4 years) with ESCC
underwent surgery with three-field lymph node dissection and were
not treated with radiotherapy or chemotherapy prior to surgery.
Histopathological examination
Esophageal cancer specimens were routinely fixed
with 10% formalin for 24–48 h, embedded in paraffin, cut into
4-µm sections and stained with hematoxylin and eosin. The
tumor stage was defined according to the tumor-node-metastasis
classification of the Union for International Cancer Control (UICC)
(18). In addition, histological
factors were graded according to the Guidelines for Clinical and
Pathological Studies on Carcinoma of the Esophagus (e.g. ly, v and
INF). The degree of lymph node metastasis was classified as: n (−),
metastasis negative; or n (+), metastasis positive. The degree of
lymphatic invasion was classified as: ly0, no lymphatic invasion;
ly1, mild lymphatic invasion; ly2, moderate lymphatic invasion; or
ly3, severe lymphatic invasion. The degree of venous invasion was
classified as: v (−), invasion negative; or v (+), invasion
positive. Tumor infiltrative patterns (INF) at the invasive front
were classified into three groups according to the general criteria
described by the Japanese Gastric Cancer Association (19): INFa, cancer nests demonstrate
expanding growth and have a clear border with pre-vesicular adipose
tissue; INFb, cancer nests are intermediate between those of INFa
and INFc; and INFc, scirrhous growth, in which cancer nests exhibit
invasive growth and where the border with adipose tissue is
unclear.
Immunohistochemical analysis
An representative specimen for immunohistochemical
analysis was selected from each patient with ESCC. Sections (5
µm) were mounted on silane-coated glass slides,
deparaffinized and autoclaved (ES-215, high-pressure steam
sterilizer, TOMY, Tokyo, Japan) at 121°C for 10 min for antigen
retrieval. The primary antibodies used in the immunohistochemical
analyses were mouse monoclonal anti-podoplanin (clone D2–40; 3X
dilution; cat. no. 413451; Nichirei Pharmaceutical, Tokyo, Japan)
and mouse monoclonal anti-vimentin (clone v9; X100 dilution; cat.
no. MO725; Dako Denmark A/S, Glostrup, Denmark). Immunoreactivity
was detected using the avidin-biotin method (Vectastain Elite ABC
kit, Vector Laboratories, Inc., Burlingame, CA, USA).
Immunohistochemical images were captured using a digital microscope
camera system (BX50 microscope and DP70 digital camera; Olympus
Corporation, Tokyo, Japan).
Evaluation of immunohistochemistry
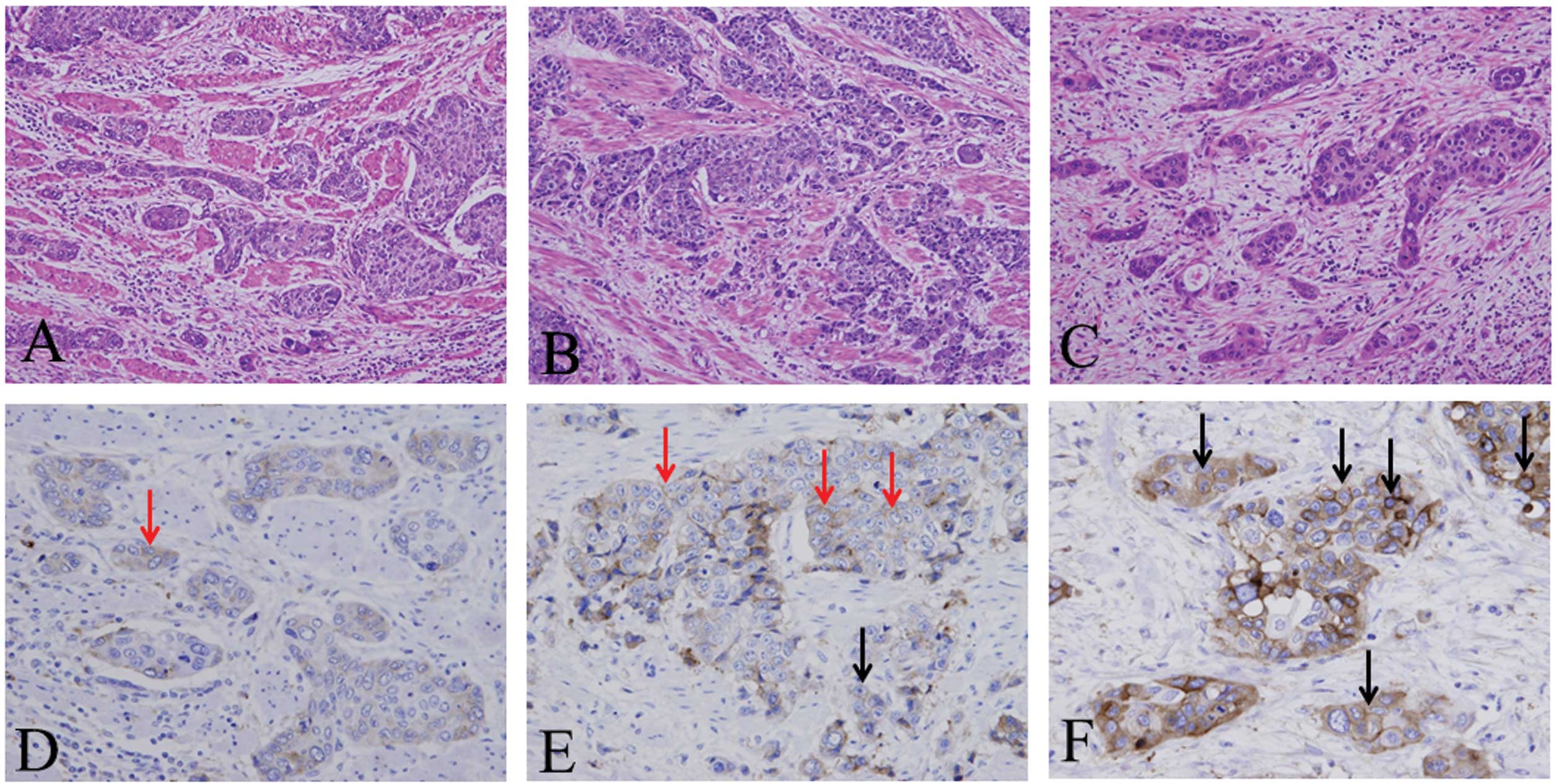
For the analysis of the immunohistochemical
expression of podoplanin, staining intensity was determined at the
invasive front of ESCC (Fig. 1),
and was classified into the following three criteria: 1) negative,
incomplete membrane expression of <10% cancer cells at the
invasive front (Fig. 1D); 2) weak,
complete membrane expression of <10% cancer cells (Fig. 1E); 3) strong, complete membrane
expression of ≥10% cancer cells (Fig.
1F). Vimentin expression was determined by the presence of
cytoplasmic staining, in particular in the tumor/stoma interface at
the invasive front of the cancer cells, and divided into the
following two criteria: Negative, <10% vimentin expression at
the invasive front of the cancer cells and positive, ≥10% vimentin
expression at the invasive front (Fig.
1E).
Statistical analysis
Univariate analysis was performed using the
χ2 test. Cox proportional hazard regression analysis was
used to determine the net effects of each predictor while
controlling the effects of the other factors by uni- and
multivariate analysis. Independent prognostic factors were analyzed
by the Cox proportional hazard regression model. Hazard ratios
(HRs) and associated 95% confidence intervals (CIs) were used to
assess the independent contributions of significant factors.
P<0.05 was considered to indicate a statistically significant
difference. Overall survival was measured from the date of surgery
to mortality from all causes. Survival curves were calculated using
Kaplan-Meier methods and analyzed using the log-rank test. All
analyses were performed using SPSS statistical software, version 21
(IBM SPSS, Armonk, NY, USA).
Results
Histological expression of podoplanin and
vimentin
Podoplanin was strongly expressed in the endothelial
cells of lymphatic vessels, which were examined as internal
controls. Non-neoplastic mucosa, obtained from the surgically
resected esophagus with ESCC, exhibited weak podoplanin expression
at the basal layer adjacent to the connective tissue papillae.
Podoplanin expression was positive in 66.2% (92/139) of samples
from patients with ESCC (Fig. 2);
weak expression was observed in 32.4% (45/139) and strong
expression in 33.8% (47/139; Fig.
1). Vimentin expression was observed in the esophageal lamina
propria, but was not detected in non-neoplastic esophageal
epithelium (Fig. 2). Vimentin
expression at the cancer invasive front was detected in 35.3%
(49/139) of samples from patients with ESCC.
Analysis of clinicopathological findings
from ESCC using χ2 statistics
The associations between podoplanin and
clinicopathological features, are summarized in Table I. Strong podoplanin expression was
significantly correlated with tumor status (P=0.001), venous
invasion (P=0.035) and UICC stage (P=0.029). The associations
between Vimentin and clinicopathological features are summarized in
Table II. Vimentin expression was
significantly associated with venous invasion (P=0.020). The
association between podoplanin and vimentin is summarized in
Table III. Podoplanin membrane
expression was strongly correlated with vimentin cytoplasmic
expression in samples from patients with ESCC (P<0.001).
Immunohistochemical double-staining demonstrated co-expression of
podoplanin and vimentin in ESCC (Fig.
3).
 | Table IPodoplanin membrane expression and
clinicopathological factors of esophageal squamous cell
carcinoma. |
Table I
Podoplanin membrane expression and
clinicopathological factors of esophageal squamous cell
carcinoma.
| Variable | Number of
patients | Podoplanin staining
intensity
| P-value |
|---|
| Negative | Weak | Strong |
|---|
| Age at surgery
(years) | | | | | 0.406 |
| <63 | 66 (47.5) | 21 (31.8) | 19 (28.8) | 26 (39.4) | |
| ≥63 | 73 (52.5) | 26 (35.6) | 26 (35.6) | 21 (28.8) | |
| Gender | | | | | 0.696 |
| Male | 128 (92.1) | 42 (32.8) | 42 (32.8) | 44 (34.4) | |
| Female | 11 (7.9) | 5 (45.4) | 3 (27.3) | 3 (27.3) | |
| Tumor status | | | | | 0.001 |
| T1 | 48 (34.5) | 24 (50.0) | 17 (35.4) | 7 (14.6) | |
| T2 | 25 (18.0) | 4 (16.0) | 13 (52.0) | 8 (32.0) | |
| T3 | 61 (43.9) | 19 (31.1) | 14 (23.0) | 28 (45.9) | |
| T4 | 5 (3.6) | 0 (0.0) | 1 (20.0) | 4 (80.0) | |
| Lymph node
metastasis | | | | | 0.164 |
| n (−) | 43 (30.9) | 18 (40.9) | 16 (36.4) | 10 (22.7) | |
| n (+) | 96 (69.1) | 29 (30.5) | 29 (30.5) | 37 (39.0) | |
| Lymphatic
invasion | | | | | 0.870 |
| ly (0, 1) | 87 (62.6) | 30 (34.5) | 29 (33.3) | 28 (32.2) | |
| ly (2, 3) | 52 (37.4) | 17 (32.7) | 16 (30.8) | 19 (36.5) | |
| Venous invasion | | | | | 0.035 |
| v (−) | 52 (37.4) | 23 (44.2) | 18 (34.6) | 11 (21.2) | |
| v (+) | 87 (62.6) | 24 (27.6) | 27 (31.0) | 36 (41.4) | |
| Histological
differentiation | | | | | 0.159 |
| Well | 38 (27.3) | 9 (23.7) | 13 (34.2) | 16 (42.1) | |
| Mod | 77 (55.4) | 25 (32.4) | 26 (33.8) | 26 (33.8) | |
| Poor | 24 (17.3) | 13 (54.2) | 6 (25.0) | 5 (20.8) | |
| Infiltration
pattern | | | | | 0.398 |
| inf a, b | 97 (69.8) | 36 (37.1) | 31 (32.0) | 30 (30.9) | |
| inf c | 42 (30.2) | 11 (26.2) | 14 (33.3) | 17 (40.5) | |
| UICC stage | | | | | 0.029 |
| IA, IB | 37 (26.6) | 16 (43.2) | 14 (37.9) | 7 (18.9) | |
| IIA, IIB | 36 (25.9) | 10 (27.8) | 16 (44.4) | 10 (27.8) | |
| IIIA, IIIB,
IIIC | 66 (47.5) | 21 (31.8) | 15 (22.7) | 30 (45.5) | |
 | Table IIVimentin expression and
clinicopathological factors of esophageal squamous cell
carcinoma. |
Table II
Vimentin expression and
clinicopathological factors of esophageal squamous cell
carcinoma.
| Variable | Number of
patients | Vimentin (−) | Vimentin (+) | P-value |
|---|
| Age at surgery
(years) | | | | 0.794 |
| <63 | 66 (47.5) | 42 (63.6) | 24 (36.4) | |
| ≥63 | 73 (52.5) | 48 (65.8) | 25 (34.2) | |
| Gender | | | | 0.163 |
| Male | 128 (92.1) | 85 (66.4) | 43 (33.6) | |
| Female | 11 (7.9) | 5 (45.5) | 6 (54.5) | |
| Tumor status | | | | 0.143 |
| T1 | 48 (34.5) | 37 (77.1) | 11 (22.9) | |
| T2 | 25 (18.0) | 16 (64.0) | 9 (36.0) | |
| T3 | 61 (43.9) | 34 (55.7) | 27 (44.3) | |
| T4 | 5 (3.6) | 3 (60.0) | 2 (40.0) | |
| Lymph node
metastasis | | | | 0.180 |
| n (−) | 44 (31.7) | 32 (72.7) | 12 (27.3) | |
| n (+) | 95 (68.3) | 58 (61.1) | 37 (38.9) | |
| Lymphatic
invasion | | | | 0.178 |
| ly (0, 1) | 87 (62.6) | 60 (69.0) | 27 (31.0) | |
| ly (2, 3) | 52 (37.4) | 30 (57.7) | 22 (42.3) | |
| Venous
invasion | | | | 0.020 |
| v (−) | 52 (37.4) | 40 (76.9) | 12 (23.1) | |
| v (+) | 87 (62.6) | 50 (57.5) | 37 (42.5) | |
| Histological
differentiation | | | | 0.582 |
| Well | 38 (27.3) | 22 (57.9) | 16 (42.1) | |
| Mod | 77 (55.4) | 52 (67.5) | 25 (32.5) | |
| Poor | 24 (17.3) | 16 (66.7) | 8 (33.3) | |
| Infiltration
pattern | | | | 0.105 |
| inf a, b | 97 (69.8) | 67 (69.1) | 30 (30.9) | |
| inf c | 42 (30.2) | 23 (54.8) | 19 (45.2) | |
| UICC stage | | | | 0.231 |
| IA, IB | 37 (26.6) | 27 (73.0) | 10 (27.0) | |
| IIA, IIB | 36 (25.9) | 25 (69.4) | 11 (30.6) | |
| IIIA, IIIB,
IIIC | 66 (47.5) | 38 (57.6) | 28 (42.4) | |
 | Table IIIPodoplanin membrane expression and
vimentin expression in esophageal squamous carcinoma. |
Table III
Podoplanin membrane expression and
vimentin expression in esophageal squamous carcinoma.
| Variable | Number of
patients | Podoplanin staining
intensity
| P-value |
|---|
| Negative | Weak | Strong |
|---|
| Vimentin | | | | | <0.001 |
| Negative | 90 (64.7) | 42 (46.7) | 31 (34.4) | 17 (18.9) | |
| Positive | 49 (35.3) | 5 (10.2) | 14 (28.6) | 30 (61.2) | |
Correlation between prognosis and
expression of podoplanin and vimentin in ESCC
Patients exhibiting strong podoplanin expression had
significantly poorer overall survival rates than those with
negative or weak expression of podoplanin (<0.001, P=0.001,
log-rank test, Fig. 4A). Patients
from whom ESCC samples were vimentin-positive also had lower
overall survival rates than patients who were negative for vimentin
expression (P=0.002, log-rank test, Fig. 4B). Multivariate analysis
demonstrated that strong podoplanin expression (HR, 3.084; 95% CI,
1.543–6.164) and lymph node metastasis (HR, 6.132; 95% CI,
2.355–15.916) were independent predictors of mortality in ESCC
(Table IV). In addition,
multivariate analysis showed that vimentin expression (HR, 2.008;
95% CI, 1.191–3.384), tumor status (HR, 1.830; 95% CI, 1.049–3.194)
and lymph node metastasis (HR, 5.77; 95% CI, 2.221–15.028) were
independent predictors of mortality in ESCC (Table V).
 | Table IVMultivariate analysis of
clinicopathological factors and patients' survival of esophageal
squamous cell carcinoma. |
Table IV
Multivariate analysis of
clinicopathological factors and patients' survival of esophageal
squamous cell carcinoma.
| Variable | Number of
patients | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Podoplanin staining
intensity |
| Negative
(reference) | 47 (33.8) | | | |
| Weak | 45 (32.4) | 1.509 | 0.717–3.174 | 0.278 |
| Strong | 47 (33.8) | 3.084 | 1.543–6.164 | 0.001 |
| Age at surgery
(years) | | | | 0.551 |
| <63 | 66 (47.5) | 0.856 | 0.514–1.426 | |
| ≥63 | 73 (52.5) | | | |
| Gender | | | | 0.604 |
| Male | 128 (92.1) | 0.685 | 0.164–2.868 | |
| Female | 11 (7.9) | | | |
| Tumor status | | | | 0.123 |
| T1, T2 | 73 (52.5) | 1.587 | 0.882–2.854 | |
| T3, T4 | 66 (47.5) | | | |
| Lymph node
metastasis | | | | <0.001 |
| n (−) | 44 (31.7) | 6.123 | 2.355–15.916 | |
| n (+) | 95 (68.3) | | | |
 | Table VMultivariate analysis of
clinicopathological factors and patients' survival of esophageal
squamous cell carcinoma. |
Table V
Multivariate analysis of
clinicopathological factors and patients' survival of esophageal
squamous cell carcinoma.
| Variable | Number of
patients | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Vimentin | | | | 0.009 |
| Negative | 90 (64.7) | 2.008 | 1.191–3.384 | |
| Positive | 49 (35.3) | | | |
| Age at surgery
(years) | | | | 0.528 |
| <63 | 66 (47.5) | 0.849 | 0.510–1.412 | |
| ≥63 | 73 (52.5) | | | |
| Gender | | | | 0.266 |
| Male | 128 (92.1) | 0.441 | 0.104–1.865 | |
| Female | 11 (7.9) | | | |
| Tumor status | | | | 0.033 |
| T1, T2 | 73 (52.5) | 1.830 | 1.049–3.194 | |
| T3, T4 | 66 (47.5) | | | |
| Lymph node
metastasis | | | | <0.001 |
| n (−) | 90 (64.7) | 5.777 | 2.221–15.028 | |
| n (+) | 49 (35.3) | | | |
Discussion
Recently, podoplanin has been shown to be a
candidate marker for cancer stem cells, which is associated with
cancer cell invasion and migration, as well as prognosis, in a
number of types of cancer, including esophageal squamous cell
carcinoma, lung squamous cell carcinoma and oral cancer (11,12,20–22).
In the present study, the focus was on the expression of podoplanin
in the cell membrane at the invasive front of ESCC tumors, since
the podoplanin molecule is a transmembrane protein. The expression
of podoplanin on the cell membrane was positively correlated with
tumor status, venous invasion and UICC stage, and was associated
with a poor prognosis in ESCC. Strong podoplanin membrane
expression was an independent predictor of mortality in patients
with ESCC (HR 3.084, 95% CI, 1.543–6.164). To the best of our
knowledge, this is the first study to report the association
between podoplanin membrane expression and the degree or presence
of certain clinicopathological factors. Studies have previously
described podoplanin expression in cancer cells, but have not
discussed podoplanin membrane expression specifically (11,12).
Rahadiani et al (11) reported that high podoplanin
expression was significantly correlated with tumor status, depth of
invasion, and lymphatic and vascular invasion, and was associated
with a poorer prognosis in ESCC. These authors demonstrated that
podoplanin was involved in cancer cell invasion and tumorigenesis
through the use of experimental procedures, such as the Matrigel
invasion assay and an in vivo mouse study. Tong et al
(12) reported that podoplanin
expression was correlated with lymph node metastasis, UICC stage
and the immunoreactivity score, and was associated with a poor
prognosis in ESCC.
Several studies have clarified that podoplanin
expression promotes EMT at molecular/histopathological levels
(9). The present study focused on
membrane expression of podoplanin, as membrane expression has been
suggested to have significant roles in EMT (9) and cell invasion processes (10). In cancer cells, EMT is a phenotypic
change, by which epithelial cells lose their polarity and
epithelial markers, such as E-cadherin, and acquire migratory
factors that are characteristic of fibroblasts, such as snail and
vimentin (23–27). Vimentin expression is an important
indicator of EMT in carcinomas (27). This transition suggests the
development of an aggressive phenotype with increased invasive and
metastatic potential (17).
Increased vimentin expression has been reported in a number of
epithelial cancers, including breast cancer, lung cancer and
esophageal squamous cell carcinoma (16,28,29).
In the present study, it was also demonstrated that vimentin
expression is associated with venous invasion and that it is an
independent predictor of prognosis in ESCC. It is hypothesized that
podoplanin is likely to be important in the process of EMT, as a
number of cancer cells in ESCC co-expressed podoplanin and
vimentin. A proportion of podoplanin-positive cancer cells also
expressed vimentin, suggesting that podoplanin may result in
vimentin-associated EMT. EMT is understood to induce a more
aggressively invasive and malignant phenotype in ESCC. Future
studies should also analyze the expression of EMT markers, such as
N-cadherin, snail and fibronectin, and the epithelial marker,
E-cadherin, in ESCC. Finally, in the present study, the membrane
expression of podoplanin was shown to be a novel
immunohistochemical indicator of a highly malignant phenotype of
human ESCC.
References
|
1
|
Chino O, Kijima H, Shimada H, et al:
Accumulation of p53 in esophageal squamous cell carcinoma. Int J
Mol Med. 8:359–363. 2001.PubMed/NCBI
|
|
2
|
Oshiba G, Kijima H, Himeno S, et al:
Stromal thrombo-spondin-1 expression is correlated with progression
of esophageal squamous cell carcinomas. Anticancer Res.
19:4375–4378. 1999.
|
|
3
|
Makuuchi H, Shimada H, Mizutani K, et al:
Clinical pathological analysis of surgically resected superficial
esophageal carcinoma to determine criteria for deciding on
treatment strategy. Diagn Ther Endosc. 3:211–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimada H, Makuuchi H, Ozawa S, et al:
Technique of the double-channel ESD method performed with an EEMR
tube. Esophagus. 8:67–70. 2011. View Article : Google Scholar
|
|
5
|
Prognostic significance of CyclinD1 and
E-Cadherin in patients with esophageal squamous cell carcinoma:
multiinstitutional retrospective analysis. Research Committee on
Malignancy of Esophageal Cancer, Japanese Society for Esophageal
Diseases. J Am Coll Surg. 192:708–718. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoneda M, Fujiwara H, Furutani A, et al:
Prognostic impact of tumor IL-6 expression after preoperative
chemoradiotherapy in patients with advanced esophageal squamous
cell carcinoma. Anticancer Res. 33:2699–2705. 2013.PubMed/NCBI
|
|
7
|
Ito E, Ozawa S, Kijima H, et al: New
invasive patterns as a prognostic factor for superficial esophageal
cancer. J Gastroenterol. 47:1279–1289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaneko MK, Kato Y, Kitano T and Osawa M:
Conservation of a platelet activating domain of Aggrus/podoplanin
as a platelet aggregation-inducing factor. Gene. 378:52–57. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martin-Villar E, Megías D, Castel S, et
al: Podoplanin binds ERM proteins to activate RhoA and promote
epithelial-mesenchymal transition. J Cell Sci. 119:4541–4553. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wicki A, Lehembre F, Wick N, et al: Tumor
invasion in the absence of epithelial-mesenchymal transition:
podoplanin-mediated remodeling of the actin cytoskeleton. Cancer
Cell. 9:261–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rahadiani N, Ikeda J, Makino T, et al:
Tumorigenic role of podoplanin in esophageal squamous-cell
carcinoma. Ann Surg Oncol. 17:1311–1323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong L, Yuan S, Feng F and Zhang H: Role
of podoplanin expression in esophageal squamous cell carcinoma: a
retrospective study. Dis Esophagus. 25:72–80. 2012. View Article : Google Scholar
|
|
13
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin H, Morohashi S, Sato F, et al:
Vimentin expression of esophageal squamous cell carcinoma and its
aggressive potential for lymph node metastasis. Biomed Res.
31:105–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mendez MG, Kojima S and Goldman RD:
Vimentin induces changes in cell shape, motility, and adhesion
during the epithelial to mesenchymal transition. FASEB J.
24:1838–1851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sobin LH, Gospodrowicz MK and Wittekind C:
TNM Classification of Malignant Tumors. 7th edition. Wiley;
Hoboken, NJ, USA: 2009
|
|
19
|
Japanese Gastric Cancer Association: Japan
classification of gastric carcinoma, 2nd English edition. Gastric
Cancer. 1:10–24. 1998. View Article : Google Scholar
|
|
20
|
Shimada Y, Ishii G, Nagai K, et al:
Expression of podoplanin, CD44, and p63 in squamous cell carcinoma
of the lung. Cancer Sci. 100:2054–2059. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan P, Temam S, El-Naggar A, et al:
Overexpression of podoplanin in oral cancer and its association
with poor clinical outcome. Cancer. 107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki H, Onimaru M, Yonemitsu Y, et al:
Podoplanin in cancer cells is experimentally able to attenuate
prolymphangiogenic and lymphogenous metastatic potentials of lung
squamoid cancer cells. Mol Cancer. 9:2872010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sudo T, Iwaya T, Nishida N, et al:
Expression of mesen-chymal markers vimentin and fibronectin: the
clinical significance in esophageal squamous cell carcinoma. Ann
Surg Oncol. 20(Suppl 3): S324–S335. 2013. View Article : Google Scholar
|
|
24
|
Wicki A and Christofori G: The potential
role of podoplanin in tumour invasion. Br J Cancer. 96:1–5. 2007.
View Article : Google Scholar
|
|
25
|
Myong NH: Loss of E-cadherin and
acquisition of vimentin in epithelial-mesenchymal transition are
noble indicators of uterine cervix cancer progression. Korean J
Pathol. 46:341–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Le Bras GF, Allison GL, Richards NF, et
al: CD44 upregulation in E-cadherin-negative esophageal cancers
results in cell invasion. PLoS One. 6:e270632011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Usami Y, Satake S, Nakayama F, et al:
Snail-associated epithelial-mesenchymal transition promotes
oesophageal squamous cell carcinoma motility and progression. J
Pathol. 215:330–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hendrix MJ, Seftor EA, Seftor RE and
Trevor KT: Experimental co-expression of vimentin and keratin
intermediate filaments in human breast cancer cells results in
phenotypic interconversion and increased invasive behavior. Am J
Pathol. 150:483–495. 1997.PubMed/NCBI
|
|
29
|
Dauphin M, Barbe C, Lemaire S, et al:
Vimentin expression predicts the occurrence of metastases in non
small cell lung carcinomas. Lung Cancer. 81:117–122. 2013.
View Article : Google Scholar : PubMed/NCBI
|