Introduction
Atherosclerosis (AS) is a multifactorial process
associated with inflammation, which occurs in response to
progressive vascular injury (1–3).
Vascular endothelium has an important role in the atherosclerotic
process, through the secretion of factors that can directly
regulate vascular tone, induce accumulation and activation of
platelets and leukocytes at the vessel wall, and cause
proliferation of vascular smooth muscle cells (4–9). The
endothelium forms barriers that define tissue compartments within
higher organisms. Tight junctions (TJ) are essential to the
functioning of the endothelial barrier (10–12).
Endothelial TJs form a barrier against passive paracellular flux,
which is regulated by complex physiological and pathophysiological
signals that acutely control TJ permeability. The permeability of
endothelial junctions is maintained by junction proteins that
cross-link to the cytoskeleton. TJ proteins consist of occludin,
claudins, junction adhesion molecules and zonulae occludentes (ZO).
Occludin is positioned at cell junctions in close proximity to
ZO-1, where they have a key role in maintaining the integrity of
TJs and cell permeability (13,14).
Vascular endothelial dysfunction (VED) is a systemic
disorder, and a critical element in the pathogenesis of AS and its
complications. VED has recently gained attention as a key issue in
cardiovascular biology, in particular with regard to its role in
the origin and pathogenesis of AS, myocardial stunning, and
restenosis following coronary artery balloon angioplasty (15). Previous studies have demonstrated
that VED reflects a vascular phenotype prone to atherogenesis and
may serve as a marker of inherent AS risk (16–18).
Therefore, reversal of VED has been suggested as a novel
therapeutic approach to inhibit or delay the incidence of AS.
Previous studies have implicated myosin light chain
kinase (MLCK) in the regulation of endothelial cell (EC) barrier
permeability through direct phosphorylation of myosin light chain
(MLC) (19–22). MLCK is able to phosphorylate
threo-nine 18 and serine 19 of MLC, which results in activation of
intracellular cytoskeletal contraction-relaxation cycles (23). Phosphorylation induces a
conformational change in MLC that enables actin-myosin interaction
and cell contraction. Eutamene et al (24) used MLCK knockout mice to
demonstrate that inhibition of MLCK activity can protect against
acute lung injury.
Previous studies have demonstrated that endothelial
permeability is increased in high-fat diet-induced AS (3,25);
however, the precise underlying mechanisms have remained to be
elucidated. The present study aimed to investigate whether the MLCK
inhibitor ML7 is able to improve VED and AS by regulating the
expression of TJ proteins ZO-1 and occludin via mechanisms
involving MLCK and MLC phosphorylation in high-fat diet-fed
rabbits.
Materials and methods
Ethics statement
All of the animal experimental and surgical
procedures conducted in the present study were approved by the
Animal Ethics Committee of the First Hospital Affiliated to Anhui
Medical University (Hefei, China), in accordance with the National
Guidelines for animal welfare (21).
Reagents and instruments
Anti-MLCK monoclonal antibody (cat. no. M7905) and
anti-phosphorylated MLC polyclonal antibody (cat. no. M6068) were
purchased from Sigma-Aldrich (St. Louis, MO, USA), monoclonal
antibodies targeting occludin (cat. no. ab167161), ZO-1 (cat. no.
ab61357) and β-actin (cat. no. ab8226) were obtained from Abcam
(Cambridge, UK). ML7, Oil red O (ORO) powder and acetylcholine
(Ach) were purchased from Sigma-Aldrich. Nitroglycerin (NTG) was
from Beijing Sihuan Pharmaceutical Co., Ltd. (Beijing, China). The
total cholesterol (TC), low-density lipoprotein cholesterol
(LDL-c), high-density lipoprotein cholesterol (HDL-c) and
triglyceride (TG) ELISA kits were purchased from Beijing BHKT
Clinical Reagent Co., Ltd. (Beijing, China). 3,3′-Diaminobenzidine
(DAB) was obtained from Pierce Biotechnology, Inc. (Rockford, IL,
USA). Polyvinylidene fluoride (PVDF) membranes were from GE
Healthcare Bio-Sciences (Little Chalfont, UK). Horseradish
peroxidase-conjugated secondary antibodies (cat. no. SP-9000-D)
were purchased from Zhongshan Jinqiao Biotechnology Co., Ltd.
(Beijing, China). Enhanced Chemiluminescence (ECL) reagents were
obtained from Engreen Biosystem (Beijing, China). Vectashield
mounting medium was from Vector Laboratories, Inc. (Burlingame, CA,
USA). The 13-MHz ultrasound probe (GES6 two-dimensional Color
Doppler Ultrasound Diagnostic Apparatus) was purchased from GE
Healthcare Bio-Sciences. The DX51 light microscope was from Olympus
Corporation (Tokyo, Japan).
Animal groups and pre-treatment of tissue
samples
A total of 49 two-month-old male New Zealand white
rabbits (weighing 1.98±0.22 kg) were obtained from Nanjing Jinling
Rabbit Farm (Nanjing, China), and were randomly divided into three
groups. The rabbits were housed individually in screen-bottomed
plastic cages, and maintained in a temperature-controlled room
(25°C) with a standard 12 h light/dark cycle. The control group
(n=14) was fed a standard diet for 12 weeks. The AS group (n=16)
was fed a high-fat diet (standard diet supplemented with 5% lard
and 2% cholesterol; Dalian Bell Pharmaceutical Co., Ltd., Dalian,
China) for 12 weeks. The ML7 group (n=19) received a high-fat diet
supplemented with ML7 (1 mg/kg/day) for 12 weeks. After 12 weeks,
following fasting overnight, the rabbits were anesthetized with 50
mg/kg ketamine hydrochloride (Jiangsu Hengrui Medicine Co., Ltd.,
Jiangsu, China). Blood was collected for cholesterol
determinations, and the aortas were excised and removed. One part
of the aorta was harvested for ORO staining, and another was fixed
in 4% formalin for immunohistochemistry (IHC) and hematoxylin &
eosin (HE) staining. The aortas of the remaining rabbits were
removed and immediately frozen at −80°C, prior to homogenization in
1X SDS lysis buffer (50 mM Tris-HCl, pH 6.8; 10% glycerol; 2% SDS)
and western blot analysis.
Transcutaneous non-invasive ultrasound
measurement of vascular endothelial function
Transcutaneous non-invasive ultrasound evaluation of
endothelial function of the abdominal aorta was performed two days
prior to the end of the experiment, as described previously
(26,27). The rabbits underwent a 12-h fast,
and the marginal ear veins were cannulated for drug infusion. The
rabbits were anesthetized with 3% pentobarbital sodium (Beijing
Propbs Biotechnology Co., Ltd., Beijing, China) via the marginal
ear vein, placed in the dorsal decubitus position and their
abdomens were shaved. After 15 min of rest in the supine position,
ultrasonic examination of the abdominal aorta was performed using a
13-MHz ultrasound probe. The transducer was lubricated with
ultrasound gel (Shandong Beno Pharmaceutical Biotechnology Co.,
Ltd., Shandong, China) and placed at the abdominal aorta with
minimal pressure, 1.0 cm below the renal artery, in order to obtain
a longitudinal axis view of the abdominal aorta. Image settings
were optimized to achieve the best and clearest definition of the
endothelial-blood interface. Once the imaging of the aorta was
considered optimal, the rabbits subsequently received the following
sequential drug perfusions via the marginal ear vein for 2 min at
10-min intervals: i) saline (1 ml/min); ii) Ach at 1.5, 3 and 6
µg/ml/min; and iii) NTG at 5 and 7.5 µg/ml/min. Images of the
abdominal aorta were continuously captured throughout the entire
procedure at the largest cross section, and the maximal diameter
was measured. The ultrasound probe remained in the same position
for the duration of the measurement. The change in diameter was
expressed as a percentage of the baseline diameter, denoted as
Ratio 1, Ratio 2, Ratio 3, Ratio 4 and Ratio 5. Ratio refers to the
aortic diastolic maximum inner diameter, under various doses of
each drug vs. baseline aortic diastolic maximum inner diameter,
under basal conditions. Ratios 1, 2 and 3 refer to aortic diastolic
maximum inner diameter under Ach (1.5, 3 and 6 µg/ml/min) vs. the
aortic diastolic maximum inner diameter under saline. Ratios 4 and
5 refer to aortic diastolic maximum inner diameter under NTG (5 and
7.5µg/ml/min) vs. the aortic diastolic maximum inner diameter under
saline.
Serum lipid measurement
Blood samples were collected via cardiac puncture,
and serum TC, LDL-c, HDL-c, and TG levels were measured using
commercially available ELISA kits. All measurements were performed
according to the manufacturer's instructions and each sample was
assayed in triplicate.
ORO staining
Lipid content in the aorta wall was assessed using
ORO staining. The ORO solution was prepared by slowly dissolving
0.5 g ORO powder in 100 ml isopropanol while heating to 60°C and
stirring until completely dissolved. The solution was then filtered
twice using Whatman filter paper (GE Healthcare Bio-Sciences) and
cooled prior to use. The aortas were immersed in ORO solution for
20 min, followed by 1 min in 70% ethanol. Subsequently, the aortas
were rinsed with 60% ethanol for 2 min followed by distilled water
for several minutes. Images of the staining were then captured
(D750; Nikon Corporation, Tokyo, Japan).
HE staining
HE staining (Beijing Century Heli Biotechnology Co.,
Ltd., Beijing, China) was performed to evaluate the pathological
and morphological changes in the arterial wall tissue. Fixed aortic
specimens were dehydrated, embedded in paraffin, sectioned (6 µm)
and stained with HE according to previously published methods
(28). The tissue sections were
deparaffinized using xylene, hydrated through a series of graded
ethanol and stained with HE. The sections were subsequently
dehydrated through a series of graded ethanol, cleared in xylene
and mounted using neutral resin. The pathological and morphological
changes of the arterial wall tissue were observed under an optical
microscope (DM4000B, Leica Microsystems, Wetzlar, Germany).
IHC analysis
The aortas were fixed in buffered parafor-maldehyde
at 4°C and embedded in paraffin. The sections (6 µm) were
deparaffinized, rehydrated and incubated in phosphate-buffered
saline (PBS) containing 3% H2O2 in order to
suppress endogenous peroxidase activity. The sections were then
blocked in species-specific normal sera (Zhongshan Jinqiao
Biotechnology Co., Ltd.) for 30–60 min in order to reduce
non-specific staining and were subsequently incubated with primary
antibodies (anti-MLCK, anti-phosphorylated MLC, anti-occludin,
anti-ZO-1; 1:1,000) or pre-immune sera at 4°C overnight. The
sections were then incubated with a horseradish
peroxidase-conjugated secondary antibody. A DAB substrate working
solution was used until the desired staining intensity was achieved
to visualize the antibodies. Nuclei were counterstained with
hematoxylin. Following thorough washing with PBS, the sections were
mounted on glass slides using Vectashield and images were captured
(Leica DM4000B).
Western blot analysis
The aortas were washed three times in PBS and then
lysed in 1X SDS lysis buffer containing 50 mM Tris-HCl (pH 6.8),
10% glycerol and 2% SDS (Sigma-Aldrich). The cell lysates were
boiled for 10 min and then centrifuged at 16,060 x g for 15 min at
room temperature. The protein samples (50 µg protein/lane) were
then separated by 10% SDS-PAGE and transferred to PVDF membranes.
The membranes were blocked in 5% bovine serum albumin
(Sigma-Aldrich) for 2 h, followed by incubation at 4°C overnight
with the appropriate primary antibodies (anti-occludin, 1:1,000;
anti-ZO1, 1:1,000; anti-β-actin, 1:5,000). Primary antibodies were
then detected following incubation with the corresponding
horseradish peroxidase-conjugated secondary antibodies. Blots were
visualized using ECL reagents and Kodak film (Eastman Kodak,
Rochester, NY, USA). The results were analyzed using Image-Pro Plus
6.0 analysis system (Media Cybernetics, Inc., Rockville, MD, USA).
To separate the membrane and cytoplasmic protein, a cell membrane
and cytoplasm protein extraction kit (Beyotime Institute of
Biotechnology, Shanghai, China) was used. Briefly, moderately
homogenized cells were disrupted by low-speed centrifugation (700 x
g, 10 min, 4°C) to remove nuclei and small amounts of precipitation
produced by the cells. Subsequently, the cells underwent high-speed
centrifugation (14,000 x g, 30 min, 4°C) in order to obtain the
cell precipitate. The precipitate contained the membrane proteins,
whereas the supernatant contained the cytoplasmic proteins.
Membrane protein extraction agents were added to the precipitate
and vortexed violently for 5 sec, in order to resuspend the
precipitate, and the sample was incubated on ice for 5 min. The
above steps were repeated twice and then centrifuged at 14,000 x g
for 5 min at 4°C. The supernatant was then collected, which
contained the membrane proteins.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Student's t-test was used to identify significant
differences between values. The correlation between vascular
endothelial function and changes in serum lipid level was evaluated
by Pearson correlation analysis. All statistical analyses were
conducted using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
ML7 reduces lipid deposition lesions in
AS rabbits
The body weight of the rabbits increased in the AS
and ML7 groups, as compared with that in the control group
(P<0.01; Table I). In addition,
the rabbits in the AS and ML7 groups exhibited arterial lesions
(Fig. 1Ba and Ca) after being fed
an atherogenic diet for 12 weeks, as compared with the control
group (Fig. 1Aa). Lipid deposition
in the lesions was detected using ORO staining (Fig. 1Ab–Cb). A reduction in the lesion
area was observed in the rabbits of the ML7 group (Fig. 1Ca and Cb), as compared with the AS
group (Fig. 1Ba and Bb).
Administration of a high-fat diet significantly increased the serum
lipid levels in the rabbits in the AS group, as compared with those
in the rabbits in the control group. As shown in Table II, the serum levels of TC, LDL-c,
HDL-c and TG were all markedly increased in the AS and ML7 groups
as compared with those in the control group (P<0.01).
Furthermore, the serum levels of TC and LDL-c were reduced, whereas
levels of HDL-c were significantly increased in the ML7 group, as
compared with those in the AS group (P<0.01).
 | Table IEffects of a high-fat diet on the
weight of experimental rabbits. |
Table I
Effects of a high-fat diet on the
weight of experimental rabbits.
| Group | 0-week body weight
(kg) | 12-week body weight
(kg) |
|---|
| Control (n=14) | 1.92±0.18 | 2.50±0.22 |
| AS (n=16) | 2.00±0.24 | 2.96±0.32a |
| ML-7 (n=19) | 2.02±0.24 | 2.82±0.29a |
 | Table IISerum lipid levels in the various
groups. |
Table II
Serum lipid levels in the various
groups.
| Lipid (mmol/l) | Control
group
(n=14) | AS group
(n=16) | ML-7
group
(n=19) |
|---|
| TC | 1.52±0.51 | 29.37±4.36a | 20.60±4.43a,b |
| LDL-c | 0.73±0.31 | 24.57±4.57a | 17.27±3.93a,b |
| HDL-c | 0.64±0.34 | 2.05±1.39a | 4.34±0.68a |
| TG | 0.23±0.10 | 2.72±2.42a | 1.62±0.81a |
ML7 improves the vascular endothelial
function of experimental AS
The vascular endothelial function of the rabbits was
compared between the three groups. Intake of the high-fat diet for
12 weeks did not produce significant alterations in
endothelium-independent vasorelaxation of the abdominal aorta
following NTG infusion; however, marked decreases were observed in
endothelium-dependent vasorelaxation of the abdominal aorta
following Ach infusion as compared with that in the control group
(P<0.05 and P<0.01; Table
III). Furthermore, treatment with ML7 significantly attenuated
the high-fat diet-induced impairment of endothelium-dependent
vasorelaxation of the abdominal aorta following Ach infusion
(P<0.05 and P<0.01; Table
III). Representative ultrasound images are shown in Fig. 2. These results indicated that ML7
markedly improved the vascular endothelial function of experimental
AS in rabbits; however, it was unable to restore vascular
endothelial function to its normal level.
 | Table IIIChanges in the abdominal aorta
diameter in response to acetylcholine or nitroglycerin infusions in
the three groups. |
Table III
Changes in the abdominal aorta
diameter in response to acetylcholine or nitroglycerin infusions in
the three groups.
| Ratio | Control group | AS group | ML-7 group |
|---|
| 1 | 8.84±3.81 | 2.04±2.07a | 5.40±3.05b,d |
| 2 | 13.56±2.86 | 4.32±2.38a | 7.79±3.60a,d |
| 3 | 16.96±3.67 | 5.35±1.96a | 10.95±3.836a,c |
| 4 | 14.08±2.04 | 14.56±4.23 | 15.36±3.88 |
| 5 | 18.55±4.05 | 17.92±4.70 | 18.29±3.52 |
In addition, a Pearson correlation analysis was
conducted to evaluate the correlation between vascular endothelial
function and the levels of serum lipids in the AS and ML7 groups.
Pearson correlation analyses showed that alterations in vascular
endothelial function were negatively correlated with changes in TC
and LDL-c levels (P<0.01 and 0.05) and had little correlation
with changes in TG and HDL-c levels in the AS and ML7 groups
(Tables IV and V). These results indicated that treatment
with ML7 improved vascular endothelial function by reducing the
serum lipid levels.
 | Table IVPearson correlation analysis between
vascular endothelial function and the levels of serum lipids in the
atherosclerosis group. |
Table IV
Pearson correlation analysis between
vascular endothelial function and the levels of serum lipids in the
atherosclerosis group.
| Ratio | TC | LDL-c | HDL-c | TG |
|---|
| 1 | −0.821a | −0.850a | 0.115 | 0.085 |
| 2 | −0.565 | −0.668b | 0.104 | 0.313 |
| 3 | −0.891a | −0.759b | −0.048 | −0.261 |
 | Table VPearson correlation analysis between
vascular endothelial function and the levels of serum lipids in the
ML-7 group. |
Table V
Pearson correlation analysis between
vascular endothelial function and the levels of serum lipids in the
ML-7 group.
| Ratio | TC | LDL-c | HDL-c | TG |
|---|
| 1 | −0.798b | −0.867a | 0.290 | −0.531 |
| 2 | −0.807a | −0.804a | 0.154 | −0.751b |
| 3 | −0.795b | −0.756b | −0.016 | −0.814a |
ML7 suppresses tight junction protein
expression in AS rabbits
A previous study by our group demonstrated that
endothelial permeability is increased in high-fat diet-fed AS
rabbits (29). Therefore, the
present study assessed the changes in endothelial TJ proteins in
response to ML7 by using HE and IHC staining as well as western
blot analysis. HE staining showed that, compared with the control
group (Fig. 3Aa and Ba),
hyperplasia was evident in the uneven aortic intima, and the ECs
had an incomplete structure in the AS group (Fig. 3Ab and Bb). Treatment with ML7
smoothed the intima, and fewer foam cells, vascular smooth muscle
cells and lipid plaques were observed underneath the endothelium
(Fig. 3Ac and Bc). For IHC, the
sections were incubated with antibodies targeting the TJ proteins
ZO-1 and occludin. The results demonstrated that ZO-1 and occludin
were not as highly expressed in the control group (Fig. 3Ad and Bd) as in the AS group
(Fig. 3Ae and Be), whereas
treatment with ML7 reduced the expression levels of ZO-1 and
occludin (Fig. 3Af and Bf).
Western blot analysis produced similar results regarding occludin
expression in the total protein lysates of rabbit aortas. It was
further demonstrated that treatment with ML7 increased occludin
expression in the precipitate, but reduced its expression in the
supernatant of lysed aortas, thus indicating that occludin
expression occurred during remodeling from cell membrane to
cytoplasm in AS (Fig. 3C).
 | Figure 3Treatment with ML7 blocks the
expression of tight junction proteins ZO-1 and occludin in AS
rabbits. (A) Expression of ZO-1 in the various rabbit groups. (a–c)
HE stain; (d–f) IHC. (B) The expression of occludin in the various
rabbit groups. (a–c) HE stain; (d–f) IHC. (C) Protein expression
levels of occludin were analyzed by western blot analysis. (Aa, Ad,
Ba, Bd and Ca) Control group; (Ab, Ae, Bb, Be and Cb) AS group;
(Ac, Af, Bc, Bf and Cc) ML7 group. Magnification, x200. AS,
atherosclerosis; ZO-1, zona occludens-1; IHC, immunohistochemistry;
HE, hematoxylin and eosin. |
ML7 attenuates MLCK expression and MLC
phosphorylation in AS rabbits
To further elucidate the precise mechanisms
underlying ML7 function, MLCK expression and MLC phosphorylation
were examined using HE staining and IHC. HE staining of MLCK
(Fig. 4Aa–c) and phosphorylated
MLC (Fig. 4Ba–c) showed that the
arterial specimens of the control group exhibited a normal
morphology of the aorta intima on the luminal side (Fig. 4Aa and Ba). In the AS group, the ECs
were shown to have an incomplete structure (Fig. 4Ab and Bb), whereas treatment with
ML7 smoothed the intima (Fig. 3Ac and
Bc). IHC staining of the sections with anti-MLCK and
anti-phosphorlyated MLC antibodies showed that the expression of
MLCK and the phosphorylation of MLC was markedly increased in AS
rabbits (Fig. 4Ae and Be) as
compared with that in the control group (Fig. 4Ad and Bd). Positive staining was
distributed regularly, and was most intense in areas surrounding
the luminal side. Treatment with ML7 attenuated MLCK expression and
MLC phosphorylation in the aortic ECs (Fig. 4Af and Bf).
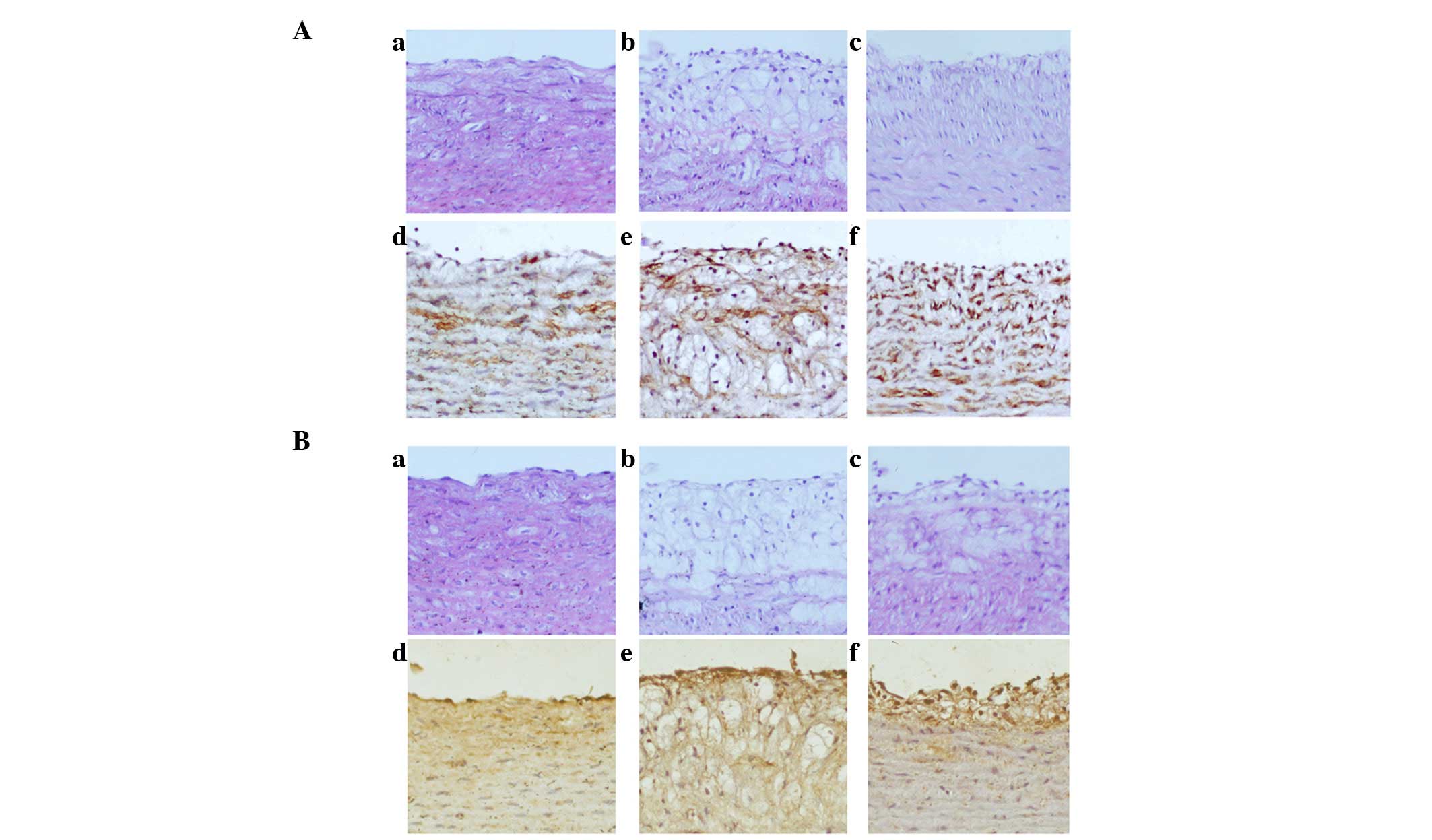 | Figure 4Expression of MLCK and phosphorylation
of MLC was increased in AS rabbits, whereas treatment with ML7
attenuated MLCK expression and MLC phosphorylation. (A) Expression
of MLCK in the various rabbit groups. (a–c) HE stain; (d–f) IHC.
(B) Phosphorylation of MLC in the various rabbit groups. (a–c) HE
stain; (d–f) IHC. (Aa, Ad, Ba and Bd) Control group; (Ab, Ae, Bb
and Be) AS group; (Ac, Af, Bc and Bf) ML7 group. Magnification,
x200. AS, atherosclerosis; MLC, myosin light chain; MLCK, MLC
kinase; IHC, immunohistochemistry; HE, hematoxylin and eosin. |
Discussion
Vascular ECs are the primary permeability barrier
between vascular tissue and blood, and are important for the
maintenance of normal biological homeostasis (30). It is generally hypothesized that AS
lesions are initiated by arterial endothelial injury. A previous
study by our group showed that endothelial permeability was
increased in high-fat diet-fed AS rabbits (29). MLCK is a protein kinase that has an
important role in the re-organization of the cytoskeleton, leading
to disruption of vascular barrier integrity (31). In the present study, higher serum
TC, LDL-c and TG levels, and typical morphological characteristics
of arterial lesions were observed in the rabbits of the AS group as
compared with those in rabbits fed a normal diet. Treatment with
the MLCK inhibitor ML7 markedly reduced serum levels of TC, LDL-c
and TG, and increased HDL-c levels, as well as inhibiting the
progression of AS in high-fat diet-fed rabbits. MLCK catalyzes the
phosphorylation of MLC, leading to cytoskeletal re-arrangements. EC
concentric contraction and gap formation are followed by
alterations to the cytoskeleton, causing lipid infiltration into
the arterial intima, which accumulate in arterial walls, ultimately
leading to atheromatous plaque formation (32–34).
Numerous studies have indicated that hyperlipidemia is an important
risk factor for the occurrence of AS (2,35).
A study on the basic mechanisms associated with
atherogenesis indicated that VED represents a key early step in the
development of AS, and is also associated with plaque progression
and the occurrence of atherosclerotic complications (17). In the present study, transcutaneous
non-invasive ultrasound evaluation of endothelial function in AS
rabbits demonstrated a marked decrease in endothelium-dependent
vasorelaxation of the abdominal aorta following Ach infusion as
compared with that in the rabbits fed a normal diet. Treatment with
ML7 attenuated the high-fat diet-induced impairment of
endothelium-dependent vasorelaxation and improved the VED by
reducing serum lipid levels. These findings suggested important
roles for ML7 in the amelioration of lipid metabolism, VED and AS
in high-fat diet-fed rabbits.
The present study also demonstrated that MLCK and
MLC phosphorylation were clearly involved in TJ regulation. The
permeability of endothelial junctions is maintained by TJ proteins,
including ZO-1 and occludin, which cross-link to the cytoskeleton
(36). The results of the present
study demonstrated that ZO-1 and occludin were highly expressed in
the AS group, whereas treatment with ML7 was able to reduce the
expression levels of ZO-1 and occludin. Western blot analysis
showed similar results regarding occludin expression in the total
protein lysates of the rabbit aortas. Furthermore, it was
demonstrated that treatment with ML7 increased occludin expression
in the precipitate, but reduced its expression in the supernatant
of lysed aortas, thus indicating that occludin expression occurred
during remodeling from cell membrane to cytoplasm.
ML7 is a selective MLCK inhibitor, which acts on the
adenosine triphosphate-binding site of the active center of MLCK
(21). The results of the present
study confirmed the crucial role of MLCK in vascular endothelial
permeability regulation. Although the number of studies on
actomyosin regulation of cell morphology has increased over the
past few years, the functional importance of the contractile
elements in controlling permeability remains to be elucidated
(37). The present study
demonstrated that MLCK expression and MLC phosphorylation were
significantly increased in AS rabbits, and treatment with ML7
attenuated MLCK expression and MLC phosphorylation in aortic ECs,
thus indicating that TJ protein expression, endothelial
permeability and gap formation may be associated with MLCK
expression and MLC phosphorylation. The present study was the
first, to the best of our knowledge, to indicate that MLCK
inhibitor ML7 may improve VED and AS by regulating the expression
of TJ proteins ZO-1 and occludin via mechanisms involving MLCK and
MLC phosphorylation in high-fat diet-fed rabbits.
In conclusion, the present study provided evidence
that ML7 is able to regulate lipid metabolism, and improve VED and
AS in high-fat diet-fed rabbits. MLCK-mediated MLC phosphorylation
was shown to have an important role in the development of AS.
However, further studies are required to define the mechanism
underlying this regulation and clarify the associated signaling
networks.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81070232, 81270372
and 30971226), the Key Project of Chinese Ministry of Education
(grant no. 212077), Grants for Scientific Research of BSKY (grant
nos. XJ201107 and XJ2008015) from Anhui Medical University, the
National Natural Science Foundation for Younger of China (grant no.
81302150) and Grants of the Cultivating Program of National Natural
Science Foundation for young scholars of the First Affiliated
Hospital of Anhui Medical University (grant no. 2012KJ10).
References
|
1
|
McDermott DH, Halcox JP, Schenke WH, et
al: Association between polymorphism in the chemokine receptor
CX3CR1 and coronary vascular endothelial dysfunction and
atherosclerosis. Circ Res. 89:401–407. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frohlich J and Al-Sarraf A: Cardiovascular
risk and atherosclerosis prevention. Cardiovasc Pathol. 22:16–18.
2013. View Article : Google Scholar
|
|
3
|
Libby P, Ridker PM and Hansson GK:
Progress and challenges in translating the biology of
atherosclerosis. Nature. 473:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalwa H, Sartoretto JL, Martinelli R, et
al: Central role for hydrogen peroxide in P2Y1 ADP
receptor-mediated cellular responses in vascular endothelium. Proc
Natl Acad Sci USA. 111:3383–3388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horio E, Kadomatsu T, Miyata K, et al:
Role of endothelial cell-derived angptl2 in vascular inflammation
leading to endothelial dysfunction and atherosclerosis progression.
Arterioscler Thromb Vasc Biol. 34:790–800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alvarez-García V, González A,
Alonso-González C, et al: Regulation of vascular endothelial growth
factor by melatonin in human breast cancer cells. J Pineal Res.
54:373–380. 2013.
|
|
7
|
d'Uscio LV, He T, Santhanam AV, et al:
Mechanisms of vascular dysfunction in mice with
endothelium-specific deletion of PPAR-δ gene. Am J Physiol Heart
Circ Physiol. 306:H1001–H1010. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sandoo A, Chanchlani N, Hodson J, et al:
Classical cardiovascular disease risk factors associate with
vascular function and morphology in rheumatoid arthritis: A
six-year prospective study. Arthritis Res Ther. 15:R2032013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robert J, Weber B, Frese L, et al: A
three-dimensional engineered artery model for in vitro
atherosclerosis research. PLoS One. 8:e798212013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chattopadhyay R, Dyukova E, Singh NK, et
al: Vascular endothelial tight junctions and barrier function are
disrupted by 15(S)-hydroxyeicosatetraenoic acid partly via protein
kinase C ε-mediated zona occludens-1 phosphorylation at threonine
770/772. J Biol Chem. 289:3148–3163. 2014. View Article : Google Scholar :
|
|
11
|
Hu ZP, Fang XL, Fang N, et al: Melatonin
ameliorates vascular endothelial dysfunction, inflammation and
atherosclerosis by suppressing the TLR4/NF-κB system in
high-fat-fed rabbits. J Pineal Res. 55:388–398. 2013.PubMed/NCBI
|
|
12
|
D'Agnillo F, Williams MC, Moayeri M and
Warfel JM: Anthrax lethal toxin downregulates claudin-5 expression
in human endothelial tight junctions. PLoS One. 8:e625762013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lampugnani MG: Endothelial cell-to-cell
junctions: Adhesion and signaling in physiology and pathology. Cold
Spring Harb Perspect Med. 2:a0065282012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Collins NT, Cummins PM, Colgan OC, et al:
Cyclic strain-mediated regulation of vascular endothelial occludin
and ZO-1: Influence on intercellular tight junction assembly and
function. Arterioscler Thromb Vasc Biol. 26:62–68. 2006. View Article : Google Scholar
|
|
15
|
Briasoulis A, Tousoulis D, Androulakis ES,
et al: Endothelial dysfunction and atherosclerosis: Focus on novel
therapeutic approaches. Recent Pat Cardiovasc Drug Discov. 7:21–32.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sawada N, Jiang A, Takizawa F, et al:
Endothelial PGC-1α mediates vascular dysfunction in diabetes. Cell
Metab. 19:246–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bonetti PO, Lerman LO and Lerman A:
Endothelial dysfunction: A marker of atherosclerotic risk.
Arterioscler Thromb Vasc Biol. 23:168–175. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Woo A, Shin W, Cuong TD, et al: Arginase
inhibition by piceatannol-3′-O-β-D-glucopyranoside improves
endothelial dysfunction via activation of endothelial nitric oxide
synthase in ApoE-null mice fed a high-cholesterol diet. Int J Mol
Med. 31:803–810. 2013.PubMed/NCBI
|
|
19
|
Yuan SY: Protein kinase signaling in the
modulation of micro-vascular permeability. Vascul Pharmacol.
39:213–223. 2002. View Article : Google Scholar
|
|
20
|
Rashid G, Bernheim J, Green J and
Benchetrit S: Parathyroid hormone stimulates endothelial expression
of atherosclerotic parameters through protein kinase pathways. Am J
Physiol Renal Physiol. 292:F1215–F1218. 2007. View Article : Google Scholar
|
|
21
|
Zhu HQ, Zhou Q, Jiang ZK, Gui SY and Wang
Y: Association of aorta intima permeability with myosin light chain
kinase expression in hypercholesterolemic rabbits. Mol Cell
Biochem. 347:209–215. 2011. View Article : Google Scholar
|
|
22
|
Pinto JR, Gomes AV, Jones MA, et al: The
functional properties of human slow skeletal troponin T isoforms in
cardiac muscle regulation. J Biol Chem. 287:37362–37370. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodella LF, Favero G, Foglio E, et al:
Vascular endothelial cells and dysfunctions: Role of melatonin.
Front Biosci (Elite Ed). 5:119–129. 2013.
|
|
24
|
Eutamene H, Theodorou V, Schmidlin F, et
al: LPS-induced lung inflammation is linked to increased epithelial
permeability: Role of MLCK. Eur Respir J. 25:789–796. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu HQ, Wang XB, Han JX, et al: Myoson
light chain kinase inhibitor attenuates atherosclerosis and
permeability via reduced endothelial tight junction in rabbits. Int
J Cardiol. 168:5042–5043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen YX, Wang XQ, Fu Y, et al: Pivotal
role of inflammation in vascular endothelial dysfunction of
hyperlipidemic rabbit and effects by atorvastatin. Int J Cardiol.
146:140–144. 2011. View Article : Google Scholar
|
|
27
|
Drolet MC, Plante E, Battistini B, et al:
Early endothelial dysfunction in cholesterol-fed rabbits: A
non-invasive in vivo ultrasound study. Cardiovasc Ultrasound.
2:102004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou B, Pan Y, Hu Z, et al:
All-trans-retinoic acid ameliorated high fat diet-induced
atherosclerosis in rabbits by inhibiting platelet activation and
inflammation. J Biomed Biotechnol. 2012:2596932012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng X, Wan Y, Xu Y, Zhou Q, Wang Y and
Zhu H: Melatonin alleviates myosin light chain kinase expression
and activity via the mitogen-activated protein kinase pathway
during atherosclerosis in rabbits. Mol Med Rep. 11:99–104.
2015.
|
|
30
|
Hirase T and Node K: Endothelial
dysfunction as a cellular mechanism for vascular failure. Am J
Physiol Heart Circ Physiol. 302:H499–H505. 2012. View Article : Google Scholar
|
|
31
|
Rossi JL, Ralay Ranaivo H, Patel F, et al:
Albumin causes increased myosin light chain kinase expression in
astrocytes via p38 mitogen-activated protein kinase. J Neurosci
Res. 89:852–861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koga T, Kwan P, Zubik L, et al: Vitamin E
supplementation suppresses macrophage accumulation and endothelial
cell expression of adhesion molecules in the aorta of
hypercholesterolemic rabbits. Atherosclerosis. 176:265–272. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun C, Wu MH and Yuan SY: Nonmuscle myosin
light-chain kinase deficiency attenuates atherosclerosis in
apolipoprotein E-deficient mice via reduced endothelial barrier
dysfunction and monocyte migration. Circulation. 124:48–57. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang B, Yan Y, Zhou J, et al: A novel
all-trans retinoid acid derivatives inhibits the migration of
breast cancer cell lines MDA-MB-231 via myosin light chain kinase
involving p38-MAPK pathway. Biomed Pharmacother. 67:357–362. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamazaki T, Nohara R, Daida H, et al
Justification for Atherosclerosis Regression Treatment (JART)
Investigators: Intensive lipid-lowering therapy for slowing
progression as well as inducing regression of atherosclerosis in
Japanese patients: Subanalysis of the JART study. Int Heart J.
54:33–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goddard LM and Iruela-Arispe ML: Cellular
and molecular regulation of vascular permeability. Thromb Haemost.
109:407–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oldenburg J and de Rooij J: Mechanical
control of the endothelial barrier. Cell Tissue Res. 355:545–555.
2014. View Article : Google Scholar : PubMed/NCBI
|


















