Introduction
Lung cancer is one of the most common types of
malignant tumor worldwide, of which non-small cell lung cancer
(NSCLC) accounts for >85%. The trend of lung cancer treatment
has been towards personalized therapy and molecular targeted
therapy is currently one of the most popular and promising fields
of advanced NSCLC treatment. Driver genes, including epidermal
growth factor receptor (EGFR) and echinoderm microtubule associated
protein like 4-anaplastic lymphoma kinase (EML4-ALK) are common
target genes, and the success of the clinical application of
inhibitors against these two molecular targets have been
demonstrated in East Asia (1,2).
Previous studies suggested that the EML4-ALK fusion is almost
mutually exclusive to EGFR or K-RAS mutation in NSCLC (3–5).
However, at least 11 patients exhibiting both an EGFR mutation and
the EML4-ALK fusion have been reported worldwide (6–13).
The present study reports a twelfth case and also the first case of
a patient of northern Han Chinese ethnicity who exhibits the two
concomitant mutations. The most effective treatment for these two
gene-positive patients remains to be elucidated with discordant
results reported previously in the literature. All protocols in the
present study were approved by the Human Clinical and Research
Ethics Committees of the First Affiliated Hospital of Xi'an
Jiaotong University (Xi'an, China) and the General Military
Hospital of Beijing PLA (Beijing, China). The patient provided
written informed consent.
Case report
A 71 year-old female who has never smoked and
originates from Tongchuan City (Shan'xi, China), was first admitted
to The First Affiliated Hospital of Xi'an Jiaotong University
(Xi'an, China) as a result of left leg pain and swelling, without
fever, in March 2013. Imaging examination, including computed
tomography (CT) and magnetic resonance imaging (MRI), revealed a 40
mm tumor in her left lower lung (Fig.
1A) and left tibia destruction with a soft tissue mass
(Fig. 2A). No significant previous
medical history and laboratory findings were reported. The surgery
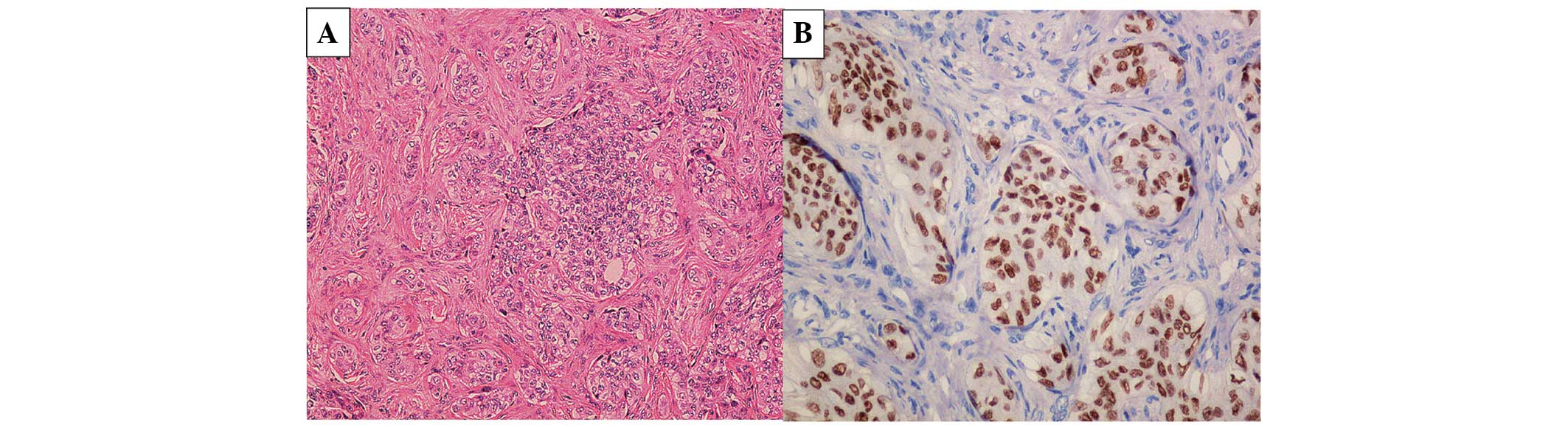
on the left tibia was performed with the pathological diagnosis of
poorly differentiated metastatic adenocarcinoma of the tibia from
the left lower lung, clinical stage IV (cT3N3M1b; Fig. 3A and B). Following diagnosis, the
patient was referred to The General Military Hospital of Beijing
PLA (Beijing, China) in April 2013. A further lung biopsy was not
performed considering the patient's clinical status. Gene detection
for mutations in EGFR (Fig. 4A)
and EML4-ALK (Fig. 4B) was
performed on a formalin-fixed, paraffin-embedded tibia tumor
specimen, by reverse transcription-quantitative polymerase chain
reaction. The genomic DNA was extracted using QIAamp DNA FFPE
Tissue kit (Qiagen, Inc., Hilden, Germany) and the total RNA was
extracted using an RNeasy Mini kit (Qiagen, Inc.), and was
reverse-transcribed into cDNA using RevertAid™ First Strand cDNA
Synthesis kit (Fermentas, Thermo Scientific, Wilmington, DE, USA).
The human EGFR mutation qualitative detection kit was the EGFR
ADx-ARMS kit and the human EML4-ALK gene expression assay kit was
the EML4-ALK ADx-ARMS kit (Amoy ADx Ltd., Xiamen, China). The
amplification products were sent to Beijing Jin Weizhi Biological
Technology Co., Ltd. (Beijing, China) for sequencing and a variant
1 of the EML4-ALK translocation (Fig.
5A) and an EGFR exon 19 deletion 2235–2249 (delE746-A750) were
detected (Fig. 5B). An ALK
rearrangement was further confirmed by fluorescence in situ
hybridization using the commercially available breakapart probe
(Guangzhou LBP Medicine Science & Technology Co., Ltd.,
Guangzhou, China; Fig. 6). The
patient was administered 250 mg gefitinib orally every day as the
first line chemotherapy and linear accelerator radiotherapy at the
left tibia by PTV 2 Gy/30 Gy/15F/23 days. Following 5 months of
EGFR-tyrosine kinase inhibitor (TKI) therapy and 15 radiotherapy
sessions, imaging examination demonstrated that the left middle
tibia cortex was absent, and the bone mineral density was increased
(Fig. 2B). Gefitinib therapy
revealed an acceptable response with a repeated chest CT (Fig. 1B), which demonstrated no obviously
enlarged lesions or novel lesions following 8 months of
therapy.
Discussion
The EGFR gene, located on the 12–14 region of
the short arm of chromosome 7, consists of 28 exons and the
majority of mutations were located within exons 19–21 of the
tyrosine kinase (TK) domain (14–16).
Deletions in exon 19, with the highest mutation rate among the
total EGFR mutation, are associated with increased gene
expression and TK inhibitor sensitivity (14,17).
The EML4-ALK fusion gene was first identified by Soda et al
in 2007 (3) in a Japanese patient
with NSCLC, which is formed by a small inversion within chromosome
2p, and at least 11 different variants have been previously
identified, with variants 1 and 3 being the most common (3–6,16,18–23).
EGFR-TK inhibitors, gefitinib and erlotinib, have
been widely used for the treatment of patients with advanced NSCLC
exhibiting the EGFR mutation. Markedly improved benefits
were observed from EGFR-TK inhibitors in a previous study, which
compared the efficacy of conventional cytotoxic chemotherapy as
first-line treatment (24). In the
2012 edition of the NCCN clinical practice guidelines of NSCLC,
EGFR mutation detection was suggested in the initial
treatment of patients with advanced NSCLC (25). Patients who harbor the EML4-ALK
mutation fail to beneft from EGFR-TKIs (5,21).
Crizotinib, an orally bioavailable ALK inhibitor, is recommended
for treating these patients (26)
and is currently under phase III clinical trials worldwide. Whether
EML4-ALK NSCLC can behave in an analogous manner to the EGFR
mutant NSCLC and whether crizotinib can become a milestone in the
treatment of NSCLC remains to be elucidated.
Patients who exhibit both mutations are extremely
rare and previous studies have suggested that an EGFR mutation and
EML4-ALK gene fusion are mutually exclusive molecular events
(3–6,18,20–22,27).
The literature for both the EGFR and EML4-ALK mutations in
NSCLC was assessed and revealed only 12 cases, including the
present case (Table I). This is
also the first case, to the best of our knowledge, in the northern
Han Chinese population identified with a concurrent EGFR
exon 19 deletion 2235–2249 (delE746-A750) and EML4-ALK variant 1.
As shown in Table I, the clinical
characteristics of the 12 cases of patients with advanced stage
NSCLC with the concomitant mutations presented in our study were as
follows: Median age, 57; 7/12 female; 9/12 Asian (8/12 Chinese,
1/12 Japanese); 9/12 light-smoker or never-smoked; pathological
type, 11/12 adenocarcinoma and 1/12 adenosquamous carcinoma; and
8/12 EGFR exon 19 or 4/12 exon 21 mutation, coexisted with the
EML4-ALK variant 1 (7/8) or variant 6 (1/8). A favorable response
was observed according to image analysis following treatment with
gefitinib for 8 months in the present case study. However, the
results reported in the literature are inconsistent and the
appropriate treatment for this subset of patients with NSCLC
remains to be elucidated.
 | Table IClinical features of 12 patients with
the EML4-ALK fusion gene and EGFR mutation. |
Table I
Clinical features of 12 patients with
the EML4-ALK fusion gene and EGFR mutation.
| Patient, ref | Ethnicity | Age/gender | Smoking history | Histology | Primary lesion | TNM stage | EGFR mutation
status | EML4ALK | First line
treatment | Assessment |
|---|
| 1, 6 | Chinese | 44, F | Never smoked | Adenocarcinoma | Left upper lung
lobe |
cT2aN3Mlb
(left ribs and thoracic vertebral bodies) stage IV | Exon 19 deletion | Variant 6 | Gefitinib | Partial response (122
days following treatment) |
| 2, 6 | Chinese | 56, F | Never smoked | Adenocarcinoma | Right upper lung
lobe |
cT4N3Mlb
(brain) stage IV | Exon 21 L858 | Variant 1 | Gefitinib | Partial response (36
days following treatment) |
| 3, 6 | Chinese | 50, M | 45 pack-years | Adenocarcinoma | Right upper lung
lobe |
cT3N0Mla
(pleura) stage IV | Exon 21 L858 | Variant 1 | Sequential
gemcitabine/carboplatin+erlotinib | Partial response (8
weeks following treatment) |
| 4, 6 | Chinese | 70, M | Never smoked | Adenocarcinoma | Right upper lung
lobe |
cT1bN3M1b,
(brain) stage IV | Exon 21 L858 | Variant 1 | Erlotinib | Partial response (105
days following treatment) |
| 5, 7 | Chinese | 56, F | Never smoked | Adenocarcinoma | Right upper lung
lobe |
T1N0M0
stage Ia | Exon 19 deletion | Variant 1 | Surgery | R0
resectiona |
| 6, 8 | Chinese | 72, F | Never smoked | Adenocarcinoma | Right upper lung
lobe | Stage IV (brain bone
metastasis) | Exon 19 deletion | Variant 1 | Gefitinib | Partial response (232
days following treatment) |
| 7, 9 | Caucasian | 48, M | Never smoked | Adeno-squamous
carcinoma | Right upper lung
lobe |
cT1N0M1
(left tenth rib) | Exon 19
deletion | – | Cisplatin +
gemcitabine six cycles | Partial response (2
months following treatment) |
| 8, 10 | Caucasian | 65, F | Never smoked | Adenocarcinoma | Right upper lung
lobe and right hilar |
pT2N2M0
stage IIIA | Exon 19
deletion | – | Erlotinib | Complete response
(25 months following treatment) |
| 9, 11 | Japanese | 39, M | Light smoker | Adenocarcinoma | Right upper lung
lobe |
cT4N3M1b
(bone) Stage IV | Exon 21 L858 | Variant 1 | Cisplatin +
docetaxel three cycles | No clinical
benefit |
| 10, 12 | Caucasian | 52, F | Heavy smoker | Adenocarcinoma | Left upper lung
lobe |
cTlbN2M0
stage IIIa | Exon 19
deletion | – | Chemotherapy +
gefitinib + radiotherapy | Stable disease (7
months following treatment) |
| 11, 13 | Chinese | 56, M | Heavy smoker | Adenocarcinoma | Right upper lung
lobe |
T4N2Mla
stage IV | Exon 19
deletion | – |
Gemcitabine/cisplatin + erlotinib +
radiotherapy + crizotinib | Complete metabolic
response (24 months following treatment) |
| 12, present
study | Chinese | 71, F | Never smoked | Adenocarcinoma | Left hilar |
cT3N3Mlb
(bone) stage IV | Exon 19
deletion | Variant 1 | Gefitinib +
erlotinib | Stable disease (7
months following treatment) |
Finally, the present study had certain limitations.
Firstly, the primary lung tumor specimen is no longer available,
therefore, our results cannot be further verified in the primary
tumor, with the possible mechanism of drug resistance, including
primary drug resistance and acquired resistance remaining unknown.
Secondly, the response of the ALK inhibitor in this patient is
unknown since no ALK-targeted agents were used.
In conclusion, the present study reported a rare
case of lung cancer, harboring both the EGFR mutation and
the EML4-ALK fusion gene. Treatment with gefitinib has demonstrated
a good response thus far. Future research and experience are
required to understand the biological features and the optimal
targeted treatment modes for this subtype of patients with lung
cancer.
References
|
1
|
Zhou Q, Zhou CC, Chen GY, et al: A
multicenter phase II study of sorafenib monotherapy in clinically
selected patients with advanced lung adenocarcinoma after failure
of EGFR-TKI therapy (Chinese Thoracic Oncology Group, CTONG 0805).
Lung Cancer. 83:369–373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Z, Zhang X, Bai H, et al: EML4-ALK
rearrangement and its clinical significance in Chinese patients
with advanced non-small cell lung cancer. Oncology. 83:248–256.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soda M, Choi YL, Enomoto M, et al:
Identification of the transforming EML4-ALK fusion gene in
non-small-cell lung cancer. Nature. 448:561–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inamura K, Takeuchi K, Togashi Y, et al:
EML4-ALK fusion is linked to histological characteristics in a
subset of lung cancers. J Thorac Oncol. 3:13–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koivunen JP, Mermel C, Zejnullahu K, et
al: EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in
lung cancer. Clin Cancer Res. 14:4275–4283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang J, Zhang X, Su J, et al: Concomitant
EGFR mutation and EML4-ALK gene fusion in non-small cell lung
cancer. J Clin Oncol. 29(Suppl): [abstr 10517]. 2011.
|
|
7
|
Zhang X, Zhang S, Yang X, et al: Fusion of
EML4 and ALK is associated with development of lung adenocarcinomas
lacking EGFR and KRAS mutations and is correlated with ALK
expression. Mol Cancer. 9:1882010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuo YW, Wu SG, Ho CC, et al: Good response
to gefitinib in lung adenocarcinoma harboring coexisting EML4-ALK
fusion gene and EGFR mutation. J Thorac Oncol. 5:2039–2040. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tiseo M, Gelsomino F, Boggiani D, et al:
EGFR and EML4-ALK gene mutations in NSCLC: A case report of
erlotinib-resistant patient with both concomitant mutations. Lung
Cancer. 71:241–243. 2011. View Article : Google Scholar
|
|
10
|
Popat S, Vieira de Araújo A, Min T, et al:
Lung adenocarcinoma with concurrent exon 19 EGFR mutation and ALK
rearrangement responding to erlotinib. J Thorac Oncol. 6:1962–1963.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka H, Hayashi A, Morimoto T, et al: A
case of lung adenocarcinoma harboring EGFR mutation and EML4-ALK
fusion gene. BMC Cancer. 12:5582012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Santelmo C, Ravaioli A, Barzotti E, et al:
Coexistence of EGFR mutation and ALK translocation in NSCLC:
Literature review and case report of response to gefitinib. Lung
Cancer. 81:294–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Zhang J, Hu Q, et al: A case of
lung adenocarcinoma harboring exon 19 EGFR deletion and EML4-ALK
fusion gene. Lung Cancer. 81:308–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shigematsu H, Lin L, Takahashi T, et al:
Clinical and biological features associated with epidermal growth
factor receptor gene mutations in lung cancers. J Natl Cancer Inst.
97:339–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kosaka T, Yatabe Y, Endoh H, et al:
Mutations of the epidermal growth factor receptor gene in lung
cancer: Biological and clinical implications. Cancer Res.
64:8919–8923. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Li Y, Yang T, et al: Clinical
significance of EML4-ALK fusion gene and association with EGFR and
KRAS gene mutations in 208 Chinese patients with non-small cell
lung cancer. PLoS One. 8:e520932013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gazdar AF, Shigematsu H, Herz J, et al:
Mutations and addiction to EGFR: The Achilles 'heal' of lung
cancers? Trends Mol Med. 10:481–486. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inamura K, Takeuchi K, Togashi Y, et al:
EML4-ALK lung cancers are characterized by rare other mutations, a
TTF-1 cell lineage, an acinar histology and young onset. Mod
Pathol. 22:508–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martelli MP, Sozzi G, Hernandez L, et al:
EML4-ALK rearrangement in non-small cell lung cancer and non-tumor
lung tissues. Am J Pathol. 174:661–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shaw AT, Yeap BY, Mino-Kenudson M, et al:
Clinical features and outcome of patients with non-small-cell lung
cancer who harbor EML4-ALK. J Clin Oncol. 27:4247–4253. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong DW, Leung EL, So KK, et al: The
EML4-ALK fusion gene is involved in various histologic types of
lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer.
115:1723–1733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi T, Sonobe M, Kobayashi M, et al:
Clinicopathologic features of non-small-cell lung cancer with
EML4-ALK fusion gene. Ann Surg Oncol. 17:889–897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takeda M, Okamoto I, Sakai K, et al:
Clinical outcome for EML4-ALK-positive patients with advanced
non-small-cell lung cancer treated with first-line platinum-based
chemotherapy. Ann Oncol. 23:2931–2936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, et al: Gefitinib or
carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med.
361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zornosa C, Vandergrift JL, Kalemkerian GP,
et al: First-line systemic therapy practice patterns and
concordance with NCCN guidelines for patients diagnosed with
metastatic NSCLC treated at NCCN institutions. J Natl Compr Canc
Netw. 10:847–856. 2012.PubMed/NCBI
|
|
26
|
Camidge DR, Bang YJ, Kwak EL, et al:
Activity and safety of crizotinib in patients with ALK-positive
non-small-cell lung cancer:updated results from a phase I study.
Lancet Oncol. 13:1011–1019. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sasaki T, Rodig SJ, Chirieac LR and Jänne
PA: The biology and treatment of EML4-ALK non-small cell lung
cancer. Eur J Cancer. 46:1773–1780. 2010. View Article : Google Scholar : PubMed/NCBI
|