Introduction
Bladder cancer is associated with a high morbidity
and mortality rate worldwide. Annually, ~350,000 new cases are
diagnosed and there are 15,000 cases of bladder cancer-associated
mortality (1). In the United
States, bladder cancer is the third most common malignant tumor in
males and the tenth in females (2). Bladder urothelial carcinoma accounts
for ~90% of all cases of bladder cancer, 75–85% of which is classed
as non-muscle invasive bladder urothelial cancer (NMIBUC), while
the remainder is muscle invasive BUC (MIBUC) (3). Transurethral resection of bladder
cancer combined with postoperative chemotherapy is the predominant
therapeutic method to treat NMIBUC, which has a five-year survival
rate of 85–90% (4,5). However, tumor recurrence occurs in
≤70% of postoperative patients and ~20% of recurrent patients will
progress to MIBUC, which has a high rate of metastasis and
mortality (4,5). Surgical technologies are improving,
however when patients undergo radical cystectomy and systemic
chemoradiotherapy, distant metastasis occurs in ~50% of the tumors
within two years and the five-year mortality rate is ≤60% (6). Given the high recurrence and
progressive rate of BUC, patients often require a lengthy and
expensive follow-up (3). Thus, the
early detection of superficial tumors that exhibit an infiltrating
tendency, and invasive tumors with metastatic potential, is
important for the development of therapeutic treatment strategies
and establishing an accurate prognosis (7,8).
Quantum dots (QDs) are luminescent semiconductor
nanocrystals. QDs, a type of fluorescent label, are widely applied
in the biological and biomedical fields due to their unique optical
properties (9). Compared with
traditional organic fluorescent probes, QDs possess a wide
absorption spectra and a narrow emission peak for simultaneous
excitation of multiple fluorescence colors, high fluorescence
intensity, long fluorescent duration, stable fluorescence of
long-term emission and strong resistance to photobleaching
(9). Previous studies have
demonstrated that QDs may be used in the labeling of live cells and
cancer cells for animal imaging and tracking in vivo
(10–13). QDs have demonstrated prognostic
potential in the early diagnosis and targeted therapy of tumors
(14–16). However, a key issue that currently
requires a solution involves the toxicity and influence of QDs on
the biological behavior of live cells. The current study aimed to
investigate the effect of QDs (CdSe-ZnS) on the toxicity,
proliferation, migration and invasion of the EJ human bladder
cancer cell line in vitro. The results aimed to provide a
theoretical basis for subsequent imaging and tracking studies in
vivo and for the early diagnosis of bladder cancer.
Materials and methods
Materials and instruments
The EJ human bladder urothelial carcinoma cell line
was donated by the Department Laboratory of Urology, Zhongnan
Hospital of Wuhan University (Wuhan, China). The QD605 (CdSe-ZnS)
Cell Tracing kit (QK605CT) was obtained from Wuhan Jiayuan Quantum
Dots Co., Ltd., (Wuhan, China). The inverted fluorescence
microscope was purchased from Olympus (IX71; Tokyo, Japan). Flow
cytometry (FCM; CyAn™ ADP Analyzer, Beckman Coulter, Inc., Brea,
CA, USA) was used for detecting the rate of cell labeling. The
ELx800 Tecan Sunrise was purchased from BioTek Instruments, Inc.,
(Winooski, VT, USA). The Transwell chambers were from Corning
Incorporated (Corning, NY, USA). Fetal bovine serum (FBS),
RPMI-1640 and 0.25% trypsin were purchased from GE Healthcare Life
Sciences (Logan, UT, USA). The cell counting kit-8 was purchased
from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan) and the
Matrigel basement membrane matrix was obtained from BD Biosciences
(Franklin Lakes, NJ, USA).
Cell culture
EJ cells were cultured in 250 ml RPMI-1640 medium
containing 10% FBS (28 ml) and 1% penicillin-streptomycin (3 ml;
Jenom, Hangzhou, China). When the cells reached ≥80% confluence,
they were subcultured. The EJ cells were maintained at 37°C in a
humidified 5% CO2 atmosphere.
Detection of QD toxicity
Synchronously growing EJ cells were trypsinized with
0.25% trypsin and resuspended in RPMI-1640 medium, and inoculated
into 96-well plates with 0.1 ml (1×104 cells) per well.
The EJ cells were then incubated at 37°C under a 5% CO2
atmosphere for 24 h. The cells were divided into three groups as
follows: Control group, where 0.1 ml RPMI-1640 medium was added;
blank control group, where RPMI-1640 medium without cells or QD605
was added; and experimental group, where QD605 at concentrations of
5, 10, 20, 40 and 80 nM was added (groups A–E, respectively). The
control, blank, and experimental groups were set up in triplicate.
Subsequent to culture for 24 h, 10 µl cell counting kit-8
was added directly to each well and the samples were incubated for
an additional 2 h. The ELx800 Tecan Sunrise was used to detect the
optical density (OD) value of each well at a wavelength of 450 nm.
Finally, the survival rate of the tumor cells was calculated as
follows: Survival rate (%) = (Experimental group OD value - blank
control group OD value)/(control group OD value - blank control
group OD value) × 100. The experiment was repeated three times and
means were calculated accordingly.
Fluorescent labeling of EJ cells with QDs
and duration of fluorescence
Fluorescent labeling of EJ Cells with QD605 was
conducted according to the manufacturer's instructions. The key
steps were as follows: Exponentially growing EJ cells
(5×105 cells/ml) were inoculated onto a 12-well plate.
When the growth density was ~80%, 0.1 ml QD605 labeling solution
[10 nM transactivator of transcription (TAT)-QD605] was added to
each well in the experimental group. The labeling solution was
replaced by an equal volume of phosphate-buffered saline (PBS) in
the control group. Each group had six equal wells. The cells were
cultivated in an incubator at 37°C with 5% CO2 for 1 h,
then the labeling solution was discarded and the cells were washed
three times with PBS in order to remove the uncombined QDs.
Subsequently, 0.5 ml RPMI-1640 medium was added to each well and
the samples were cultured for an additional 2 h. The cells of three
randomly selected wells were then resuspended in PBS following
digestion with 0.25% trypsin and FCM was used to detect the
labeling rate. The experiment was repeated three times and the mean
labeling rate was determined. In addition, one randomly selected
well in the experimental group was used to assess the fluorescent
durability of QD605-labeled EJ cells.
Influence of QDs on cell growth
Synchronously growing EJ cells were seeded into four
24-well plates with 0.3 ml (3×104 cells) per well.
Following culture for 6 h, the four plates were divided into four
groups. For the control group, 0.05 ml RPMI-1640 medium was added.
For the experimental groups A–C, different final concentrations (5,
10 and 20 nM, respectively) of QD605 labeling solution (0.05 ml)
diluted with RPMI-1640 medium were added. Each group was cultured
for 1.5 h, then 0.3 ml RPMI-1640 medium was added to each well for
continuous culture. After 2 h, three wells of cells were randomly
selected from each group for detecting the labeling rate of QDs by
FCM. Detection of fluorescent labeling was conducted according to
the above-mentioned method. Culture was continued for the remaining
wells. The number of cells was counted in three randomly selected
wells from each group. The cells were trypsinized into a single
cell suspension and counted with a hemocytometer (XIE QIU JI SHU
BAN; Shanghai Biochemical Reagent Refinement Instrument Co., Ltd.,
Shanghai, China). This was conducted over seven consecutive days.
The cell growth curve was constructed according to the mean values
obtained by cell counting.
Influence of QDs on cell migration
EJ cells were labeled with QD605 (10 nM) according
to the above-mentioned method and used for subsequent experiments.
Two different methods were performed for assessing cell migration.
For the Transwell assay, a Transwell chamber (Corning Incorporated)
was used, which was separated into an upper and lower chamber by a
polycarbonate membrane (pore diameter, 8 µm; Corning
Incorporated). In the upper chamber, 0.2 ml EJ/QD605 or EJ cell
suspension (5×104 cells) was added and in the lower
chamber, 0.6 ml RPMI-1640 medium containing 10% FBS was added. The
samples were incubated at 37°C under a 5% CO2 atmosphere
for 24 h. Each group was set up in triplicate. The cells that
migrated to the lower chamber below the polycarbonate membrane were
stained with 0.2% crystal violet (Fortuneibo-tech Co., Ltd.)
subsequent to fixing with formaldehyde (Goodbio-tech Co., Ltd,
Wuhan, China). Five fields of visions (magnification, ×400) were
randomly selected and the number of cells in each field was counted
using an Olympus IX71 microscope (Olympus Corporation, Tokyo,
Japan).
The second method performed was the scratch assay.
Synchronously-growing EJ/QD605 and EJ cells were inoculated into a
6-well plate (105 cells/well), and each group was plated
in triplicate. Following culture for 24 h, the cell layer was
scratched with a 10-µl pipette tip, and the cells were
washed three times with PBS in order to remove the detached cells.
Serum-free RPMI-1640 medium (Jenom) was used for continuous
culture. At 0 and 24 h subsequent to scratching, five fields of
vision (magnification, ×100; Olympus IX71 microscope) were randomly
selected, and the width of the scratch was measured. The repair
rate of the scratch was calculated as follows: Repair rate (%) =
(0-h width - 24 h width)/0 h width × 100. The mean number of cells
that had migrated and the mean repair rate of the scratches were
considered to indicate the migratory capacity of the EJ/QD605 or EJ
cells.
Influence of QDs on cell invasion
The cell invasion experiment was also conducted
using Transwell assay. However, in contrast to the cell migration
assay, the basement membrane was reconstructed using
Matrigel:RPMI-1640 at a 1:8 ratio. A total of 40 µl Matrigel
that was diluted with serum-free RPMI-1640 (1:8) was added to the
surface of each upper chamber membrane and allowed to set at 37°C
for 1 h. The Matrigel was then air-dried with ultraviolet
irradiation overnight. To rehydrate the basement membrane, 0.2 ml
serum-free RPMI-1640 (1:8) was added and the plates were incubated
for 1 h at 37°C. Subsequently, EJ/QD605 or EJ cells
(3×105 cells) were added into the upper chamber, and all
remaining steps were conducted using the same protocol as for the
Transwell cell migration assay. Briefly, the cells that invaded to
the lower chamber below the polycarbonate membrane were stained
with 0.2% crystal violet (Fortuneibo-tech Co., Ltd., Shanghai,
China) for 25 min, prior to being fixed for 30 min with
formaldehyde (Goodbio-tech Co., Ltd). The mean number of cells that
had invaded to the chamber below the polycarbonate membrane was
assessed to determine the invasive ability of EJ/QD605 or EJ
cells.
Statistical analysis
All statistical analyses were performed with the
SPSS 17.0 statistical package (SPSS, Inc., Chicago, IL, USA). The
statistical differences between the groups were analyzed using
Student's t-test. All experimental values are expressed as the mean
± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
High concentrations of QDs reduced the
survival rate of EJ cells
Following 24 h of incubation with QDs, the survival
rate of EJ cells declined as the concentration of QDs increased.
The survival rate between the experimental group and control groups
was not identified to be significantly different when the
concentration of QDs used was 5, 10 or 20 nM (P>0.05); however,
with 40 and 80 nM QDs, significant differences were observed
(P<0.001). The mean survival rate of the EJ cells remained high,
at 95.7% and 92.6%, with 40 and 80 nM QDs respectively (Table I).
 | Table ISurvival rate of EJ cells at varying
concentrations of QD605. |
Table I
Survival rate of EJ cells at varying
concentrations of QD605.
| Concentration of
QDs, nM (n=27) | Survival rate, %
| P-value |
|---|
| Experimental
group | Control group |
|---|
| 5 | 101±2.69 | 100 | 0.062 |
| 10 | 99.2±2.57 | 100 | 0.092 |
| 20 | 97.9±5.44 | 100 | 0.055 |
| 40 | 95.7±5.59 | 100 | <0.001 |
| 80 | 92.6±6.50 | 100 | <0.001 |
Labeling rate and durable fluorescence of
EJ cells with QDs
The fluorescence of traditional organic fluorescent
materials decreases rapidly following excitation. However, the
fluorescence of QDs continues to be emitted for a long time. The
mean labeling rate of EJ cells following labeling for 2 h was 98.5%
(Fig. 1). In line with cell
proliferation over time, fluorescent intensity gradually reduced.
Subsequent to labeling for 1 day, a large quantity of QD605 was
present in the cytoplasm due to endocytosis (Fig. 2).
QDs exhibited no significant effect on
the proliferation of EJ cells
The growth curves of unlabeled EJ cells or EJ cells
labeled with different concentrations (5, 10 and 20 nM) of
TAT-QD605 are presented in Fig. 3.
The results demonstrate no significant differences between the
growth curves of EJ/QD605 and EJ cells, indicating that the
presence of TAT-QDs in the cytoplasm does not affect the growth of
EJ cells.
QDs exhibited no significant effect on EJ
cell migration
Two methods were performed to detect the effect of
QDs on EJ cell migration. In the Transwell assay, the mean number
of EJ/QD605 and EJ cells that penetrated the polycarbonate membrane
without Matrigel was 50.13±1.41 and 49.87±1.49, respectively.
Statistical analysis identified no significant difference between
the number of QD605-labeled EJ cells and unlabeled EJ cells that
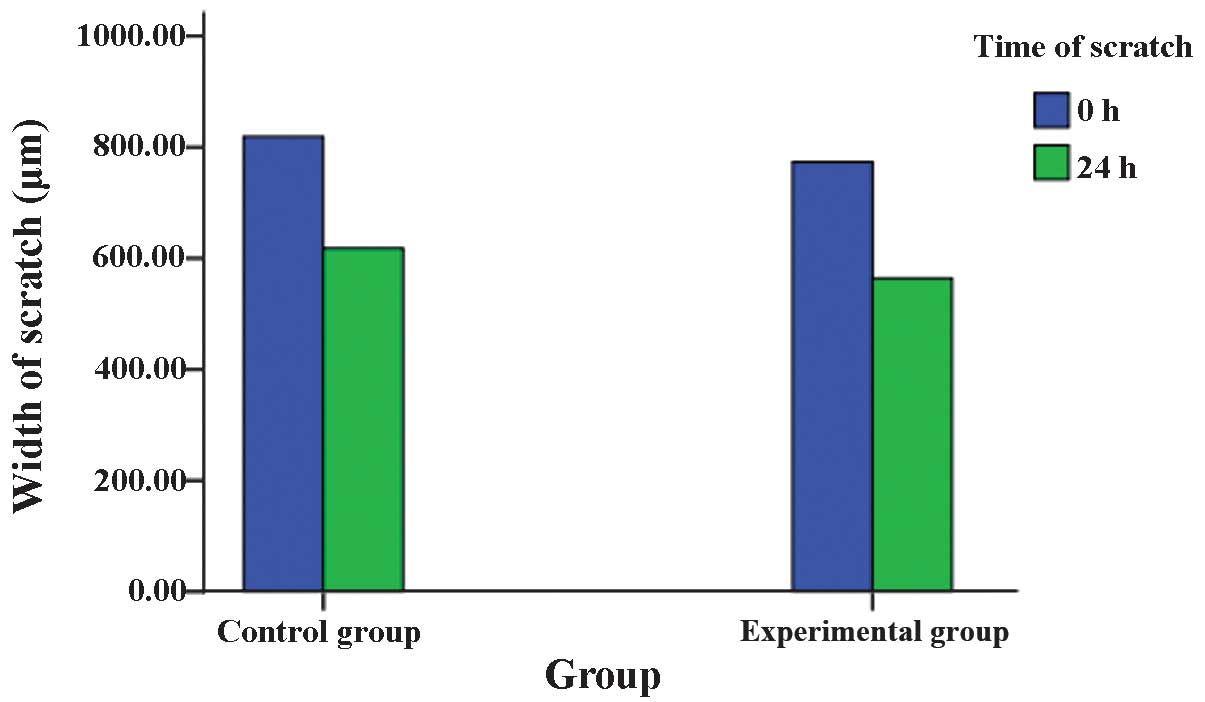
had migrated (P>0.05). In the scratch test, the repair rate of
the scratch in EJ/QD605 and EJ cells was 27.03±1.32% and
24.43±1.17%, respectively, and there was no significant difference
between QD605-labeled and unlabeled EJ cells (P>0.05). The two
assays indicated that the labeling of EJ cells with QDs does not
affect their migratory ability (Figs.
4Figure 5–6).
QDs exhibited no significant effect on EJ
cell invasion
The Matrigel on the surface of the upper chamber
membrane served as an artificial basement membrane. In this assay,
the tumor cells are required to degrade the matrix to invade, thus
mimicking metastasis. Thus, the ability of a cell to penetrate
through Matrigel indirectly reflects its invasive capability. The
results collected demonstrated that the mean number of EJ/QD605 and
EJ cells that penetrated the membrane was 41.47±0.88 and
40.53±1.10, respectively. The invasive capacity of the QD-labeled
EJ cells and unlabeled EJ cells was not significantly different
(P>0.05), suggesting that the labeling of EJ cells with QDs does
not affect their invasive ability (Fig. 7).
Discussion
QDs are luminescent semiconductor nanocrystals with
unique fluorescent properties (9).
Compared with the traditional organic fluorescent materials, such
as fluorescein isothiocyanate, Cy3 and Cy5, QDs have 10–20 times
greater fluorescence intensity and their fluorescence durability is
100–1,000 times that of organic fluorescence (17). In addition, the emission wavelength
of QDs can be adjusted by changing the size and composition of the
QDs, and the emission spectrum ranges from visible light to
infrared light. Thus, fluorescent labeling imaging of deep tissues
can be performed in vitro using near infrared or infrared
photon imaging (18).
Additionally, QDs have a wide absorption spectrum and narrow
emission peak, thus enabling simultaneous imaging of multiple
molecules (19–21).
QDs have a broad range of potential applications in
cell labeling, imaging, tracking in vivo and monitoring
biological macromolecular interactions due to their optical
properties (9). A non-invasive
in vivo study indicated that there may be potential for the
use of QDs in the development and early diagnosis of cancer and the
transfer process of therapeutic agents (22). However, the application of QDs is
limited in biological and biomedical fields due to their toxicity
towards cells, solubility in water and ability to penetrate cell
membranes. Research advancements have enabled the modification of
the surface of QDs with different molecules, in order to reduce
their cytotoxicity, increase their water solubility and improve
their ability to penetrate cell membranes. These properties are of
great importance in intracellular imaging, tracing and drug
targeting transport. Dubertret et al (23) used modified QDs during the
development of clawed frog embryo cells and identified that QDs
exerted no influence on cell growth, development, migration, signal
transduction and other physiological activities. Mattheakis et
al (24) and Pinaud et
al (25) fluorescently labeled
normal mammalian cells with QDs that exhibited wrapped inner cores.
The cells were incubated for 2 weeks, and there was no apparent
difference between the growth of the QD-labeled and unlabeled
cells.
In the current study, the cytotoxicity of different
concentrations of QD605 (CdSe-ZnS) was assessed in EJ cells. The
viability of EJ cells was marginally reduced with increasing
concentrations of QD605, however the reduction was not significant.
At the high concentration (80 nM), the survival rate of EJ cells
remained high (92.6%). At conventionally used concentrations (5, 10
and 20 nM QD605), the survival rate was not significantly different
between the experimental and control groups.
Cell-penetrating peptides (CPPs) are short peptides
with <20 amino acids and a positive charge (26,27).
They function in transmembrane transduction and are able to pass
through the majority of the cellular membrane without resulting in
cellular injury. TAT protein is the 48–60 polypeptide fragment of
the human immunodeficiency virus-1 and was the first CPP to be
discovered. It has been widely used in the delivery of
extracellular bioactive molecules, including small interfering RNA
(28–30), nucleic acids (31,32),
proteins (33,34) and QDs (35), into cells (36). Previous studies have demonstrated
that the modification of the surface of QDs with CPPs improves the
labeling rate of cells (37,38).
Xue et al (39) developed
sulfhydryl-modified QDs combined with the TAT protein to obtain
TAT-QD compounds. The QGY liver cancer and the MCF-7 human breast
cancer cell lines were cultivated with TAT-QDs and these cells were
assessed by laser confocal scanning microscopy (39). The penetration of TAT-QDs into the
QGY and MCF-7 cells was observed to increase compared with
unmodified QDs, by 2.1 and 1.5 times, respectively. In addition,
compared with alternative delivery methods, this method was
observed to exhibit a lower level of cytotoxicity in all cell lines
examined (40).
Few studies have focused upon the effect of
TAT-conjugated QDs on the proliferation, migration and invasion of
cancer cells, thus investigation of this will be beneficial for
future non-invasive and visual studies of cancer in vivo
with CPPs. In the current study, the co-culture of TAT-QD605 with
EJ cells resulted in the rapid entry of QD605 into the cytoplasm,
and the mean fluorescent labeling rate of EJ cells increased to
98.4% subsequent to 2 h of culture. The intracellular TAT-QD605 did
not affect the proliferation, migration and invasion of EJ cells.
These results demonstrate that peptide-conjugated QDs have
potential for application in labeling, imaging and tracking of live
cells in vitro and in animals in vivo, as well as for
investigation of tumor development and early diagnosis.
Acknowledgments
The authors would like to thank Professor Dai-Wen
Pang and Dr. Jun Peng (College of Chemistry and Molecular Sciences
and State Key Laboratory of Virology of Wuhan University, Wuhan,
China) for his technical assistance in the current study. The
present study was supported by grants from the National Natural
Science Foundation of China (grant no. 81272826).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA A Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
3
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Böhle A, Palou-Redorta J and Rouprêt M; European
Association of Urology (EAU): EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder, the 2011 update. Eur Urol.
59:997–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Milowsky MI, Stadler WM and Bajorin DF:
Integration of neoadjuvant and adjuvant chemotherapy and cystectomy
in the treatment of muscle-invasive bladder cancer. BJU Int.
102:1339–1344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
et al: Radical cystectomy in the treatment of invasive bladder
cancer: Long-term results in 1,054 patients. J Clin Oncol.
19:666–675. 2001.PubMed/NCBI
|
|
6
|
Shariat SF, Karakiewicz PI, Palapattu GS,
Lotan Y, Rogers CG, Amiel GE, Vazina A, Gupta A, Bastian PJ,
Sagalowsky AI, et al: Outcomes of radical cystectomy for
transitional cell carcinoma of the bladder: A contemporary series
from the bladder cancer research consortium. J Urol. 176:2414–2422.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu RJ, Stein JP, Cai J, Miranda G, Groshen
S and Skinner DG: Superficial (pT2a) and deep (pT2b) muscle
invasion in pathological staging of bladder cancer following
radical cystectomy. J Urol. 176:493–498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nieder AM, Simon MA, Kim SS, Manoharan M
and Soloway MS: Radical cystectomy after bacillus Calmette-Guérin
for high-risk Ta, T1 and carcinoma in situ: Defining the risk of
initial bladder preservation. Urology. 67:737–741. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu WW, Chang E, Drezek R and Colvin VL:
Water-soluble quantum dots for biomedical applications. Biochem
Biophys Res Commun. 348:781–786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Larson DR, Zipfel WR, Williams RM, Clark
SW, Bruchez MP, Wise FW and Webb WW: Water-soluble quantum dots for
multiphoton fluorescence imaging in vivo. Science. 300:1434–1436.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akerman ME, Chan WC, Laakkonen P, Bhatia
SN and Ruoslahti E: Nanocrystal targeting in vivo. Proc Natl Acad
Sci USA. 99:12617–12621. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Algar WR, Tavares AJ and Krull UJ: Beyond
labels: A review of the application of quantum dots as integrated
components of assays, bioprobes, and biosensors utilizing optical
transduction. Anal Chim Acta. 673:1–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosenthal SJ, Chang JC, Kovtun O, McBride
JR and Tomlinson ID: Biocompatible quantum dots for biological
applications. Chem Biol. 18:10–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Michalet X, Pinaud FF, Bentolila LA, Tsay
JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS and Weiss S:
Quantum dots for live cells, in vivo imaging, and diagnostics.
Science. 307:538–544. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Geissler D, Charbonnière LJ, Ziessel RF,
Butlin NG, Löhmannsröben HG and Hildebrandt N: Quantum dot
biosensors for ultrasensitive multiplexed diagnostics. Angew Chem
Int Ed Engl. 49:1396–1401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Medintz IL, Uyeda HT, Goldman ER and
Mattoussi H: Quantum dot bioconjugates for imaging, labelling and
sensing. Nat Mater. 4:435–446. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao X, Cui Y, Levenson RM, Chung LW and
Nie S: In vivo cancer targeting and imaging with semiconductor
quantum dots. Nat Biotechnol. 22:969–976. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith AM, Ruan G, Rhyner MN and Nie S:
Engineering luminescent quantum dots for in vivo molecular and
cellular imaging. Ann Biomed Eng. 34:3–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baiiey RE and Nie SM: Alloyed
semiconductor guantum dots:Tuning the optical properties without
changing the particle size. J Am Chem Soc. 125:7100–7106. 2003.
View Article : Google Scholar
|
|
20
|
Han M, Gao X, Jack Z, Su JZ and Nie S:
Quantum-dot-tagged microbeads for multiplexed optical coding of
biomolecules. Nat Biotechnol. 19:631–635. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wehrenberg BL, Wang CJ and Philippe GS:
Interband and intraband optical studies of PbSe colloidal quantum
dots. J Phys Chem B. 106:10634–10640. 2002. View Article : Google Scholar
|
|
22
|
Mattoussi H, Palui G and Na HB:
Luminescent quantum dots as platforms for probing in vitro and in
vivo biological processes. Adv Drug Deliv Rev. 64:138–166. 2012.
View Article : Google Scholar
|
|
23
|
Dubertret B, Skourides P, Norris DJ,
Noireaux V, Brivanlou AH and Libchaber A: In vivo imaging of
quantum dots encapsulated in phospholipid micelles. Science.
298:1759–1762. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mattheakis LC, Dias JM, Choi YJ, Gong J,
Bruchez MP, Liu J and Wang E: Optical coding of mammalian cells
using semiconductor quantum dots. Anal Biochem. 327:200–208. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pinaud F, King D, Moore HP and Weiss S:
Bioactivation and cell targeting of semiconductor CdSe/ZnS
nanocrystals with phytochelatin-related peptides. J Am Chem Soc.
126:6115–6123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang T, Zhang Z, Zhang Y, Lv H, Zhou J,
Li C, Hou L and Zhang Q: Dual-functional liposomes based on
pH-responsive cell-penetrating peptide and hyaluronic acid for
tumor-targeted anticancer drug delivery. Biomaterials.
33:9246–9258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Milletti F: Cell-penetrating peptides:
Classes, origin, and current landscape. Drug Discov Today.
17:850–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakase I, Tanaka G and Futaki S:
Cell-penetrating peptides (CPPs) as a vector for the delivery of
siRNAs into cells. Mol Biosyst. 9:855–861. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng CJ and Saltzman WM: Enhanced siRNA
delivery into cells by exploiting the synergy between targeting
ligands and cell-penetrating peptides. Biomaterials. 32:6194–6203.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ezzat K, Zaghloul EM, El Andaloussi S,
Lehto T, El-Sayed R, Magdy T, Smith CI and Langel U: Solid
formulation of cell-penetrating peptide nanocomplexes with siRNA
and their stability in simulated gastric conditions. J Control
Release. 162:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bai H, You Y, Yan H, Meng J, Xue X, Hou Z,
Zhou Y, Ma X, Sang G and Luo X: Antisense inhibition of gene
expression and growth in gram–negative bacteria by cell-penetrating
peptide conjugates of peptide nucleic acids targeted to rpoD gene.
Biomaterials. 33:659–667. 2012. View Article : Google Scholar
|
|
32
|
Nakase I, Akita H, Kogure K, Gräslund A,
Langel U, Harashima H and Futaki S: Efficient intracellular
delivery of nucleic acid pharmaceuticals using cell-penetrating
peptides. Acc Chem Res. 45:1132–1139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu BR, Liou JS, Chen YJ, Huang YW and Lee
HJ: Delivery of nucleic acids, proteins, and nanoparticles by
arginine-rich cell-penetrating peptides in rotifers. Mar Biotechnol
(NY). 15:584–595. 2013. View Article : Google Scholar
|
|
34
|
Liu BR, Huang YW and Lee HJ: Mechanistic
studies of intracellular delivery of proteins by cell-penetrating
peptides in cyanobacteria. BMC Microbiol. 13:572013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Santra S, Yang H, Stanley JT, Holloway PH,
Moudgil BM, Walter G and Mericle RA: Rapid and effective labeling
of brain tissue using TAT-conjugated CdS: Mn/ZnS quantum dots. Chem
Commun (Camb). 3144–3146. 2005. View
Article : Google Scholar
|
|
36
|
Hyndman L, Lemoine JL, Huang L, Porteous
DJ, Boyd AC and Nan X: HIV-1 Tat protein transduction domain
peptide facilitates gene transfer in combination with Cationic
liposomes. J Control Release. 99:435–444. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen B, Liu Q, Zhang Y, Xu L and Fang X:
Transmembrane delivery of the cell-penetrating peptide conjugated
semiconductor quantum dots. Langmuir. 24:11866–11871. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lei Y, Tang H, Yao L, Yu R, Feng M and Zou
B: Applications of mesenchymal stem cells labeled with Tat peptide
conjugated quantum dots to cell tracking in mouse body. Bioconjug
Chem. 19:421–427. 2008. View Article : Google Scholar
|
|
39
|
Xue FL, Chen JY, Guo J, Wang CC, Yang WL,
Wang PN and Lu DR: Enhancement of intracellular delivery of CdTe
quantum dots (QDs) to living cells by tat conjugation. J Fluoresc.
17:149–154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vives E, Schmidt J and Pèlegrin A:
Cell-penetrating and cell-targeting peptides in drug delivery.
Biochim Biophys Acta. 1786:126–138. 2008.PubMed/NCBI
|