Introduction
Gastric cancer is one of the leading causes of
cancer-related mortality worldwide (1,2). It
remains difficult to cure, primarily because the majority of
patients present with advanced-stage disease. The pathogenesis of
gastric cancer is complex and relates to multiple factors.
Dysregulation of intracellular signaling pathways represents a
common pathogenic mechanism and may be amenable to drug
targeting.
Hedgehog (Hh) signaling is critical during embryonic
development. It is involved in the patterning of the neural tube,
lung, skin, axial skeleton and gastrointestinal tract (3–5). In
multiple adult tissues, Hh signaling remains active and contributes
to differentiation, proliferation and maintenance (6,7).
Deregulation of the Hh pathway is associated with congenital
defects and cancer. Specifically, Hh signaling is constitutively
active in a number of types of cancer, including gastric cancer
(8), pancreatic cancer (9), prostate cancer (10), breast cancer (11), basal cell carcinoma (12) and small cell lung cancer (13). Elevated expression of Hh target
genes patched gene 1 (Ptch1) or Gli1 occurs in >2/3 of primary
gastric cancers (1). Sonic
hedgehog (Shh) signaling promotes motility and invasiveness of
gastric cancer cells through cross-talk with the transforming
growth factor-β pathway (14,15).
Recent studies have observed the presence of Smoothened (Smo)
and/or Ptch1 mutations at a low frequency in different histological
subtypes of gastric cancer (16).
Rab23, another mediator of the Hh pathway, is also associated with
gastric cancer (17,18). These studies suggest that the
molecules involved in the Hh pathway may be potential candidates
for the treatment of gastric cancer (19).
Tectonic1 (TCTN1), which is a member of the tectonic
family, has been identified as a regulator of the Hh pathway
(20). TCTN1 functions in Hh
signal transduction to fully activate the pathway in the presence
of high Hh levels, and to repress the pathway by modulating Hh
signal transduction downstream of Smo and Rab23 in the absence of
Hh signals (20,21). The expression pattern of TCTN1
resembles certain proteins in the Hh pathway. TCTN1-null mice
showed similar phenotypes to those with Shh mutations. Recently,
TCTN1 was reported to form a complex with multiple ciliopathy
proteins associated with Meckel and Joubert syndromes (21,22).
Abolition of TCTN1 leads to tissue-specific defects in ciliogenesis
and ciliary membrane composition (21). However, there have been no studies
regarding the function of TCTN1 in human cancer thus far.
Accumulating evidence has shown that there is a
close association between abnormal cell proliferation and cancer.
In order to investigate the role of TCTN1 in gastric cancer, the
present study investigated the biological role of TCTN1 in cell
growth via an RNA interference lentivirus system in three gastric
cancer cell lines. Cell proliferation, colony formation and cell
cycle progression were investigated in gastric cancer cells
following TCTN1 knockdown.
Materials and methods
Cell culture
SGC-7901, MGC80-3 and AGS human gastric cancer cell
lines and the 293T human embryonic kidney cell line (HEK293T) were
obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). SGC-7901 cells were maintained in RPMI-1640
(Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS; Biowest, Kansas City, MO, USA). AGS cells were cultured in
F-12 (Sigma-Aldrich, St. Louis, CA, USA) with 10% FBS. MGC80-3 and
HEK293T cells were cultured in Dulbecco's modified Eagle's medium
(Hyclone) with 10% FBS. Cells were maintained at 37°C in a
humidified atmosphere of 5% CO2.
Lentiviral vector construction
The short hairpin RNA (shRNA) sequence
(5′-GCTCAGATGCATCAGTTCCTTC TCG AGAAGGAACTGATGCATCTGAGCTTTTTT-3′)
was designed to target the human TCTN1 gene (NM_001082537.2). The
control shRNA sequence was
5′-TTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAA-3′. The shRNA
oligos were annealed, ligated and inserted into NheI/PacI double
digested pFH-L vectors (Shanghai Hollybio, Shanghai, China). The
generated plasmids were termed pFH-L-shTCTN1 or pFH-L-shCon.
Lentiviral packaging and cell
infection
The reconstructed plasmids were then transfected
into HEK293T cells using Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA) together with the helper plasmids
pVSVG-I and pCMV-R8.92 (Shanghai Hollybio). Supernatants containing
either the lentivirus expressing the TCTN1 shRNA (Lv-shTCTN1) or
the control shRNA (Lv-shCon) were harvested 72 h after
transfection. The lentiviruses were purified using
ultracentrifugation (4°C, 100,000 ×g, 2h). As the lentivirus
carried a green fluorescence protein (GFP) reporter, the viral
titer was determined by counting GFP-expressing cells under a
fluorescence microscope (Olympus BX50; Olympus Corporation, Tokyo,
Japan) as described in previous studies (23,24).
For cell infection, SGC-7901 (5×104
cells/well), MGC80-3 (5×104 cells/well) and AGS
(8×104 cells/well) were seeded into 6-well plates and
incubated with recombinant lentivirus at a multiplicity of
infection of 30, 6 or 20, respectively. At 72 h post infection,
cells were observed under a fluorescence microscope. The infection
efficiency was calculated as the number of GFP positive cells
versus the total number of cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Five days after lentivirus infection, SGC-7901,
MGC80-3 and AGS cells were collected and extracted for total RNA
using TRIzol reagent (Invitrogen Life Technologies) according to
manufacturer's instructions. cDNA that was adjusted to a final
concentration of 30 ng/µl was synthesized by using M-MLV
reverse transcriptase (Promega Corporation, Madison, CA, USA).
RT-qPCR was then performed to analyze the expression level of TCTN1
using actin as an endogenous control. Primers were designed as
follows: Forward: 5′-CCTTTGCGTGAATGTTGTTC-3′ and reverse:
5′-AGAGGGACTGGCTGGGTATT-3′ for TCTN1; and forward:
5′-GTGGACATCCGCAAAGAC-3′ and reverse: 5′-AAAGGGTGTAACGCAACTA-3′ for
actin. The RT-qPCR reaction system (Takara, Tokyo, Japan) was set
up as follows: 10 µl of 2X SYBR premix ex taq, 0.8 µl
of 2.5 µM forward and reverse primers, 5 µl cDNA and
4.2 µl ddH2O. The reaction conditions were an
initial denaturation step at 95°C for 1 min and 40 cycles of
denaturation at 95°C for 5 sec followed by annealing extension at
60°C for 20 sec. The absorbance values were obtained at the
extension stage and expression levels were calculated using
2−ΔΔCt methods. Results were presented as Ct values,
which were defined as the threshold PCR cycle number at which an
amplified product is first detected. The average Ct was calculated
for TCTN1 and actin, and ΔCt was determined as the ratio of the
mean of the triplicate Ct values for TCTN1 to the mean of the
triplicate Ct values for actin. The experiment was repeated at
least three times on a BioRad Connet Real-Time PCR platform
(Bio-Rad CFX96; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
3-(4, 5-Dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) assay
Three days after lentivirus infection, SGC-7901
(2.5×103 cells/well), MGC80-3 (2×103
cells/well) and AGS (2×103 cells/well) were reseeded in
96-well plates and cultured at 37°C. The number of viable cells was
measured at daily intervals (days 1, 2, 3, 4 and 5). At each time
point, 10 µl of 5 mg/ml MTT was added to the cells. After
incubation for 3 h, acidic isopropanol (10% SDS, 5% isopropanol and
0.01 mol/l HCl) was added. The absorbance value of each well was
recorded at a wavelength of 595 nm (Epoch; BioTek, Winooski, VT,
USA). Each experiment was repeated at least three times.
Colony formation assay
Three days after lentivirus infection, MGC80-3 cells
were reseeded into 6-well plates at a density of 400 cells/well.
The medium was changed at three-day intervals. After 7 days of
culture at 37°C, cells were fixed with 4% paraformaldehyde and
crystal violet staining was performed. The colony number in each
well was counted under a microscope (Olympus CH-2; Olympus
Corporation). Image analysis was conducted using Image-Pro® Plus
Version 6.0 (Media Cybernetics, MD, USA). Each experiment was
repeated at least three times.
Flow cytometric analysis
Three days after lentivirus infection, MGC80-3 cells
were reseeded in 6-cm dishes at a density of 2×105
cells/dish. When the confluence of MGC80-3 cells was ~70%, cells
were collected and fixed with 70% cold ethanol overnight at 4°C.
Propidium iodide (PI; (C1052, Beyotime Institute of Biotechnology,
Haimen, China) was then added to stain the nuclei following the
manufacturer's instructions. The fluorescence of PI was measured
using flow cytometry with Cell Lab Quanta Beckman Coulter (Gallios,
Beckman Coulter, San Jose, CA, USA). Each experiment was repeated
at least three times.
Statistical analysis
All data were presented as the mean ± standard error
of three independent experiments. SPSS 13.0 (SPSS, Inc., Chicago,
IL, USA) was used for all statistical analyses. Comparison of data
was analyzed using Student's t-test. P<0.05 were considered to
indicate a statistically significant difference.
Results
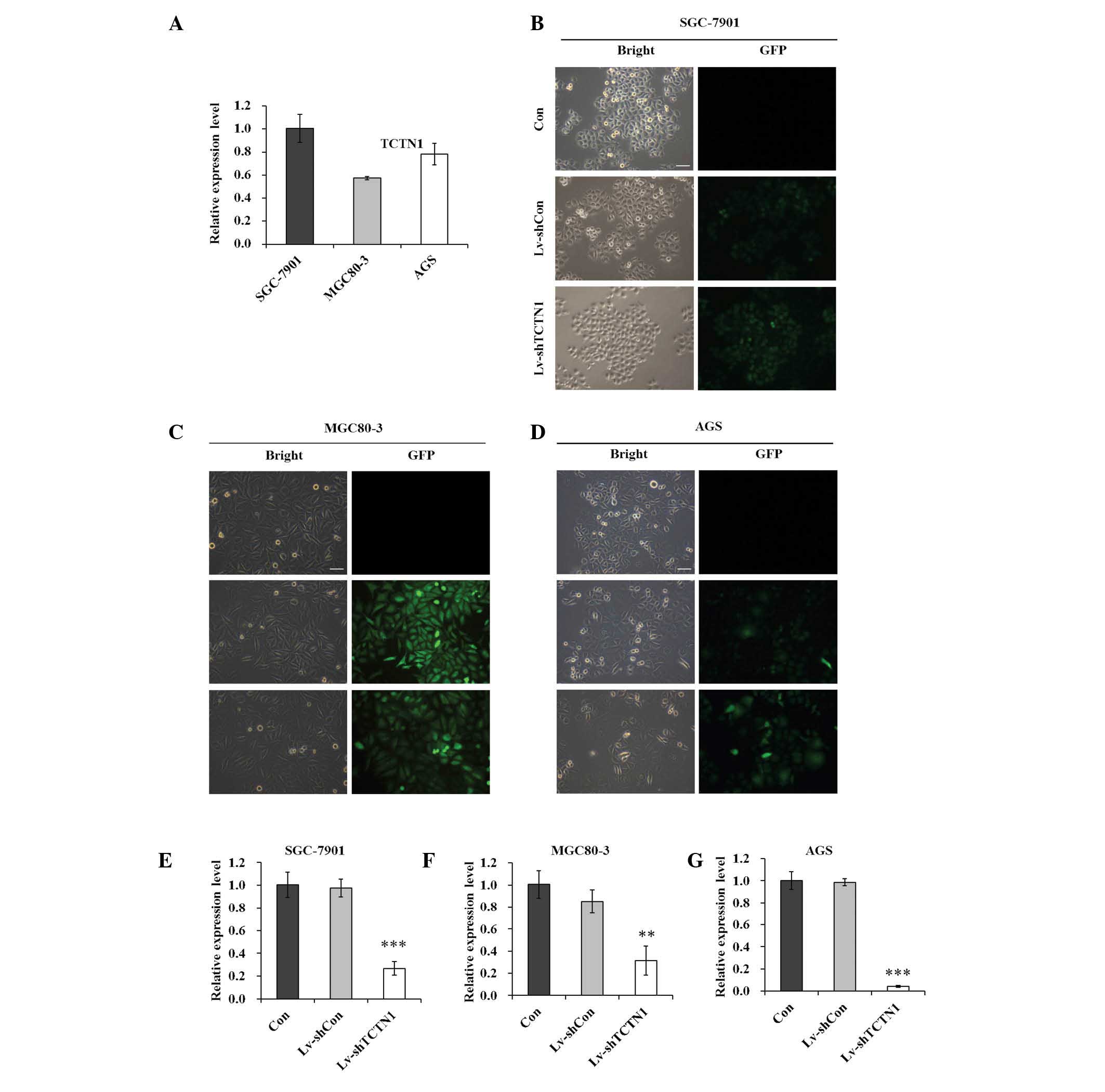
TCTN1 is suppressed efficiently in
MGC80-3 cells by Lv-shTCTN1
The expression of TCTN1 was initially analyzed in
different human gastric cancer cell lines by RT-qPCR. As shown in
Fig. 1A, TCTN1 mRNA signals were
detected in the three gastric cancer cell lines, SGC-7901, MGC80-3
and AGS.
Lentiviral vectors were then constructed expressing
shTCTN1 and shCon, and recombinant lentivirus was obtained,
Lv-shTCTN1 and Lv-shCon. As shown in Fig. 1B–D, the infection efficiency of
recombinant lentivirus was detected in SGC-7901, MGC80-3 and AGS
cells. More than 80% of cells presented GFP-positive signals,
suggesting that recombinant lentivirus could deliver exogenous
shRNA into gastric cancer cells with high efficiency. Furthermore,
at day 5 post lentivirus infection, the mRNA levels of TCTN1 were
significantly reduced in Lv-shTCTN1 groups, by 72.6, 63.2 and 95.8%
in SGC-7901, MGC80-3 and AGS, compared with the Lv-shCon groups
(Fig. 1E–G). These results
indicated that TCTN1 could be efficiently downregulated by the
constructed Lv-shTCTN1.
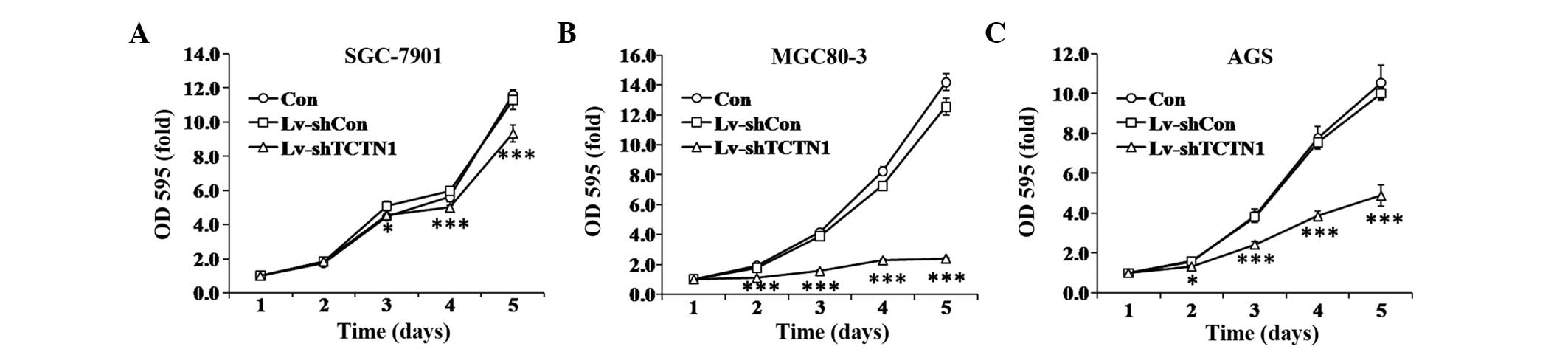
Cell proliferation is inhibited following
knockdown of TCTN1 in MGC80-3 cells
To investigate the function of TCTN1 in gastric
cancer, the effect of TCTN1 knockdown on cell proliferation was
examined by an MTT assay. As shown in Fig. 2A–C, the proliferation rates of
Lv-shTCTN1 infected cells were significantly decreased, compared
with Lv-shCon infected and uninfected cells. While Lv-shCon
infected cells did not show significant difference compared with
uninfected cells. The inhibition of proliferation was stronger in
MGC80-3 and AGS cells (P<0.001) compared with SGC-7901 cells
(P<0.05). These results suggested that TCTN1 may be essential
for gastric cancer cell proliferation.
Colony formation is suppressed in MGC80-3
cells after TCTN1 knockdown
The colony forming ability of MGC80-3 cells was
analyzed following knockdown of TCTN1 using crystal violet
staining. As shown in Fig. 3A, the
size of single colonies was smaller and the number of colonies
formed was fewer in Lv-shTCTN1 groups than in control groups. The
colony formation ability of Lv-shTCTN1 infected cells was
significantly decreased by ~33 fold compared with Lv-shCon infected
and uninfected cells (Fig. 3B).
These results indicated that TCTN1 knockdown could impair the
colony formation ability of gastric cancer cells.
Cell cycle of MGC80-3 cells is blocked at
G2/M phase following TCTN1 knockdown
To determine the mechanisms underlying impaired cell
proliferation and colony formation, the cell cycle distribution of
MGC80-3 cells was analyzed with PI staining using flow cytometry.
As shown in Fig. 4, at day 3 post
Lv-shTCTN1 infection, there were fewer cells in
G0/G1 phase, while cell population in
G2/M phase was significantly increased. The percentages
of cells in S phase did not change significantly. The results
suggested that downregulation of TCTN1 in MGC80-3 cells induced
cell cycle arrest at G2/M phase.
Discussion
TCTN1, as a regulator of the Hh pathway, has been
demonstrated to have an important role in regulating the Hh
signaling pathway (20). The Hh
pathway is important in development and cancer. Previous studies
have demonstrated that inhibition of the Hh pathway using small
interfering (si)RNA or chemical inhibitor could lead to growth
suppression and apoptosis in several types of cancer, such as
esophageal adenocarcinoma, ovarian cancer and endometrial cancer
(25–27). Generally, siRNA targeting is
effective over a short period of time, and gene transcription
returns to normal after 3–7 days (28). However, lentivirus-based vectors
have been used as a successful tool for gene targeting to deliver
siRNA with long-term silencing efficiency (29). Therefore, in the present study, the
biological role of TCTN1, a regulator of the Hh pathway, was
examined via lentivirus-mediated siRNA in three gastric cancer cell
lines. Knockdown of TCTN1 markedly inhibited the proliferation and
colony formation ability of gastric cancer cells, suggesting that
TCTN1 is essential for gastric cancer growth.
Cell proliferation is directly linked to cell cycle
progression. The cell cycle distribution of MGC80-3 cells was
determined following lentivirus infection by flow cytometric
analysis. The cell numbers in the G1 phase were
decreased, and the cell population in G2/M phase was
markedly increased in TCTN1 knockdown cells, indicating that
downregulation of TCTN1 arrested cell cycle at G2/M
phase. However, Chen et al (25) found that treatment of ovarian
cancer with cyclopamine (inhibitor of Hh pathways) induced
G1 arrest and apoptosis. The context differences between
gastric cancer and ovarian cancer may also contribute to the
results. Also, TCTN1 could have other functions in addition to
regulating the Hh pathway. Recently, calcium signaling particularly
Orai1/Ca2+ release-activated Ca2+ channel M1
has been shown to be the most important regulatory signal in cell
cycle arrest as well as cancer cell proliferation (30,31).
Whether TCTN1 induced G2/M phase cell cycle arrest due
to regulation of calcium signaling in gastric cancer remains to be
investigated. Understanding the regulatory mechanism of TCTN1 in
gastric cancer may provide a rational basis for drug
development.
In conclusion, the present study demonstrated the
novel biological functions of TCTN1 in human gastric cancer.
Knockdown of TCTN1 suppressed the growth of gastric cancer cells
through inducing G2/M phase arrest. The results suggest
that TCTN1 may serve as a potential therapeutic target in human
gastric cancer. However, the relative importance of TCTN1 in
gastric carcinogenesis is not completely understood and warrants
further investigation.
Acknowledgments
This study was supported by grants from the Natural
Science Foundation of Zhejiang Province (grant no. Y205757), the
Medical Research Fund of Zhejiang Province (grant no. 2009A029) and
the Outstanding Research Personnel Training Funds of Zhejiang
Cancer Hospital.
References
|
1
|
Wu WK, Cho CH, Lee CW, et al:
Dysregulation of cellular signaling in gastric cancer. Cancer Lett.
295:144–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Danaei G, Vander Hoorn S, Lopez AD, Murray
CJ and Ezzati M: Comparative risk assessment collaborating group
(Cancers): Causes of cancer in the world: comparative risk
assessment of nine behavioural and environmental risk factors.
Lancet. 366:1784–1793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramalho-Santos M, Melton DA and McMahon
AP: Hedgehog signals regulate multiple aspects of gastrointestinal
development. Development. 127:2763–2772. 2000.PubMed/NCBI
|
|
4
|
Dessaud E, McMahon AP and Briscoe J:
Pattern formation in the vertebrate neural tube: a sonic hedgehog
morphogen-regulated transcriptional network. Development.
135:2489–2503. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chiang C, Litingtung Y, Lee E, et al:
Cyclopia and defective axial patterning in mice lacking Sonic
hedgehog gene function. Nature. 383:407–413. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang L, Xie G, Fan Q and Xie J: Activation
of the hedgehog-signaling pathway in human cancer and the clinical
implications. Oncogene. 29:469–481. 2010. View Article : Google Scholar
|
|
7
|
Varjosalo M and Taipale J: Hedgehog:
functions and mechanisms. Genes Dev. 22:2454–2472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saqui-Salces M and Merchant JL: Hedgehog
signaling and gastrointestinal cancer. Biochim Biophys Acta.
1803:786–795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kelleher FC: Hedgehog signaling and
therapeutics in pancreatic cancer. Carcinogenesis. 32:445–451.
2011. View Article : Google Scholar
|
|
10
|
Shaw G, Price AM, Ktori E, et al: Hedgehog
signalling in androgen independent prostate cancer. Eur Urol.
54:1333–1343. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hui M, Cazet A, Nair R, Watkins DN,
O'Toole SA and Swarbrick A: The Hedgehog signalling pathway in
breast development, carcinogenesis and cancer therapy. Breast
Cancer Res. 15:2032013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saran A: Basal cell carcinoma and the
carcinogenic role of aberrant Hedgehog signaling. Future Oncol.
6:1003–1014. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watkins DN, Berman DM and Baylin SB:
Hedgehog signaling: progenitor phenotype in small-cell lung cancer.
Cell Cycle. 2:196–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu H, Hu Z, Wen J, Wang K and Liu Y:
TGF-beta promotes invasion and metastasis of gastric cancer cells
by increasing fascin1 expression via ERK and JNK signal pathways.
Acta Biochim Biophys Sin (Shanghai). 41:648–656. 2009. View Article : Google Scholar
|
|
15
|
Feng R, Xiao C and Zavros Y: The role of
Sonic Hedgehog as a regulator of gastric function and
differentiation. Vitam Horm. 88:473–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang XD, Inzunza H, Chang H, et al:
Mutations in the hedgehog pathway genes SMO and PTCH1 in human
gastric tumors. PLoS ONE. 8:e544152013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun HJ, Liu YJ, Li N, et al:
Sublocalization of Rab23, a mediator of Sonic hedgehog signaling
pathway, in hepatocellular carcinoma cell lines. Mol Med Rep.
6:1276–1280. 2012.PubMed/NCBI
|
|
18
|
Hou Q, Wu YH, Grabsch H, et al:
Integrative genomics identifies RAB23 as an invasion mediator gene
in diffuse-type gastric cancer. Cancer Res. 68:4623–4630. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katoh Y and Katoh M: Hedgehog signaling
pathway and gastrointestinal stem cell signaling network (review).
Int J Mol Med. 18:1019–1023. 2006.Review. PubMed/NCBI
|
|
20
|
Reiter JF and Skarnes WC: Tectonic, a
novel regulator of the Hedgehog pathway required for both
activation and inhibition. Genes Dev. 20:22–27. 2006. View Article : Google Scholar :
|
|
21
|
Garcia-Gonzalo FR, Corbit KC,
Sirerol-Piquer MS, et al: A transition zone complex regulates
mammalian ciliogenesis and ciliary membrane composition. Nat Genet.
43:776–784. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alazami AM, Alshammari MJ, Salih MA, et
al: Molecular characterization of Joubert syndrome in Saudi Arabia.
Hum Mutat. 33:1423–1428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tiscornia G, Singer O and Verma IM:
Production and purification of lentiviral vectors. Nat Protoc.
1:241–245. 2006. View Article : Google Scholar
|
|
24
|
Sakoda T, Kasahara N, Hamamori Y and Kedes
L: A high-titer lentiviral production system mediates efficient
transduction of differentiated cells including beating cardiac
myocytes. J Mol Cell Cardiol. 31:2037–2047. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X, Horiuchi A, Kikuchi N, et al:
Hedgehog signal pathway is activated in ovarian carcinomas,
correlating with cell proliferation: it's inhibition leads to
growth suppression and apoptosis. Cancer Sci. 98:68–76. 2007.
View Article : Google Scholar
|
|
26
|
Feng YZ, Shiozawa T, Miyamoto T, et al:
Overexpression of hedgehog signaling molecules and its involvement
in the proliferation of endometrial carcinoma cells. Clin Cancer
Res. 13:1389–1398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zaidi AH, Komatsu Y, Kelly LA, et al:
Smoothened inhibition leads to decreased proliferation and induces
apoptosis in esophageal adenocarcinoma cells. Cancer Invest.
31:480–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McManus MT SP: Gene silencing in mammals
by small interfering RNAs. Nat Rev Genet. 3:737–747. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Devi G: siRNA-based approaches in cancer
therapy. Cancer Gene Ther. 13:819–829. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou MF, Kuo HC, Li JH, et al: Orai1/CRACM1
overexpression suppresses cell proliferation via attenuation of the
store-operated calcium influx-mediated signalling pathway in A549
lung cancer cells. Biochim Biophys Acta. 1810:1278–1284. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang IH, Tsai YT, Chiu SJ, et al:
Involvement of STIM1 and Orai1 in EGF-mediated cell growth in
retinal pigment epithelial cells. J Biomed Sci. 20:412013.
View Article : Google Scholar : PubMed/NCBI
|