Introduction
In the Asian population, non-alcoholic fatty liver
disease is an increasing public health concern, which encompasses
fibrosis, cirrhosis and hepatocarcinoma (1–4).
Owing to this, it is necessary to develop a novel drug, which is
more efficient and lowers the necessary dosage in order to treat
this disease and prevent side effects.
The mechanism of carbon tetrachloride
(CCl4)-induced liver acute injury and fibrosis is
hypothesized to act through the trichloromethyl free radical
(CCl3•) produced from its metabolism by the cytochrome
P450 enzyme (5). The metabolic
products of the trichloromethyl free radical are converted to the
trichloromethyl peroxy radical (CCl3OO•) (6). Liver fibrosis is associated with an
activated inflammatory response through expression of
pro-inflammatory cytokines (7).
Interleukin (IL)-6 is a cytokine that is able to trigger the
inflammatory cascade and cell death in hepatocytes. In addition,
connective tissue growth factor (CTGF) level is increased in
patients with liver cirrhosis (8,9).
TGF-β is a key mediator of cirrhosis (10,11).
Anti-cirrhosis drugs are able to prevent and reverse the process of
fibrosis through the inhibition of TGF-β and CTGF.
Silymarin is a promising treatment against
CCl4-induced acute liver injury. Silymarin is a herbal
liver-protective drug with four flavonolignan isomers, including
60–70% silybin, 20% silychristin, 10% silydianin and 5% isosilybin
(12,13). San Huang Shel Shin Tang (SHSST) is
also a cocktail-like traditional herbal decoction used for liver
protection in China. SHSST is composed of 50% Rheum
officinale Baill, 25% Scutellaria baicalnsis Geprgi and
25% Coptis chinensis Franch. Rheum has been reported to have
a liver protective effect folowing CCl4-induced injury
in rats (14,15). Scutellaria and Coptis
were also identified to have similar hepatoprotective effects in
acute hepatotoxicity (16–18). The coinciding liver protective
effects between Rheum officinale, Scutellaria baicalnsis and
Coptis chinensis are due to their shared bioactive
compounds, including potent flavonoids, such as baicalein (19–23).
SHSST and silymarin are potential liver protection
drugs, but are limited by poor water solubility and poor
bioavailability (30%) (24–26).
A formulation approach is necessary for increasing the solubility
of these drugs. β-cyclodextrin (β-CD) modification is able to
increase the solubility and spectral properties of the hydrophobic
drugs, without altering their intrinsic ability to permeate the
cell membrane (27–29). Thus, SHSST was modified to a
SHSST-β-CD-complex (SHSSTc) and evaluated in the present study.
Janus kinase (JAK) phosphorylation is mediated
through IL-6 activation (30).
Furthermore, signal transducer and activator of transcription 3
(STAT3) is a downstream protein of JAK and regulates the hepatocyte
regeneration (30). In addition,
extracellular-signal-regulated kinase 5 (ERK5) and nuclear factor
of activated T cells (NFAT) are also implicated in liver
regeneration (31,32). In the present study, silymarin and
baicalein were used in the evaluation of inflammation-regulated
IL-6 expression and fibrosis-regulated TGF-β expression in
CCl4-induced acute injury. Additionally, proteins
involved in the regulation of liver regeneration were also analyzed
in association with SHSSTc-mediated liver protection effects.
Materials and methods
Preparation of SHSSTc and drug
treatment
The SHSST complex with β-CD was prepared by
co-precipitation. β-CD (70.0 g) was dissolved in distilled water
(85 ml) at 70°C in a water bath for 1 h. SHSST (10.0 g) in 15 ml
ethanol was slowly added to the β-CD solution with continuous
agitation for 6 h. Following this, 40 ml of ethanol was added
dropwise to regulate the solubility of the hydrophobic solute in
the β-CD solution. Subsequently, the solution was refrigerated
overnight at 4°C. The precipitated SHSSTc (SHSST:β-CD=1:9 in
weight) was recovered by filtration and washed with ethanol to
remove unencapsulated SHSST. This residue was dried in a vacuum at
−20°C for 48 h. The final powder was stored at 4°C until use.
Silymarin and baicalein were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The silymarin, baicalein, SHSST
and SHSSTc stock solutions for treatments were prepared by
dissolving in distilled deionized water at 100 mg/ml each.
CCl4 was dissolved in olive oil at a concentration of 4%
v/v.
High performance liquid chromatography
(HPLC) analysis
Baicalein, SHSST and SHSSTc were dissolved in the
mobile phase solution (69% 60 mM phosphoric acid water solution and
31% acetonitrile, pH 3.2) and analyzed by HPLC with ultraviolet
detection, using a C-18 column (Mightysil RP-18 GP column; Kanto
Chemical Co., Iwiki, Japan) and the flow rate was 1.0 ml/min.
Baicalein was detected using a UV-VIS detector (SPD 20A/20AV;
Shimadzu Corporation, Kyoto, Japan) at an absorbance of 279 nm.
Animal model
A total of 42 Sprague-Dawley rats were purchased
from BioLASCO Taiwan Co., Ltd (Taipei, Taiwan) and divided into the
following seven groups (n=6): Control (group I), CCl4
intraperitoneal injection treatment (group II), CCl4
intraperitoneal injection combined with silymarin (100 mg/kg/day)
oral treatment (group III), CCl4 intraperitoneal
injection combined with baicalein (30 mg/kg/day) oral treatment
(group IV), CCl4 intraperitoneal injection combined with
SHSST (30 mg/kg/day) oral treatment (group V), CCl4
intraperitoneal injection combined with sSHSSTc (30 mg/kg/day) oral
treatment (group VI) and CCl4 intraperitoneal injection
combined with SHSSTc (300 mg/kg/day) oral treatment (group VII).
After 4 weeks of pretreatment, the CCl4 intraperitoneal
injection (0.2 ml/kg) was applied to all groups with the exception
of the control group. Subsequently, the liver tissue was collected
from all rats 48 h after CCl4 intraperitoneal injection.
The study was approved by the ethics committee of the Institutional
Animal Care and Use Committee (100-4-B) of the China Medical
University (Taichung, Taiwan).
Hematoxylin and eosin (H&E)
staining
The livers of rats in each group were soaked in 10%
formalin, dehydrated through graded alcohols and embedded in
paraffin wax. Subsequently, the 0.2 µm-thick paraffin
sections were cut into slices from these paraffin-embedded tissue
blocks. The tissue sections were deparaffinized by immersing in
xylene and rehydrated. All slices were stained with H&E and
then rinsed with water. Each slide was dehydrated through graded
alcohols. Finally, the samples were soaked in xylene twice.
Photomicrographs were obtained using a Zeiss Axiophot microscope
(Carl Zeiss Inc., Oberkochen, Germany).
Masson's trichrome staining
The livers of rats in each group were soaked in 10%
formalin, dehydrated through graded alcohols and embedded in
paraffin wax. Subsequently, the 0.2 µm-thick paraffin
sections were cut into slices from these paraffin-embedded tissue
blocks. The tissue sections were deparaffinized by immersing in
xylene and rehydrated. The samples were then stained with Masson's
trichrome staining to investigate histological and fibrotic
alterations in the liver. Photomicrographs were obtained using a
Zeiss Axiophot microscope.
Tissue protein extraction
Liver tissue extracts of six rats in each group were
obtained by homogenizing in a lysis buffer (0.05 M Tris-HCl, pH
7.4, 0.15 M NaCl, 0.25% deoxycholic acid, 1% NP-40 and 1 mM EDTA)
at a ratio of 100 mg tissue/1 ml buffer. The homogenates were
placed on ice and then centrifuged at 18,900 × g for 40 min. The
supernatants were collected and stored at −80°C for further
experiments.
Western blot analysis
The protein concentration of liver tissue extracts
was determined using the Lowry protein assay. Protein samples were
separated using a 12% SDS polyacrylamide gel electrophoresis with a
constant voltage of 75 V for 2 h. The proteins were then
transferred onto Hybond-C membranes (GE Healthcare, Amersham, UK)
at 50 volts for 3 h. Polyvinylidene difluoride membranes were
incubated in 3% bovine serum albumin in Tris-buffered saline
(Sigma-Aldrich). Primary antibodies, including goat polyclonal
immunoglobulin G (IgG) IL-6 (cat no. SC-1266; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), p-JAK (cat no. SC-21870;
Santa Cruz Biotechnology, Inc.), rabbit polyclonal IgG STAT-3 (cat
no. SC-483; Santa Cruz Biotechnology, Inc.), mouse monoclonal
IgG2a α-tubulin (cat no. SC-5286; Santa Cruz
Biotechnology, Inc.), goat polyclonal IgG ERK5 (cat no. SC-1284;
Santa Cruz Biotechnology, Inc.), rabbit polyclonal IgG p-GATA4 (cat
no. SC-32823; Santa Cruz Biotechnology, Inc.), rabbit polyclonal
IgG Smad-3 (cat no. SC-8332; Santa Cruz Biotechnology, Inc.),
rabbit monoclonal IgG NFAT-3 (cat no. 2188; Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit monoclonal TGF-β (cat
no. 3709, Cell Signaling Technology, Inc.) and goat polyclonal IgG
CTGF (cat no. SC-14939, Santa Cruz Biotechnology, Inc.) were added
to the membranes for binding to the target proteins. Finally,
horseradish peroxidase-labeled antibodies (donkey anti-goat
IgG-HRP, sc-2020; goat anti-mouse IgG-HRP, sc-2005; goat
anti-rabbit IgG-HRP, sc-2004; Santa Cruz Biotechnology, Inc.) were
used and images were captured using a LAS-4000 camera (GE
Healthcare).
Statistical analysis
Data are expressed as the mean ± standard deviation
of three independent experiments. Statistical analysis was
performed using a one-way analysis of variance. For paired samples,
Student's t-test was applied. Statistical analysis was conducted
using SigmaPlot, version 10.0 (Systat Software, Inc., San Jose, CA,
USA) and P<0.05 was considered to indicate a statistically
significant difference.
Results
In the process of precipitating SHSSTc
(SHSST:β-CD=1:9 weight), washing and filtration is necessary to
unencapsulate SHSST. The β-CD complex modification increased the
formula weight of the SHSSTc. The bioactive components, including
baicalein, were compared between SHSSTc and SHSST. HPLC analysis
revealed that SHSST contained 54.5 mg/g baicalein and SHSSTc
contained 4.6 mg/g baicalein (Fig.
1). This result demonstrated that the concentration of
baicalein present in SHSST was 11.8 times greater than that in
SHSSTc.
The liver biopsy with H&E staining is shown in
(Fig. 2). CCl4 induced
cell death surrounding the microvascular and caused vacuole-like
structures. The hepatocytes were protected with a complete
structure in the groups pretreated with silymarin, baicalein,
SHSST, SHSSTc (low dose) and SHSSTc (high dose). Masson's trichrome
staining is useful in the detection of cirrhosis with collagen
indicated in blue. In the CCl4-induced fibrosis group,
marked collagen accumulation was observed. The collagen
accumulation decreased in the silymarin, baicalein and SHSST
treatment groups. In addition, collagen accumulation was
undetectable in the high dose and low dose SHSSTc treatment groups
(Fig. 2).
IL-6 signaling pathway analysis revealed an increase
in IL-6 protein level after 48 h of CCl4-induced
inflammation (Fig. 3). However,
IL-6 expression decreased significantly following pretreatment with
silymarin and partially decreased following pretreatment with
baicalein, SHSST and SHSSTc. After 48 h CCl4-induced
inflammation, p-JAK expression was partially increased, however, no
suppression was observed in the groups pretreated with silymarin,
baicalein, SHSST and SHSSTc. The STAT3 expression level was only
decreased in the group pretreated with silymarin after 48 h of
CCl4-induced inflammation.
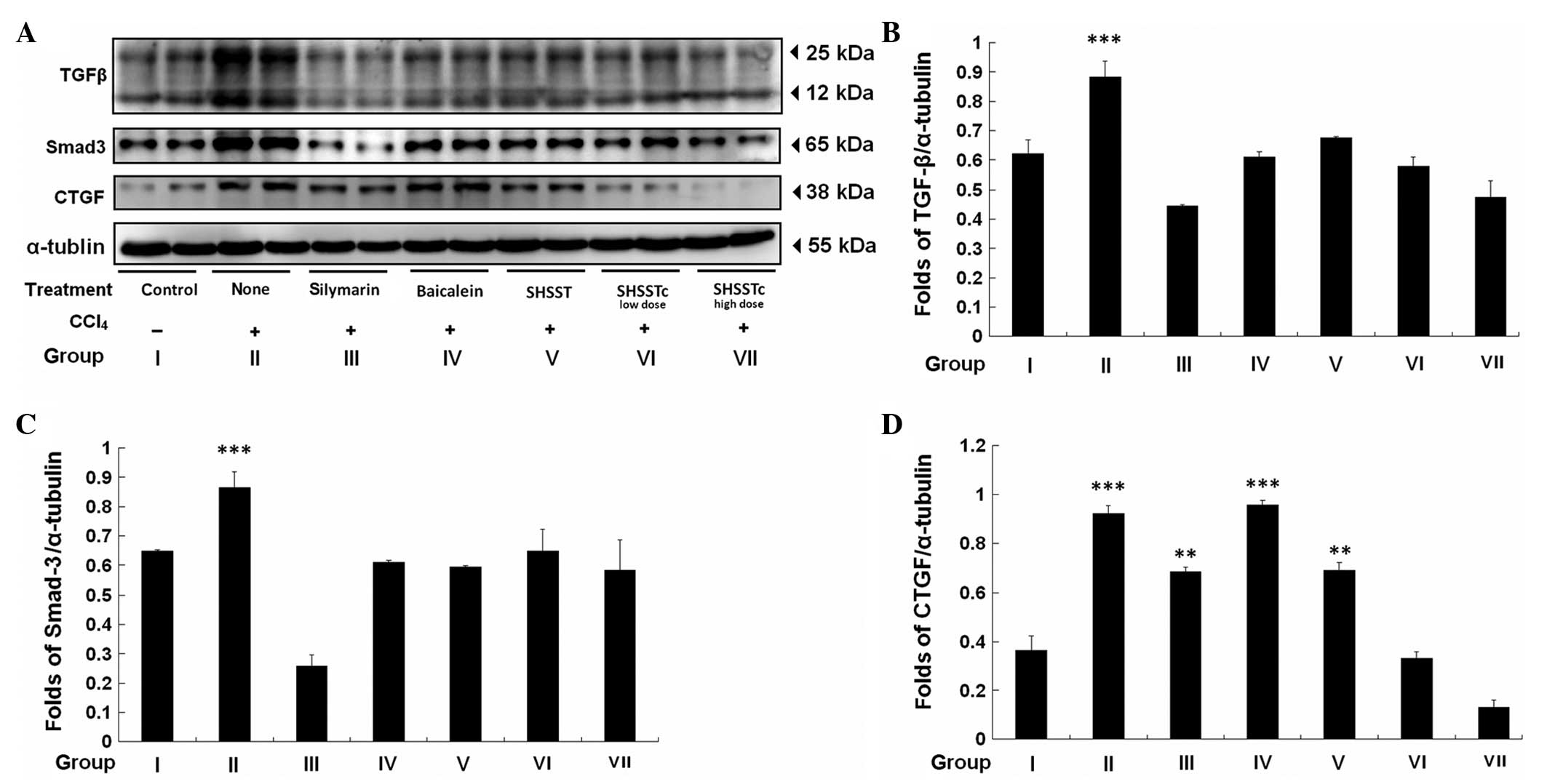
The levels of TGF-β, Smad-3 and CTGF were increased
48 h after CCl4-induced acute injury (Fig. 4). Following pretreatment with
silymarin, the TGF-β signaling pathway was significantly inhibited
in the SHSSTc high and low dose pretreatment groups. SHSSTc exerted
the same protective mechanism and exhibited greater suppression of
the CTGF level compared with SHSST and silymarin treatment.
Pretreatment with baicalein and SHSST was also able to partially
reduce the protein level of TGF-β, Smad-3 and CTGF.
Proteins involved in the regulation of hepatocyte
regeneration, including ERK5, p-NFAT-3 and p-GATA-4 were analyzed
(Fig. 5). The
CCl4-induced injury did not stimulate regeneration in
the liver without pretreatment with any drugs. The pretreatment of
silymarin and baicalein was able to increase ERK5 and the
expression of p-NFAT-3. p-GATA-4 expression was increased in the
liver of the SHSSTc low dose pretreatment group and partially
increased in the silymarin, baicalein and SHSST pretreatment
groups.
Discussion
In the present study, hydrophobic SHSST was altered
to hydrophilic SHSSTc through β-CD complex modification. Baicalein
was selected as the standard used in the bioactive ingredient for
HPLC analysis of SHSST and SHSSTc. The results demonstrated that
SHSST contained 11.8 times more baicalein as SHSSTc at the same
weight. The protective effects of pretreatment with SHSSTc (low
dose and high dose) were similar to silymarin, baicalein and SHSST
in the CCl4-induced acute liver injury.
Hepatocyte renewal in mild inflammation is mediated
through IL-6 and downstream activation of JAK/STAT3 (33). In the present study,
CCl4-induced acute injury increased the expression of
IL-6. Pretreatment with hepatoprotective drugs, including
silymarin, baicalein, SHSST and SHSSTc, reduced the protein level
of IL-6. However, alterations in p-JAK expression were not
detectable in all drug pretreatment groups due to the short
half-life of p-JAK. A previous study identified that the half-life
of JAK activation was <20 sec (34). However, downstream STAT3 protein
expression in each group was similar to that of IL-6. Although the
activation of STAT3 is regulated during hepatocyte regeneration,
peroxisome proliferator-activated receptor leads to the degradation
of STAT3 through hepatic oxidative stress and loses its original
function (30).
In cirrhosis, TGF-β is important as a negative
regulator of proliferation and an inducer of CTGF synthesis
(35,36). Several approaches aim at inhibition
of TGF-β function as a priority target for the development of
antifibrotic drugs. In the present study, CCl4-induced
acute injury caused TGF-β/Smad-3/CTGF activation, which was
decreased significantly by pretreatment with silymarin and SHSSTc
(high dose). SHSSTc exerted a greater suppression on CTGF level
than SHSST and silymarin. The results confirmed the antifibrotic
effects of silymarin through inhibition of TGF-β/Smad-3/CTGF and
also suggested the SHSSTc exhibited a more significant antifibrotic
effect than silymarin (37).
ERK5 is activated by TGF-β in hepatocytes, which
also regulates the proliferation and migration of hepatic stellate
cells (38,39). In the present study, all pretreated
groups exhibited partially enhanced ERK5 expression (Fig. 5). The increase in p-NFAT expression
levels only occurred in the silymarin and baicalein groups and the
lack of NFAT activation may cause incomplete liver regeneration
(32). In addition, the
development of the mammalian liver is dependent on GATA-4
activation (40). However, GATA-4
phosphorylation was only induced in the SHSSTc low dose treatment
group.
In conclusion, the β-CD complex modification of
SHSST promoted the water solubility and increased the
bioavailability of SHSST. Thus, the dose necessary for original
SHSST in cirrhosis therapy can be reduced at least 10 times through
using SHSSTc. In addition, SHSSTc exerts stronger antifibrotic
effects than silymarin and baicalein and acts through the
inhibition of the TGF-β/Smad-3/CTGF signaling pathway similarly to
silymarin and baicalein.
Acknowledgments
This study was supported in part by the Taiwan
Ministry of Health and Welfare Clinical Trial and Research Center
of Excellence (grant no. MOHW104-TDU-B-212-113002).
References
|
1
|
Loguercio C and Fredico A: Oxidative
stress in viral and alcoholic hepatitis. Free Radic Biol Med.
34:1–10. 2003. View Article : Google Scholar
|
|
2
|
Vitaglione P, Morisco F, Caporaso N and
Fogliano V: Dietary antioxidant compounds and liver health. Crit
Rev Food Sci Nutr. 44:575–586. 2004. View Article : Google Scholar
|
|
3
|
Diehl AM: Cytokine regulation of liver
injury and repair. Immunol Rev. 174:160–171. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marra F, Valente AJ, Pinzani M and Abboud
HE: Cultured human liver fat-storing cells produce monocyte
chemotactic protein-1. Regulation by proinflammatory cytokines. J
Clin Invest. 92:1674–1680. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Basu S: Carbon tetrachloride-induced lipid
peroxidation: eicosanoid formation and their regulation by
antioxidant nutrients. Toxicology. 189:113–127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zira A, Kostidis S, Theocharis S, Sigala
F, Engelsen SB, Andreadou I and Mikros E: 1H NMR-based metabonomics
approach in a rat model of acute liver injury and regeneration
induced by CCl4 administration. Toxicology. 303:115–124.
2013. View Article : Google Scholar
|
|
7
|
Ramadori G and Armbrust T: Cytokines in
the liver. Eur J Gastroenterol Hepatol. 13:777–784. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kugelmas M, Hill DB, Vivian B, et al:
Cytokines and NASH: a pilot study of the effects of lifestyle
modification and vitamin E. Hepatology. 38:413–419. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diehl AM, Goodman Z and Ishak KG:
Alcohollike liver disease in nonalcoholics. A clinical and
histologic comparison with alcohol-induced liver injury.
Gastroenterology. 95:1056–1062. 1988.PubMed/NCBI
|
|
10
|
Ramadori G, Rieder H and Knittel T:
Biology and pathobiology of sinusoidal liver cells. Hepatic
Transport and Bile Secretion. Tavoloni N and Berk PD: Raven Press;
New York, NY: pp. 83–102. 1993
|
|
11
|
Leask A: Focal adhesion kinase: a key
mediator of transforming growth factor beta signaling in
fibroblasts. Adv Wound Care (New Rochelle). 2:247–249. 2013.
View Article : Google Scholar
|
|
12
|
Attama AA, Nzekwe IT, Nnamani PO, Adikwu
MU and Onugu CO: The use of solid self-emulsifying systems in the
delivery of diclofenac. Int J Pharm. 262:23–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Parveen R, Baboota S, Ali J, Ahuja A,
Vasudev SS and Ahmad S: Effects of silymarin nanoemulsion against
carbon tetrachloride-induced hepatic damage. Arch Pharm Res.
34:767–774. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang JB, Zhao HP, Zhao YL, Jin C, Liu DJ,
Kong WJ, Fang F, Zhang L, Wang HJ and Xiao XH: Hepatotoxicity or
hepatoprotection? Pattern recognition for the paradoxical effect of
the Chinese herb Rheum palmatum L in treating rat liver injury.
PLoS One. 6:e244982011. View Article : Google Scholar
|
|
15
|
Fang F, Wang JB, Zhao YL, Jin C, Kong WJ,
Zhao HP, Wang HJ and Xiao XH: A comparative study on the tissue
distributions of rhubarb anthraquinones in normal and
CCl4-injured rats orally administered rhubarb extract. J
Ethnopharmacol. 137:1492–1497. 2011.
|
|
16
|
Chien CF, Wu YT and Tsai TH: Biological
analysis of herbal medicines used for the treatment of liver
diseases. Biomed Chromatogr. 25:21–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nan JX, Park EJ, Kim YC, Ko G and Sohn DH:
Scutellaria baicalensis inhibits liver fibrosis induced by bile
duct ligation or carbon tetrachloride in rats. J Pharm Pharmacol.
54:555–563. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye X, Feng Y, Tong Y, Ng KM, Tsao S, Lau
GK, Sze C, Zhang Y, Tang J, Shen J and Kobayashi S:
Hepatoprotective effects of Coptidis rhizoma aqueous extract on
carbon tetrachloride-induced acute liver hepatotoxicity in rats. J
Ethnopharmacol. 124:130–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Püssa T, Raudsepp P, Kuzina K and Raal A:
Polyphenolic composition of roots and petioles of Rheum rhaponticum
L. Phytochem Anal. 20:98–103. 2009. View
Article : Google Scholar
|
|
20
|
Wang CZ, Calway TD, Wen XD, Smith J, Yu C,
Wang Y, Mehendale SR and Yuan CS: Hydrophobic flavonoids from
Scutellaria baicalensis induce colorectal cancer cell apoptosis
through a mitochondrial-mediated pathway. Int J Oncol.
42:1018–1026. 2013.PubMed/NCBI
|
|
21
|
Liu L and Chen Z: Analysis of four
alkaloids of Coptis chinensis in rat plasma by high performance
liquid chromatography with electrochemical detection. Anal Chim
Acta. 737:99–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu SC, Lin JH, Weng SW, Chueh FS, Yu CC,
Lu KW, Wood WG and Chung JG: Crude extract of Rheum palmatum
inhibits migration and invasion of U-2 OS human osteosarcoma cells
by suppression of matrix metalloproteinase-2 and -9. Biomedicine.
3:120–129. 2013. View Article : Google Scholar
|
|
23
|
Hsu SC and Chung JG: Anticancer potential
of emodin. Biomedicine. 2:108–116. 2012. View Article : Google Scholar
|
|
24
|
Madaus R, Halbach G and Trost W: Salt of
silymarin group with aminopolyhydroxy alcohols. US Patent 3994925A.
Filed January 21, 1974; issued November 30, 1976.
|
|
25
|
Comoglio A, Tomasi A, Malandrino S, Poli G
and Albano E: Scavenging effect of silipide, a new
silybin-phospholipid complex, on ethanol-derived free radicals.
Biochem Pharmacol. 50:1313–1316. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giacomelli S, Gallo D, Apollonio P,
Ferlini C, Distefano M, Morazzoni P, Riva A, Bombardelli E, Mancuso
S and Scambia G: Silybin and its bioavailable phospholipids complex
(IdB 1016) potentiate in vitro and in vivo the activity of
cisplatin. Life Sci. 70:1447–1459. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsai YS, Tsai HH, Wu CP and Tsai FJ:
Preparation, characterisation and activity of the inclusion complex
of paeonol with β-cyclodextrin. Food Chem. 120:837–841. 2010.
View Article : Google Scholar
|
|
28
|
Yuan C, Jin Z, Xu X, Zhuang H and Shen W:
Preparation and stability of the inclusion complex of astaxanthin
with hydroxypropyl-β-cyclodextrin. Food Chem. 109:264–268. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vyas A and Saraf S and Saraf S:
Cyclodextrin based novel drug delivery systems. J Incl Phenom
Macrocycl Chem. 62:23–42. 2008. View Article : Google Scholar
|
|
30
|
Kim JH, Qu A, Reddy JK, Gao B and Gonzalez
FJ: Hepatic oxidative stress activates the Gadd45b gene via
degradation of the transcriptional repressor STAT3. Hepatology.
59:695–704. 2014. View Article : Google Scholar :
|
|
31
|
Li Z, Cheng Z, Wang G, Hao X, Zhang L and
Xu C: 6 Paths of ERK5 signaling pathway regulate hepatocyte
proliferation in rat liver regeneration. Indian J Biochem Biophys.
49:165–172. 2012.PubMed/NCBI
|
|
32
|
Pierre KB, Jones CM, Pierce JM, Nicoud IB,
Earl TM and Chari RS: NFAT4 deficiency results in incomplete liver
regeneration following partial hepatectomy. J Surg Res.
154:226–233. 2009. View Article : Google Scholar :
|
|
33
|
Ray S, Ju X, Sun H, Finnerty CC, Herndon
DN and Brasier AR: The IL-6 trans-signaling-STAT3 pathway mediates
ECM and cellular proliferation in fibroblasts from hypertrophic
scar. J Invest Dermatol. 133:1212–1220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giese B, Au-Yeung CK, Herrmann A,
Diefenbach S, Haan C, Küster A, Wortmann SB, Roderburg C, Heinrich
PC, Behrmann I and Müller-Newen G: Long term association of the
cytokine receptor gp130 and the Janus kinase Jak1 revealed by FRAP
analysis. J Biol Chem. 278:39205–39213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gressner AM, Weiskirchen R, Breitkopf K
and Dooley S: Roles of TGF-β in hepatic fibrosis. Front Biosci.
7:d793–d807. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bauer S, Eisinger K, Wiest R, Karrasch T,
Scherer MN, Farkas S, Aslanidis C and Buechler C: Connective tissue
growth factor level is increased in patients with liver cirrhosis
but is not associated with complications or extent of liver injury.
Regul Pept. 179:10–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeong DH, Lee GP, Jeong WI, Do SH, Yang
HJ, Yuan DW, Park HY, Kim KJ and Jeong KS: Alterations of mast
cells and TGF-β1 on the silymarin treatment for CCl(4)-induced
hepatic fibrosis. World J Gastroenterol. 11:1141–1148. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marchetti A, Colletti M, Cozzolino AM,
Steindler C, Lunadei M, Mancone C and Tripodi M: ERK5/MAPK is
activated by TGFβ in hepatocytes and required for the
GSK-3β-mediated Snail protein stabilization. Cell Signal.
20:2113–2118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rovida E, Navari N, Caligiuri A, Dello
Sbarba P and Marra F: ERK5 differentially regulates PDGF-induced
proliferation and migration of hepatic stellate cells. J Hepatol.
48:107–115. 2008. View Article : Google Scholar
|
|
40
|
Watt AJ, Zhao R, Li J and Duncan SA:
Development of the mammalian liver and ventral pancreas is
dependent on GATA4. BMC Dev Biol. 7:372007. View Article : Google Scholar : PubMed/NCBI
|