Introduction
Glioma, the most common type of primary neoplasm of
the brain, is the main cause of brain tumor-associated mortality.
It can be divided into four sub-types according to the World Health
Organization's classification of central nervous system tumors
published in 2007 (1).
Glioblastoma (GBM), the most malignant type of glioma, accounts for
more than half of all gliomas in adults (2). Despite enormous efforts in the
development and application of GBM therapies, the prognosis of
patients with GBM remains poor due to the highly migratory and
invasive nature of GBM cells (3,4). The
identification of the molecular mechanisms underlying the
aggressive biological behavior of GBM may aid in the development of
novel effective therapeutics with the aim of improving the
prognosis of patients with GBM.
The hedgehog signaling pathway is essential for
embryonic patterning and cancer development (5–7). The
hedgehog protein family includes desert hedgehog, Indian hedgehog
and sonic hedgehog (SHH). Among them, SHH is the most widely
expressed and the most potent protein. Once SHH binds to its
receptor Patched 1, the transmembrane protein Smoothened is
released, causing the transport of the transcription factor
glioma-associated oncogene (Gli) into the nucleus, where it
regulates the transcription of target genes that control cell
differentiation, survival and growth (5,6).
Three Gli genes (Gli1, Gli2 and Gli3) have been identified in
mammalian tissues, among which Gli1 is considered to be the only
loyal marker of hedgehog pathway activity (8–10).
Several recent studies have shown that the hedgehog pathway is
associated with the migration and invasion of ovarian, pancreatic,
esophageal and gastric carcinomas (6,11–13).
However, to date, the implication of the hedgehog pathway in the
aggressiveness of GBM has not been elucidated. Furthermore, the
mechanisms by which the hedgehog signaling pathway is involved in
GBM migration and invasion require further elucidation.
Matrix metalloproteinase-2 (MMP-2) and MMP-9,
zinc-dependent endopeptidases, are able to degrade nearly all
extracellular matrix components to promote cancer-cell invasion
(14). It has been demonstrated
that MMP-2 and MMP-9 are mostly associated with migration and
invasion in multiple tumor types (14–16).
Further studies have shown that the expression of MMP-2 and MMP-9
in several cancer types is mediated by numerous signaling pathways,
which include the hedgehog signaling pathway, the wnt/β-catenin
pathway, the phosphoinositide-3 kinase (PI3K)/AKT pathway and the
mitogen-activated protein kinase (MAPK) pathway (10,17,18).
It has been suggested that the hedgehog signaling pathway is
cross-linked with other signaling pathways. However, in GBM, it has
remained elusive whether there is a link between the hedgehog
signaling pathway and the PI3K/AKT pathway or whether the hedgehog
signaling pathway regulates the expression of MMP-2 and MMP-9 via
the PI3K/AKT.
The present study examined the role of the hedgehog
pathway in the migration and invasion of GBM, specifically focusing
on the link between the hedgehog signaling pathway and the PI3K/AKT
signaling pathway. Specifically, the present study assessed whether
the hedgehog pathway is involved in human GBM migration and
invasion by induction of MMP-2 and MMP-9 via the PI3K/AKT signaling
pathway.
Materials and methods
Human samples
Fifty-one patients with GBM who underwent surgery at
the Second Affiliated Hospital of Harbin Medical University
(Harbin, China) between March 2010 and July 2013 were enrolled in
the present study. All tumor specimens were pathologically
diagnosed as GBM. Written informed consent was given by the
patients for their information to be stored in the hospital
database and used for research. Approval was obtained from the
Research Ethics Committee of the Second Affiliated Hospital of
Harbin Medical University (Harbin, China).
Reagents
Dulbecco's modified Eagle's medium [(DMEM)/F12] was
obtained from GE Healthcare Life Sciences (Logan, UT, USA). Mouse
anti-MMP-2 monoclonal antibody (cat. no. SC-13595) and mouse MMP-9
monoclonal antibody (cat. no. SC-21733) were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Rabbit monoclonal
phospho-AKTS473 antibody (cat. no. #4060) and mouse AKT
monoclonal antibody (cat. no. #2920) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Rabbit polyclonal
β-actin antibody (cat. no. BS-0061R) was purchased from Bioss
(Beijing, China). Biotin-labelled goat anti-rabbit secondary
antibody (cat. no. ZDR-5306) and biotin-labelled goat anti-mouse
secondary antibody (cat. no. ZDR-5307) were purchased from ZSJB-BIO
(Beijing, China). Recombinant human sonic hedgehog N-terminal
peptide (rhSHH; cat. no. 1314-SH) was purchased from Toronto
Research Chemicals (Toronto, Canada). Cyclopamine (cat. no.
BML-GR334) and GM6001 (cat. no. BML-EI300), were obtained from Enzo
Life Sciences, Inc. (Farmingdale, NY, USA). Ly294002 (cat. no.
L9908) was from purchased from Sigma-Aldrich (St. Louis, MO,
USA)
Cell lines and culture conditions
The human glioma U251 cell line, purchased from the
Chinese Academy of Science Cell Bank (Shanghai, China), was grown
in DMEM/F12 supplemented with 100 U/ml penicillin (Beyotime
Institute of Biotechnology, Haimen, China), 100 mg/ml streptomycin
(Beyotime Institute of Biotechnology) and 10% fetal bovine serum
(FBS; GE Healthcare Life Sciences) in a humidified incubator (5%
CO2 and 37°C).
Cell adhesion assay
The U251 cells were incubated for 24 h with various
concentrations of cyclopamine (0, 5.0 and 10.0 µm) or rhSHH
(0, 0.5 and 1.0 µg/ml). The 96-well plates were coated with
5 mg/ml fibronectin (Sigma-Aldrich) and blocked with 1% bovine
serum albumin (ZSGB-BIO, Beijing, China) for 4 h. U251 cells
(20,000 cells/well) were then seeded into the 96-well plates and
incubated for 1 h at 37°C in 5% CO2. Subsequently, the
medium was gently removed by aspiration and the wells were washed
twice with phosphate-buffered saline (PBS) to remove any
non-adherent cells. The adherent cells were quantified using an MTT
assay, as we previously described (19).
Migration and invasion assay
The U251 cells were either untreated or pre-treated
with GM6001 (15 µm), anti-MMP-2 (20 µg/ml) or
anti-MMP-9 neutralizing antibodies (20 µg/ml) for 1 h. The
cell invasion assay was performed using a 24-well Transwell chamber
(cat no. 3422; Corning-Costar Inc., Corning, NY, USA) with a pore
size of 8 µm. Polycarbonate filters were coated with 20
µl diluted Matrigel (1:1 in DMEM/F12; Corning Incorporated,
Corning, NY, USA) on the upper side. 1×105 cells/well
were suspended in 0.2 ml serum-free medium with various
concentrations of cyclopamine (0, 5.0 and 10.0 µm) or rhSHH
(0, 0.5 and 1.0 µg/ml), and then seeded in the upper
chamber, while medium (0.5 ml) containing 10% FBS as a
chemoattractant was added to the lower chamber. After a 24-h
incubation period at 37°C with 5% CO2, the non-invaded
cells and the Matrigel on the upper surface of the filter were
removed using a cotton swab and cells on the lower surface of the
filter were fixed with methanol (Beyotime Institute of
Biotechnology) for 10 min, stained with hematoxylin (ZSGB-BIO,
Beijing, China) for 2 min, rinsed in running tap water for 10 min,
differentiated with 0.3% acid alcohol (ZSGB-BIO) for 1 min, rinsed
in running tap water for 10 min, and finally stained with eosin
(ZSGB-BIO) for 2 min. The migration assay was performed as
described in the invasion assay but with a shorter incubation
period (12 h) and no matrigel coating. A Leica DM2700 M light
microscope (Leica Microsystems GmbH, Wetzlar, Germany) was used for
evaluating cell number. The cell number on the lower surface of the
filter was evaluated by counting three random microscopic fields
(magnification, ×400) and the numbers of migrated or invaded cells
were averaged.
RNA extraction and reverse transcription
quantitative polymerase chain reaction analysis
The U251 cells were incubated for 24 h with various
concentrations of cyclopamine (0, 5.0 and 10.0 µm) or rhSHH
(0, 0.5 and 1.0 µg/ml), and then incubated with or without
Ly294002 (25 µM) for 1 h. Total RNA was isolated from cells
using TRIzol (Invitrogen Life Technologies, Inc., Carlsbad, CA,
USA) according to the manufacturer's instructions. 1 µg RNA
was reversely transcribed into cDNA using a High-Capacity cDNA
reverse transcription kit (Applied Biosystems, Thermo Fisher
Scientific, Waltham, MA, USA). qPCR was performed using SYBR Green
PCR Master Mix (Applied Biosystems) in a total volume of 20
µl on an ABI Prism 7500 sequence detection system (Applied
Biosystems). GAPDH, MMP-2 and MMP-9 primers were as follows: MMP-2
sense, 5′-GGTTGTCTGAAGTCACTGCACAGT-3′ and anti-sense,
5′-CTCGGTAGGGACATGCTAAGTAGAG-3′; MMP-9 sense,
5′-GCTGGGCTTAGATCATTCCTCA-3′ and anti-sense,
5′-AGGGCGAGGACCATAGAGGT-3′; GAPDH sense, 5′-CCTCCCGCTTCGCTCTCT-3′
and anti-sense, 5′-CTGGCGACGCAAAAGAAGA-3′. All primers were
synthesized by the Invitrogen Life Technologies. The reaction
conditions were as follows: 94°C for 5 min, 35 cycles at 94°C for
30 sec, 55°C for 30 sec, 72°C for 2 min and a final extension at
72°C for 5 min. The mRNA expression levels were normalized to GAPDH
using the standard ΔΔCt method.
Western blot analysis
The U251 cells were incubated for 24 h with various
concentrations of cyclopamine (0, 5.0 and 10.0 µm) or rhSHH
(0, 0.5 and 1.0 µg/ml), and then incubated with or without
Ly294002 (25 µM) for 1 h. Cells were washed with PBS and
total protein was extracted from cells with lysis buffer [50 mmol/l
Tris-HCl (pH 7.4; Sigma-Aldrich, St. Louis, MO, USA), 150 mmol/l
NaCl (Beyotime Institute of Biotechnology), 0.5% NP-40
(Sigma-Aldrich), 1 mmol/l EDTA (Sigma-Aldrich), 25 mmol/l NaFl
(Beyotime Institute of Biotechnology), 10 mmol/l Na3VO4
(Sigma-Aldrich) and 1 mmol/l phenylmethanesulfonylfluoride (pH 7.4;
Sigma-Aldrich)] on ice for 15 min. After centrifugation, the
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology) was used to determine the protein concentration.
Equal amounts of 80 µg protein were loaded onto 15% SDS-PAGE
gels and transferred to 0.45-µm polyvinylidene difluoride
membranes (Millipore, Billerica, MA, USA). After blocking with 5%
non-fat milk for 1 h, the membranes were incubated overnight at 4°C
with antibody against phospho-AKTS473 (1:1,000), AKT
(1:1,000), β-actin (1:1,000), MMP-2 (1:500) or MMP-9 (1:500). After
three washes with PBS containing Tween 20 for 5 min, the membranes
were incubated with horseradish peroxidase-conjugated secondary
antibodies against rabbit (1:2,000) or mouse (1:2,000) for 1 h at
room temperature. Protein bands were detected by enhanced
chemiluminescence (cat. no. PK-MB902-500-500; Thermo Fisher
Scientific) and quantified by Quality One Bio-Rad Gel Imaging 4.52
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunohistochemical staining
Formalin-fixed and paraffin-embedded GBM tissues
were sliced into 4-µm sections. The tumor sections were then
dewaxed, re-hydrated and immersed in 3% H2O2
(ZSGB-BIO). Antigen retrieval was achieved by heating the sections
at 95°C for 10 min in 0.01 mol/l citrate buffer (pH 6.0; ZSGB-BIO).
To reduce non-specific reactivity, the sections were treated with
10% normal goat serum (ZSGB-BIO) for 60 min. Subsequently, the
sections were incubated overnight at 4°C with primary antibodies
against phospho-AKTS473 (1:50), Gli1 (1:50; Abcam,
Cambridge, UK), MMP-2 (1:100) and MMP-9 (1:100). Following three
washes with PBS for 5 min and incubation with secondary antibody
(anti-rabbit/anti-mouse; ZSGB-BIO) for 1 h at room temperature,
antibodies were visualized done using a 3,3′-diaminobenzidine
substrate kit (cat. no. ZLI-9017; ZSGB-BIO). Immunohistochemical
analysis was performed as previously described using a Leica
DM2700M light microscope (20).
Statistical analysis
Values are expressed as the mean ± standard
deviation. SPSS 19.0 statistical software (International Business
Machines, Armonk, NY, USA) was used for statistical analysis.
Statistical significance was determined by Student's t-test
or Spearman's correlation test. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
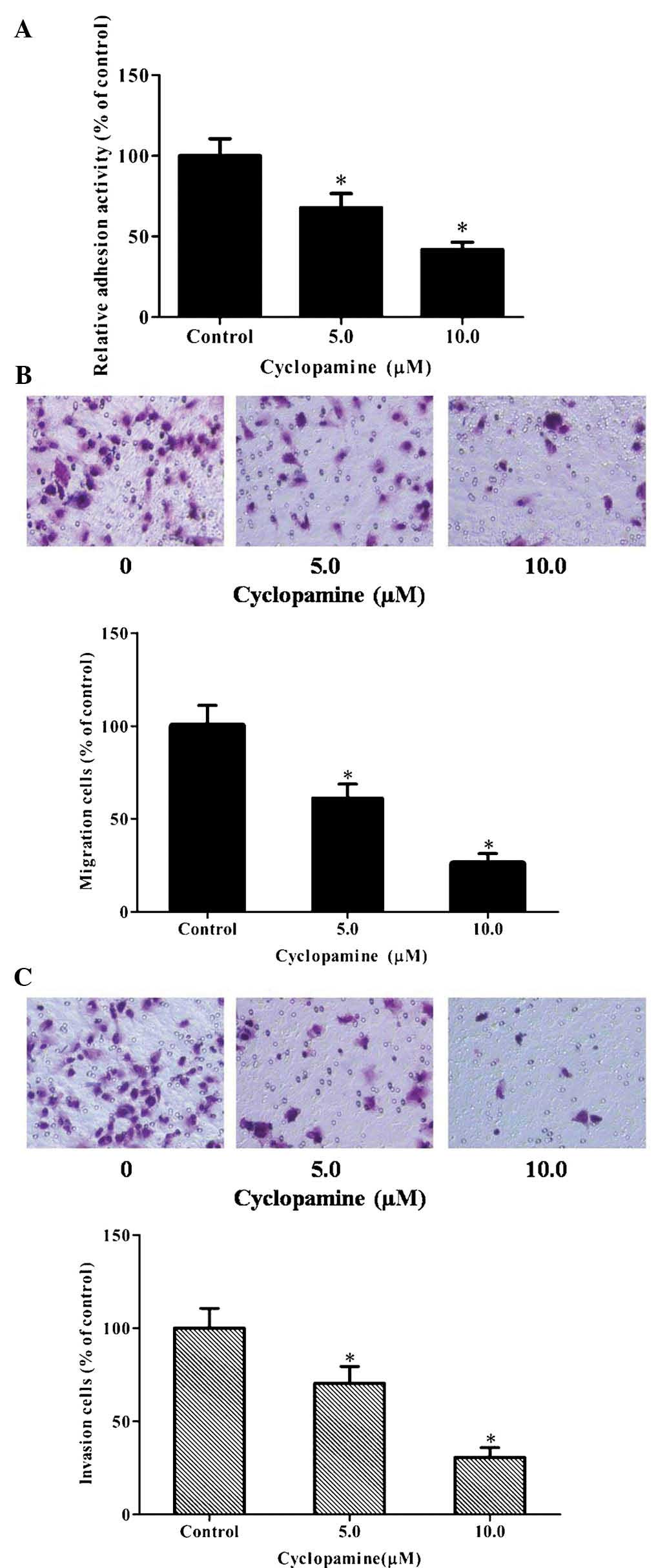
Inhibition of hedgehog signaling inhibits
the adhesion, invasion and migration of GBM cells
Cyclopamine, a specific antagonist of the hedgehog
pathway, was used to evaluate the effects of the hedgehog pathway
on the adhesion, invasion and migration of U251 cells. The results
showed that cyclopamine suppressed the adhesion of U251 cells in a
dose-dependent manner (Fig. 1A).
Furthermore, Transwell migration and invasion assays were performed
following treatment of the cells with 0, 5.0 or 10.0 µM
cyclopamine for 12 h (cell migration) and 24 h (cell invasion),
respectively. As demonstrated in Fig.
1B and C, cyclopamine significantly reduced U251-cell migration
and invasion in a dose-dependent manner. These results demonstrated
that inactivation of the hedgehog pathway markedly inhibited the
adhesion, invasion and migration of U251 cells.
Enhancement of hedgehog signaling
enhances the adhesion, invasion and migration of GBM cells
To determine the effects of hedgehog signaling on
cell attachment, U251 cells were treated with rhSHH and their
adhesion ability was evaluated. As illustrated in Fig. 2A, rhSHH dose-dependently increased
the adhesion ability of U251 cells. Furthermore, Transwell
migration and invasion assays were performed following treatment of
the cells with 0, 0.5 or 1.0 µg/ml rhSHH for 12 h (cell
migration) or 24 h (cell invasion), respectively. As shown in
Fig. 2B and C, the number of
migrated or invaded cells was increased by rhSHH in a
dose-dependent manner. These results demonstrated that activation
of the hedgehog pathway markedly increased the adhesion, invasion
and migration of U251 cells.
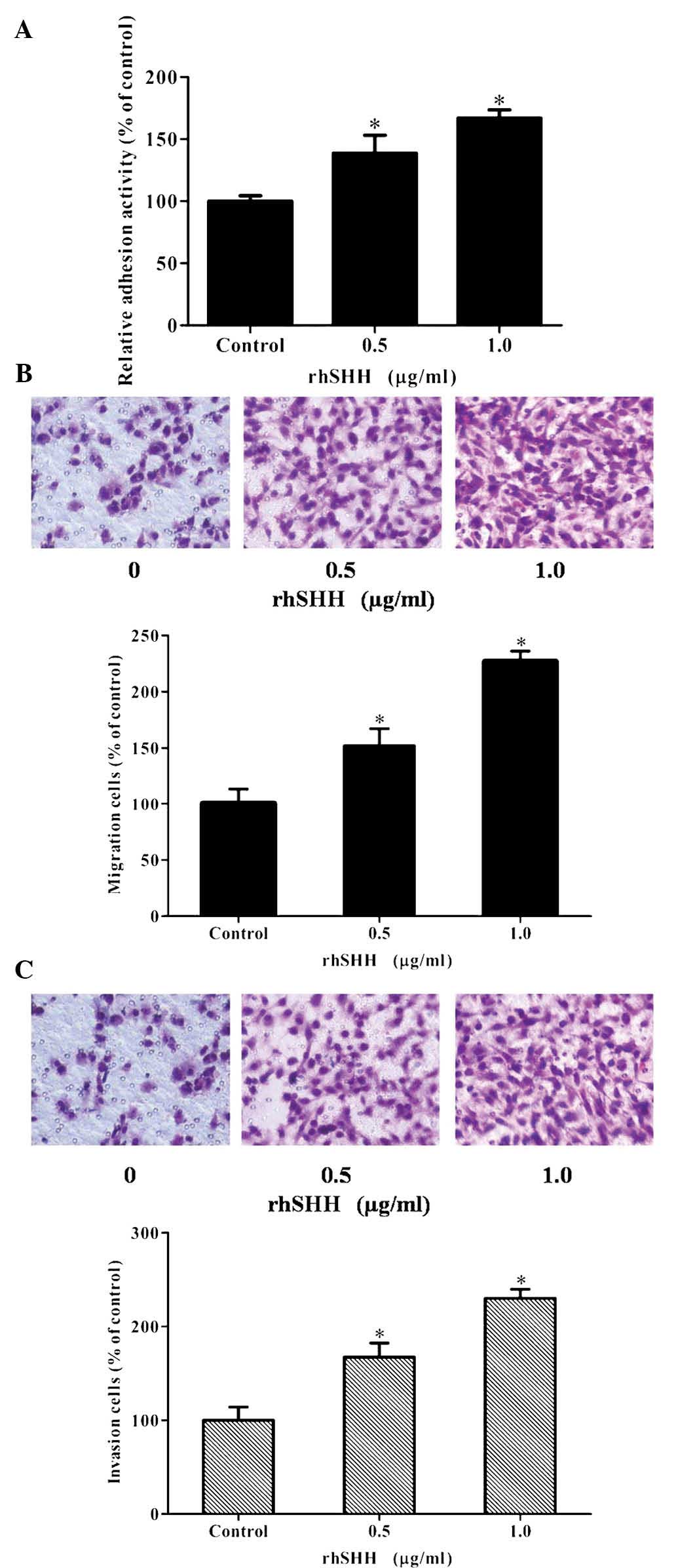 | Figure 2rhSHH enhances the adhesion, invasion
and migration of U251 cells. (A) U251 cells were incubated for 24 h
with various concentrations of rhSHH. Cells were seeded into
96-well plates coated with fibronectin. After 1 h, the adhered
cells were analyzed by MTT assay. The adhesion rate was expressed
as a percentage of the control (0 µg/ml). (B) U251 cells
were seeded in the upper wells without matrigel coating and treated
with various concentrations of rhSHH. After 12 h, cells on the
bottom side of the filter were fixed, stained and counted
(magnification, ×400). The migration rate was expressed as a
percentage of the control (0 µg/ml). (C) U251 cells were
seeded in the upper wells with matrigel coating and treated with
various concentrations of rhSHH. After 24 h, cells on the bottom
side of the filter were fixed, stained and counted (magnification,
×400). The invasion rate was expressed as a percentage of the
control (0 µg/ml). Values are expressed as the mean ±
standard deviation of three independent experiments.
*P<0.001, compared with controls. rhSHH, recombinant
human sonic hedgehog N-terminal peptide. |
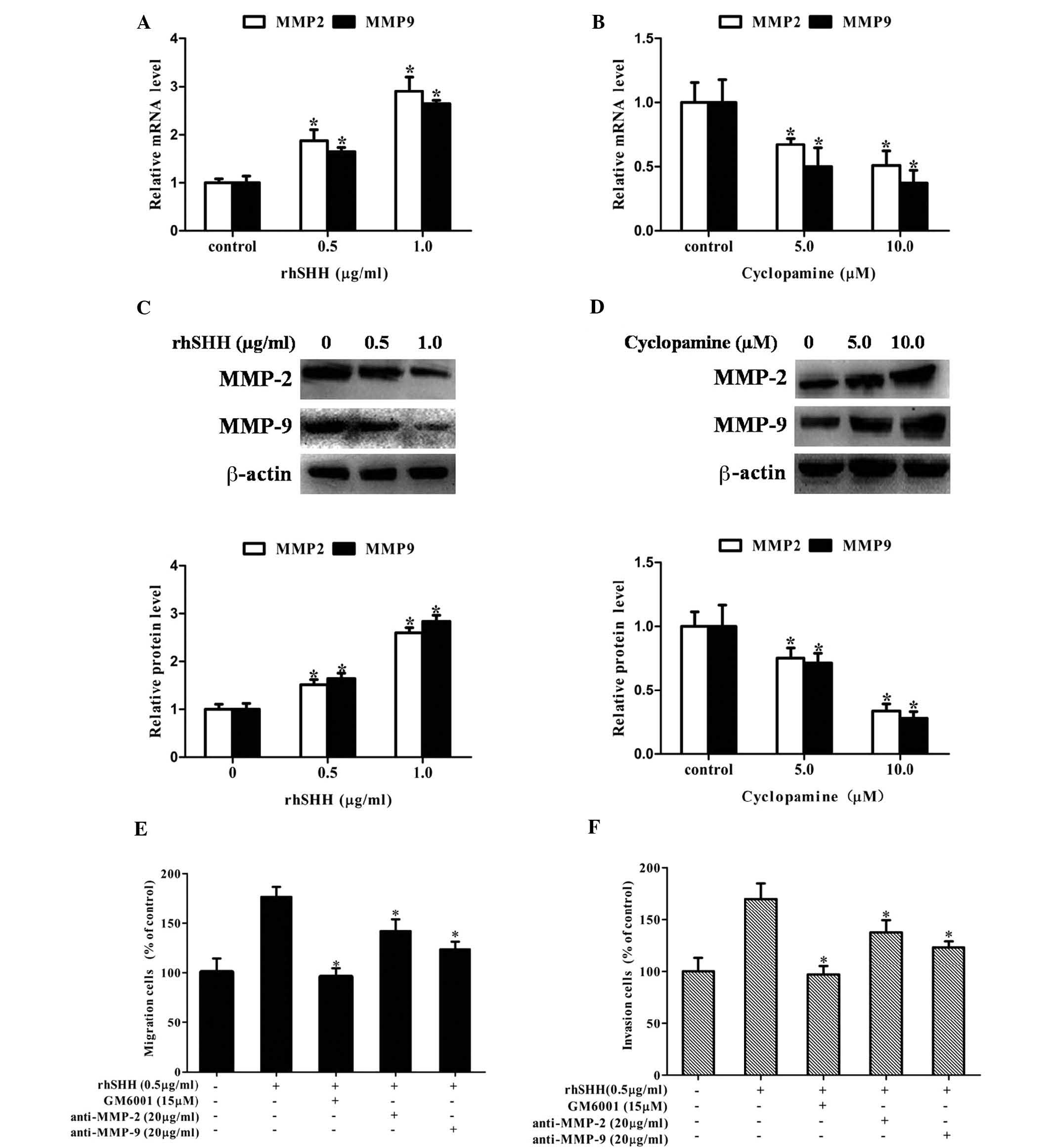
Hedgehog signaling enhances cell
migration and invasion by increasing the expression of MMP-2 and
MMP-9 in GBM
It is known that MMP-2 and MMP-9 have important
roles in tumor migration and invasion (14–16).
Therefore, the present study investigated whether the hedgehog
pathway regulated the expression of MMP-2 and MMP-9. It was
observed that treatment with rhSHH dose-dependently upregulated the
mRNA levels of MMP-2 and MMP-9 in U251 cells (Fig. 3A), while cyclopamine treatment
decreased the mRNA levels of MMP-2 and MMP-9 in U251 cells in a
concentration-dependent manner (Fig.
3B). Similarly, rhSHH dose-dependently upregulated the protein
levels of MMP-2 and MMP-9 in U251 cells (Fig. 3C), while cyclopamine treatment
decreased the protein levels of MMP-2 and MMP-9 in U251 cells in a
concentration-dependent manner (Fig.
3D). Furthermore, MMP-2 neutralizing antibody (anti-MMP-2),
MMP-9 neutralizing antibody (anti-MMP-9) or GAM6001 were able to
inhibit the rhSHH-induced cell migration and invasion (Fig. 3E and F). These results demonstrated
that hedgehog signaling enhanced the expression of MMP-2 and MMP-9
to promote cell migration and invasion of GBM.
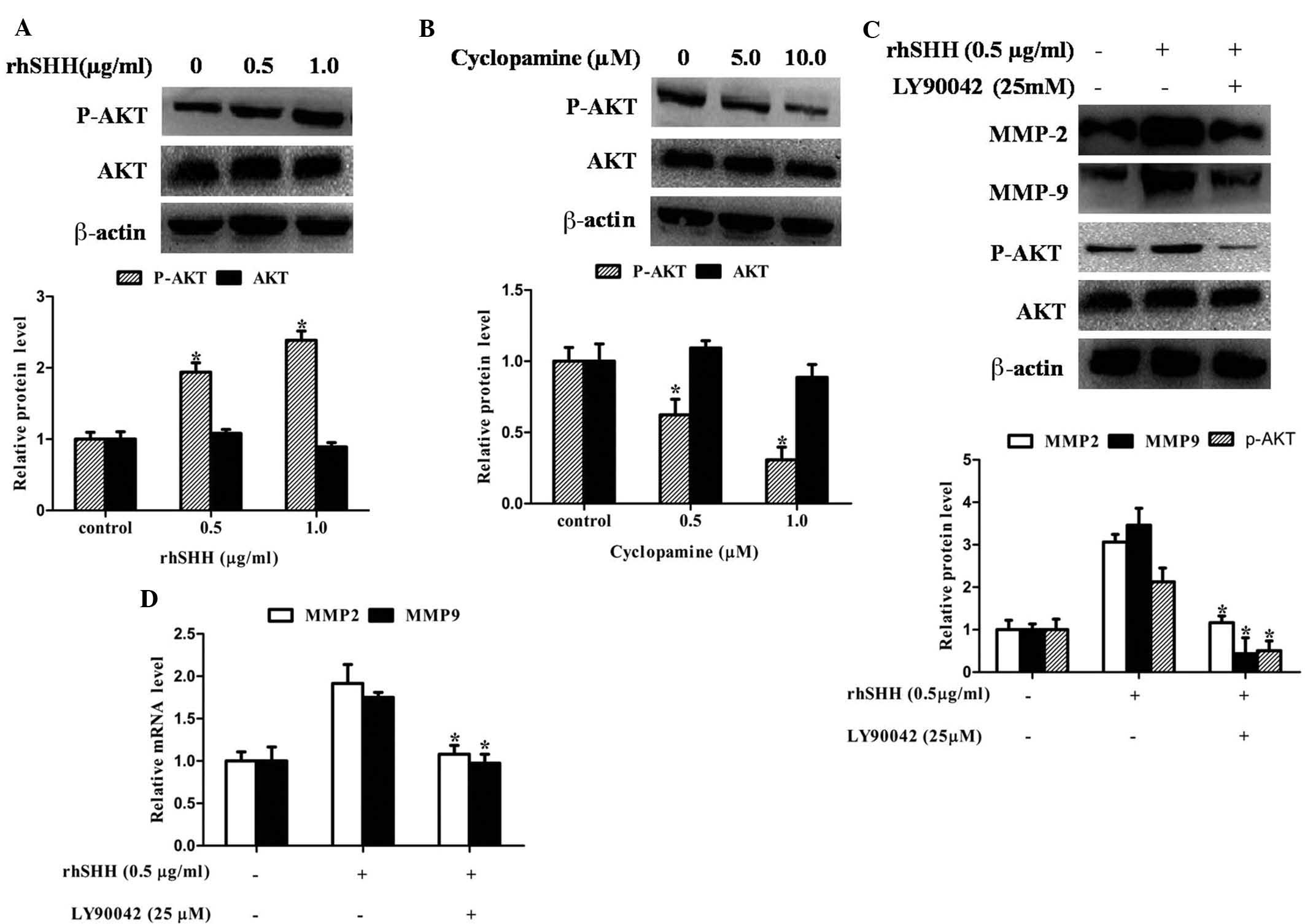
Hedgehog signaling regulates the
expression of MMP-2 and MMP-9 through the PI3K/AKT pathway in GBM
cells
In human glioma cells, the PI3K/Akt pathway is one
of the major signaling pathways for the invasive process.
Therefore, the present study determined whether activation of the
hedgehog pathway is associated with alterations of the PI3K/Akt
pathway in GBM. Fig. 4A and B
shows that AKT phosphorylation was significantly enhanced by rhSHH,
while it was significantly inhibited by cyclopamine. However, the
total protein expression of AKT was not altered by rhSHH and
cyclopamine. To confirm whether MMP-2 and MMP-9 expression,
mediated by the hedgehog pathway, was associated with the PI3K/AKT
pathway in GBM cells, this pathway was blocked using PI3K inhibitor
Ly294002. Fig. 4C and D shows that
Ly294002 significantly decreased rhSHH-induced mRNA and protein
expression of MMP-2 and MMP-9 as well as phosphorylation of AKT.
These results revealed that hedgehog signaling regulated the
expression of MMP-2 and MMP-9 through the PI3K/AKT pathway in GBM
cells.
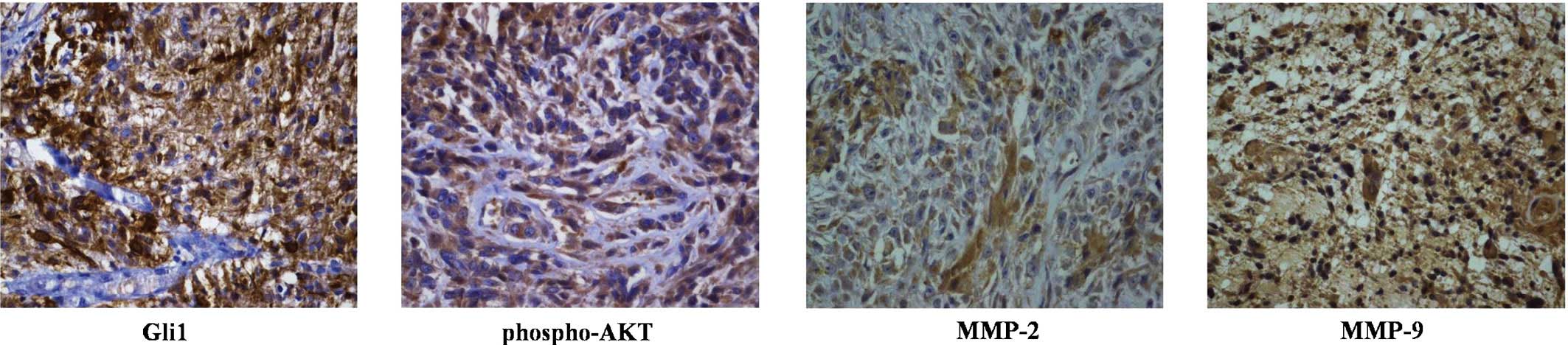
Gli1 expression is correlated with
phospho-AKT, MMP-2 and MMP-9 expression in GBM
The present study further examined the correlation
between Gli1, phospho-AKT, MMP-2 and MMP-9 expression in GBM. The
expression of Gli1, phospho-AKT, MMP-2 and MMP-9 in the 51 GBM
samples was evaluated using immunohistochemical analysis (Fig. 5). As shown in Table I, Gli1 protein expression was
positively correlated with the expression of phospho-AKT (r=0.582;
P<0.001), MMP-2 (r=0.380; P=0.013) and MMP-9 (r=0.329; P=0.019).
These results further indicated that the expression of MMP-2 and
MMP-9 is mediated by the hedgehog signaling pathway via PI3K/AKT
signaling.
 | Table ICorrelation between Gli1,
phospho-AKT, MMP-2 and MMP-9 protein expression. |
Table I
Correlation between Gli1,
phospho-AKT, MMP-2 and MMP-9 protein expression.
| Variable | Gli1 expression
| P-value | r |
|---|
| Negative (n) | Positive (n) |
|---|
| Phospho-AKT
expression | | | <0.001 | 0.582 |
| Negative | 13 | 7 | | |
| Positive | 3 | 28 | | |
| MMP-2
expression | | | 0.013 | 0.380 |
| Negative | 8 | 5 | | |
| Positive | 8 | 30 | | |
| MMP-9
expression | | | 0.019 | 0.329 |
| Negative | 9 | 8 | | |
| Positive | 7 | 27 | | |
Discussion
Glioblastoma is the most common type of malignant
tumor of the central nervous system. Migration and invasion are the
leading reasons for treatment failure and cancer-associated
mortality of GBM patients (3,4).
Therefore, it is necessary to develop novel prevention and
treatment strategies for GBM, for which an enhanced knowledge of
the underlying molecular mechanisms of migration and invasion of
GBM is required. The present study posed and verified the
hypothesis that the hedgehog signaling pathway activates the
PI3K/AKT pathway, which subsequently upregulates the expression of
MMP-2 and MMP-9 at the mRNA and protein level, thereby mediating
GBM migration and invasion.
The hedgehog signaling pathway has a vital role in
the control of cell differentiation, cell proliferation and
embryonic development (5,7,12).
According to recent studies, aberrant activation of hedgehog
signaling is linked to the aggressiveness of multiple tumor types,
including GBM (21,22). The results of the present study are
therefore consistent with those of previous studies. The present
study demonstrated that activation of hedgehog signaling by rhSHH
promoted the adhesion, invasion and migration of GBM cells, whereas
inhibition of this signaling with cyclopamine, an antagonist of the
hedgehog pathway, suppressed the adhesion, invasion and migration
of GBM cells. These results suggested that the hedgehog signaling
pathway has a tumor-promoting role in GBM that regulates cell
invasion and migration.
MMP-2 and MMP-9 are important members of the MMP
family, which and promote cancer-cell migration and invasion by
degrading extracellular matrix components (2,14–17).
An increasing number of studies demonstrated that MMP-2 and MMP-9
have important roles in GBM angiogenesis and can be regulated by
multiple pathways (23–25). The present study examined MMP-2 and
MMP-9 expression to explore the association between the hedgehog
pathway and cell invasion and migration. It was found that rhSHH
dose-dependently upregulated mRNA and protein levels of MMP-2 and
MMP-9 in U251 cells, whereas cyclopamine decreased mRNA and protein
levels of MMP-2 and MMP-9 in U251 cells in a
concentration-dependent manner. In addition, U251 cells were
treated with MMP-2- or MMP-9-neutralizing antibody or with GAM6001,
and their effects on rhSHH-induced migration and invasion of GBM
cells was evaluated. A Transwell assay revealed that MMP-2- and
MMP-9 neutralizing antibody or GAM6001 were able to reverse
rhSHH-induced cell migration and invasion. These results indicated
that hedgehog signaling promotes cell migration and invasion by
enhancing the expressions of MMP-2 and MMP-9 in GBM cells.
Accumulating evidence revealed that the PI3K/Akt
pathway is activated in multiple types of cancer, including kidney,
lung, liver and ovarian cancer, and has an important role in
migration and invasion (26–28).
Several studies reported that the PI3K/Akt pathway is mediated by
the SHH pathway. For example, Yoo et al (29) reported that hedgehog signaling
promotes the metastasis of gastric cancer through activation of the
PI3K/Akt pathway. Morton et al (30) also demonstrated that inhibition of
the hedgehog pathway markedly regulated pancreatic tumorigenesis
through inhibition of the PI3K/AKT pathway. However, it remains
elusive whether such association also exists in GBM. In the present
study, AKT phosphorylation of GBM cells was significantly enhanced
by rhSHH and was significantly inhibited by cyclopamine, verifying
the hypothesis that the PI3K/Akt pathway is mediated via the
hedgehog pathway in GBM cells. Furthermore, the present study
demonstrated that the PI3K inhibitor Ly294002 significantly
decreased rhSHH-induced MMP-2 and MMP-9 mRNA and protein expression
as well as phosphorylation of AKT. More importantly,
immunohistochemical analysis showed that Gli1 protein expression
was closely correlated with phospho-AKT, MMP-2 and MMP-9 in GBM
samples. All of these results suggested that hedgehog signaling
regulates the expression of MMP-2 and MMP-9 through the PI3K/AKT
pathway in GBM.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, that the hedgehog
signaling pathway promotes the invasion and migration of GBM cells
by enhancing MMP-2 and MMP-9 expression via the PI3K/AKT pathway.
These results provided a novel molecular mechanism of GBM-cell
aggressiveness based on the hedgehog signaling pathway and may
assist in the discovery of novel therapeutic targets to control the
aggressiveness of GBM.
Acknowledgments
The present study was supported by the National High
Technology Research and Development Program of China (863 program;
grant no. 2012AA02A508), the National Basic Research Program of
China (973 program; grant no. 2012CB517803), the National Natural
Science Foundation of China (grant nos. 81070217 and 81270340) and
the Research Foundation of the Chinese Ministry of Health (grant
no. w2011bx059).
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeAngelis LM: Brain tumors. N Engl J Med.
344:114–123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsumura H, Ohnishi T, Kanemura Y, Maruno
M and Yoshimine T: Quantitative analysis of glioma cell invasion by
confocal laser scanning microscopy in a novel brain slice model.
Biochem Biophys Res Commun. 269:513–520. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou X, Zhan W, Bian W, Hua L, Shi Q, Xie
S, Yang D, Li Y, Zhang X, Liu G and Yu R: GOLPH3 regulates the
migration and invasion of glioma cells though RhoA. Biochem Biophys
Res Commun. 433:338–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hao K, Tian XD, Qin CF, Xie XH and Yang
YM: Hedgehog signaling pathway regulates human pancreatic cancer
cell proliferation and metastasis. Oncol Rep. 29:1124–1132.
2013.PubMed/NCBI
|
|
6
|
Qin CF, Hao K, Tian XD, Xie XH and Yang
YM: Combined effects of EGFR and Hedgehog signaling pathway
inhibition on the proliferation and apoptosis of pancreatic cancer
cells. Oncol Rep. 28:519–526. 2012.PubMed/NCBI
|
|
7
|
Fuller K, O'Connell JT, Gordon J, Mauti O
and Eggenschwiler J: Rab23 regulates Nodal signaling in vertebrate
left-right patterning independently of the Hedgehog pathway. Dev
Biol. 391:182–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ju B, Chen W, Spitsbergen JM, Lu J, Vogel
P, Peters JL, Wang YD, Orr BA, Wu J and Henson HE: Activation of
Sonic hedgehog signaling in neural progenitor cells promotes glioma
development in the zebrafish optic pathway. Oncogenesis. 3:e962014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Onishi H and Katano M: Hedgehog signaling
pathway as a new therapeutic target in pancreatic cancer. World J
Gastroenterol. 20:2335–2342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsushita S, Onishi H, Nakano K,
Nagamatsu I, Imaizumi A, Hattori M, Oda Y, Tanaka M and Katano M:
Hedgehog signaling pathway is a potential therapeutic target for
gallbladder cancer. Cancer Sci. 105:272–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Q, Gao G and Luo S: Hedgehog
signaling pathway and ovarian cancer. Chin J Cancer Res.
25:346–353. 2013.PubMed/NCBI
|
|
12
|
Wan J, Zhou J, Zhao H, Wang M, Wei Z, Gao
H, Wang Y and Cui H: Sonic hedgehog pathway contributes to gastric
cancer cell growth and proliferation. Biores Open Access. 3:53–59.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu W, You Z, Li T, Yu C, Tao G, Hu M and
Chen X: Correlation of hedgehog signal activation with
chemoradiotherapy sensitivity and survival in esophageal squamous
cell carcinomas. Jpn J Clin Oncol. 41:386–393. 2011. View Article : Google Scholar
|
|
14
|
Aparna M, Rao L, Kunhikatta V and
Radhakrishnan R: The role of MMP-2 and MMP-9 as prognostic markers
in the early stages of tongue squamous cell carcinoma. J Oral
Pathol Med. 44:345–352. 2015. View Article : Google Scholar
|
|
15
|
Zhou M, Qin S, Chu Y, Wang F, Chen L and
Lu Y: Immunolocalization of MMP-2 and MMP-9 in human rheumatoid
synovium. Int J Clin Exp Pathol. 7:3048–3056. 2014.PubMed/NCBI
|
|
16
|
Yang W, Li Q and Pan Z:
Sphingosine-1-phosphate promotes extravillous trophoblast cell
invasion by activating MEK/ERK/MMP-2 signaling pathways via
S1P/S1PR1 axis activation. PLoS One. 9:e1067252014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu WH and Chang LS: Caffeine induces
matrix metalloproteinase-2 (MMP-2) and MMP-9 down-regulation in
human leukemia U937 cells via Ca2+/ROS-mediated suppression of
ERK/c-fos pathway and activation of p38 MAPK/c-jun pathway. J Cell
Physiol. 224:775–785. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang MH, Oh SC, Lee HJ, Kang HN, Kim JL,
Kim JS and Yoo YA: Metastatic function of BMP-2 in gastric cancer
cells: The role of PI3K/AKT, MAPK, the NF-κB pathway, and MMP-9
expression. Exp Cell Res. 317:1746–1762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du WZ, Feng Y, Wang XF, Piao XY, Cui YQ,
Chen LC, Lei XH, Sun X, Liu X, Wang HB, et al: Curcumin suppresses
malignant glioma cells growth and induces apoptosis by inhibition
of SHH/GLI1 signaling pathway in vitro and vivo. CNS Neurosci Ther.
19:926–936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Chen L, Bao Z, Li S, You G, Yan W,
Shi Z, Liu Y, Yang P, Zhang W, et al: Inhibition of STAT3 reverses
alkylator resistance through modulation of the AKT and β-catenin
signaling pathways. Oncol Rep. 26:1173–1180. 2011.PubMed/NCBI
|
|
21
|
Morgenroth A, Vogg AT, Ermert K,
Zlatopolskiy B and Mottaghy FM: Hedgehog signaling sensitizes
glioma stem cells to endogenous nano-irradiation. Oncotarget.
5:5483–5493. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dixit D, Ghildiyal R, Anto NP, Ghosh S,
Sharma V and Sen E: Guggulsterone sensitizes glioblastoma cells to
Sonic hedgehog inhibitor SANT-1 induced apoptosis in a Ras/NFκB
dependent manner. Cancer Lett. 336:347–358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Das G, Shiras A, Shanmuganandam K and
Shastry P: Rictor regulates MMP-9 activity and invasion through
Raf-1-MEK-ERK signaling pathway in glioma cells. Mol Carcinog.
50:412–423. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Senft C, Priester M, Polacin M, Schröder
K, Seifert V, Kögel D and Weissenberger J: Inhibition of the
JAK-2/STAT3 signaling pathway impedes the migratory and invasive
potential of human glioblastoma cells. J Neurooncol. 101:393–403.
2011. View Article : Google Scholar
|
|
25
|
Zhao Y, Xiao A, Dipierro CG, Abdel-Fattah
R, Amos S, Redpath GT, Carpenter JE, Pieper RO and Hussaini IM:
H-Ras increases urokinase expression and cell invasion in
genetically modified human astrocytes through Ras/Raf/MEK signaling
pathway. Glia. 56:917–924. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Porta C and Figlin RA:
Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney
cancer and the therapeutic potential of
phosphatidylinositol-3-kinase/Akt inhibitors. J Urol.
182:2569–2577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang E, Feng X, Liu F, Zhang P2, Liang J
and Tang X: Roles of PI3K/Akt and c-Jun signaling pathways in human
papillomavirus type 16 oncoprotein-induced HIF-1α, VEGF and IL-8
expression and in vitro angiogenesis in non-small cell lung cancer
cells. PLoS One. 9:e1034402014. View Article : Google Scholar
|
|
28
|
Mazzoletti M and Broggini M: PI3K/AKT/mTOR
inhibitors in ovarian cancer. Curr Med Chem. 17:4433–4447. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK,
Kim HK, Kim JS and Oh SC: Sonic hedgehog pathway promotes
metastasis and lymphangiogenesis via activation of Akt, EMT and
MMP-9 pathway in gastric cancer. Cancer Res. 71:7061–7070. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morton JP, Mongeau ME, Klimstra DS, Morris
JP, Lee YC, Kawaguchi Y, Wright CV, Hebrok M and Lewis BC: Sonic
hedgehog acts at multiple stages during pancreatic tumorigenesis.
Proc Natl Acad Sci USA. 104:5103–5108. 2007. View Article : Google Scholar : PubMed/NCBI
|