Introduction
Postoperative immunosuppression is a generalized
state in several types of surgery, which is involved in
postoperative septic complications and tumor metastasis formation
(1). To assess the changes in
immune state underlying surgical stress, cell-mediated immunity
(CMI) is often investigated (1-3).
Although surgical procedures always cause an increase in the total
number of circulating white blood cells, critical individual
leukocyte subpopulations, including lymphocytes, are suppressed in
number and function (2,3). A decrease in the lymphocyte
proliferation rate (1) and
increase in apoptosis (4,5) are the two predominant factors leading
to the reduction in circulating lymphocyte numbers. In addition,
surgery also shifts the balance of T-helper (Th)1/Th2 cells towards
anti-CMI Th2 dominance, which is relevant to the immunosuppression
of CMI (2).
Lidocaine is widely used as an analgesic and
anti-hyperalgesic and exhibits antibacterial actions and beneficial
effects on the inflammatory response (6–9).
Previous studies have revealed that intraoperative systemic
lidocaine benefits patients by reducing postoperative pain,
analgesic consumption, postoperative nausea and vomiting, and even
the duration spent in hospital (10–12).
Data also demonstrates that lidocaine can protect rats from cecal
ligation and puncture assault-associated septic organ failure
(7,9). However, whether lidocaine exerts a
protective effect on CMI in patients undergoing surgery for the
removal of a primary tumor remains to be elucidated.
Peri-operative immune changes occur primarily as a
result of surgical trauma and subsequent neuroendocrine responses,
thus efforts to reduce the immunosuppressive effects of surgery
require initiation in the pre-operative period (1). Therefore, in the present study,
lidocaine was administered intravenously between the pre-anesthesia
period and the point of discharge from the operating room. It was
hypothesized that lidocaine may have a beneficial effect on CMI
during the postoperative period in patients with cervical cancer
undergoing radical hysterectomy.
Patients and methods
Patients
A total of 30 adult female patients aged between 25
and 65 years old, undergoing radical hysterectomy were recruited in
the present prospective study between August 2013 and January 2014.
Patients were excluded if they had a weight <45 kg or >65 kg;
a history of allergies to local anesthetics, bradycardia or heart
block; severe respiratory, renal or hepatic disease, previous
history of opioid medication use or a psychiatric medical
history.
The study protocol was approved by the ethics
committee of Qilu Hospital of Shandong University (Jinan, China)
and performed according to the Declaration of Helsinki. Written
informed consent was obtained from all participants prior to
enrolment.
All participants were randomized into two groups,
according to a computer-generated random number table, in which the
patients received either intravenous lidocaine (Shanghai Zhi Pharma
Co., Ltd., Shanghai, China) or normal saline (control group). The
solutions were prepared in a 20 cc syringe and labeled only with a
case number by a nurse in a blinded-manner. The patients assigned
to the lidocaine group received an intravenous bolus infusion of
1.5 mg/kg lidocaine 10 min prior to the induction of anesthesia,
followed by continuous infusion at 1.5 mg/kg/h using a Graseby 3100
syringe pump (Graseby Medical, Ltd., Watford, UK) until discharge
from the operating room. The patients in the control group received
the same volume of normal saline. All surgical procedures were
performed by the same team of surgeons to avoid individual
variability in operative techniques. The characteristics of the
patients are listed in Table
I.
 | Table IDemographic and clinical
characteristics of the participants in the control and lidocaine
groups. |
Table I
Demographic and clinical
characteristics of the participants in the control and lidocaine
groups.
| Characteristic | Control | Lidocaine | P-value |
|---|
| ASA (n) | | | 1.00 |
| I | 10 | 11 | |
| II | 5 | 4 | |
| Age (years) | 48.6±5.6 | 44.2±11.8 | 0.20 |
| Height (cm) | 156.4±8.9 | 155.8±9.7 | 0.86 |
| Weight (kg) | 56.9±7.6 | 56.0±6.5 | 0.11 |
| Duration of surgery
(min) | 129.3±24.4 | 132.3±25.1 | 0.74 |
| Duration of
anesthesia (min) | 158.0±16.9 | 152.3±14.1 | 0.32 |
Following the administration of 0.1 mg/kg midazolam
(Jiangsu Nhwa Pharmaceutical Co., Ltd., Xuzhou, China), 1–2 mg/kg
propofol (Fresenius Kabi AG, Bad Homburg vor der Höhe, Germany), 2
µg/kg fentanyl (Yichang Humanwell Pharmaceutical Co., Ltd.,
Yichang, China) and 0.6 mg/kg rocuronium (Taizhou Xianju
Pharmaceutical Co., Ltd., Taizhou, China) intravenously, the
patients were intu-bated and ventilated to maintain the end-tidal
CO2 volume between 30 and 40 mmHg. Anesthesia was
maintained using 1.5–3% sevoflurane (Jiangsu Hengrui Medicine Co.,
Ltd., Lianyungang, China) in 1 l/min O2. Fentanyl and
rocuronium were added, according to heart rate or the bispectral
index (S/5; GE Healthcare Life Sciences, Helsinki, Finland).
Noninvasive arterial blood pressure, electrocardiography and pulse
oximetry (S/5) were monitored continuously. During surgery, the
patients received intravenous infusion of lactated Ringer's
solution (Shandong Hualu Pharmaceutical Co., Ltd., Muping, China)
at a rate of 6–12 ml/kg/h.
Peripheral blood lymphocyte (PBL)
isolation
A 10 ml blood sample was drawn from the median
cubital vein of each patient 1 day prior to surgery, following
discharge from the operating room and at 48 h post-surgery. Serum
was isolated at room temperature by centrifugation (1,000 × g, 5
min) and stored at −20°C prior to assessment. Lymphocytes were
isolated using Ficoll-paque density centrifugation (Haoyang
Biological Manufacture Co., Ltd., Tianjin, China) at 1,000 x g for
15 min at −20°C. The cell viability was assayed by 0.4% trypan blue
exclusion, which was >95% in all cases. The collected cells were
resuspended at a density of 1×106 cells/ml in RPMI 1640
medium (Thermo Fisher Scientific, Shanghai, China) supplemented
with 10% heat-inactivated fetal calf serum, and cultured at 37°C in
a humidified 5% CO2 atmosphere.
Cell proliferation
The suspended cells (100 µl; 1×106
cells/ml) were cultivated with 5 µg/ml phytohemagglutinin (PHA)
(Sigma-Aldrich, St. Louis, MO, USA) in a 96-well plate at 37°C and
5% CO2 for 24 h. RPMI 1640 was used as a blank control
in each group. Subsequently, 10 µl Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added to
each well 4 h prior to the end of stimulation, when the optical
density values were measured at 450 nm (Model 722; INESA
Instrument, Shanghai, China). Data are expressed as the mean ±
standard deviation of four wells.
Detection of early apoptosis using
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
staining
Detection of early apoptosis in the PBLs was
performed, according to the manufacturer's instructions, using an
Annexin V FITC kit (Bogoo, Shanghai, China). In brief, the PBLs
(2×106) were resuspended in 2 ml ice-cold
phosphate-buffered saline and washed twice. The collected PBLs were
initially suspended in 400 µl 1X binding buffer, following which 5
µl Annexin V-FITC was added for 15 min in the dark followed by 10
µl PI for 5 min in the dark. A negative control was used for each
sample, in which the PBLs were incubatedwith binding buffer alone.
For the positive control, the cells were incubated with Annexin
V-FITC or PI alone. The cell sample was measured immediately using
a flow cytometer.
Flow cytometry (FC)
The FC data were acquired within 24 h of staining
using CellFit version 2.0 software and a FACSCalibur cytometer (BD
Biosciences, San Jose, CA, USA). An argon ion laser excitation of
488 nm was used. The emitted light was detected by logarithmic
amplification through barrier filters, which were specific for the
emission range of the different fluorophores: 530/22 nm for FITC
(fluorescence channel FL1) and 575/42 nm (FL2) for PI. The results
of the lymphocyte typing and lymphocyte early apoptosis detection
were obtained by quadrant analyses of FL1, vs. FL2 channel dot
plots and presented as the percentage of gated lymphocytes.
ELISA assay
The level of high-mobility group protein B1 (HMGB1),
interferon (IFN)-γ and interleukin (IL)-4 in the serum were
determined using ELISA kits (R&D Systems, Inc., Minneapolis,
MN, USA), according to the manufacturer's instructions.
Statistical analysis
SigmaPlot 12.5 (Systat Software, Inc., San Jose, CA,
USA) was used for statistical analysis. The data distribution was
evaluated using Levene's test. The normally distributed data are
expressed as the mean ± standard deviation and were compared using
one-way analysis of variance or an unpaired t-test. Descriptive
variables were subjected to χ2 analysis or Fisher's
exact test, as appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
Preoperative clinical parameters of
patients
All 30 patients recruited in the present study
completed the study. As shown in Table
I, no significant differences were identified between the
groups in terms of the American Society of Anesthesiologists class,
age, height, weight, duration of surgery or anesthesia (13).
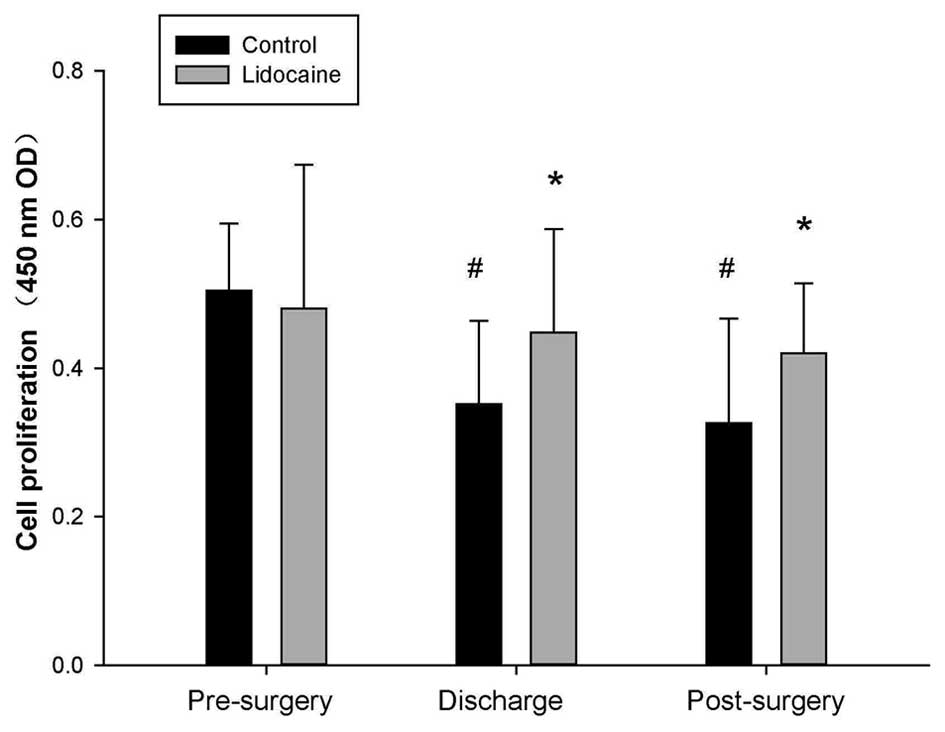
Lidocaine protects the proliferation of
PBLs
The number of blood lymphocytes always falls
peri-operatively, and decreases in the lymphocyte proliferation
rate are considered to be one of its causes (1). Therefore, the present study initially
examined the proliferation rate of PBLs using a CCK-8 assay. The
results, as shown in Fig. 1,
demonstrated that lidocaine improved the proliferation rate of the
surgical stressor-induced lymphocytes. Therefore, lidocaine may
exert a protective effect on lymphocyte function.
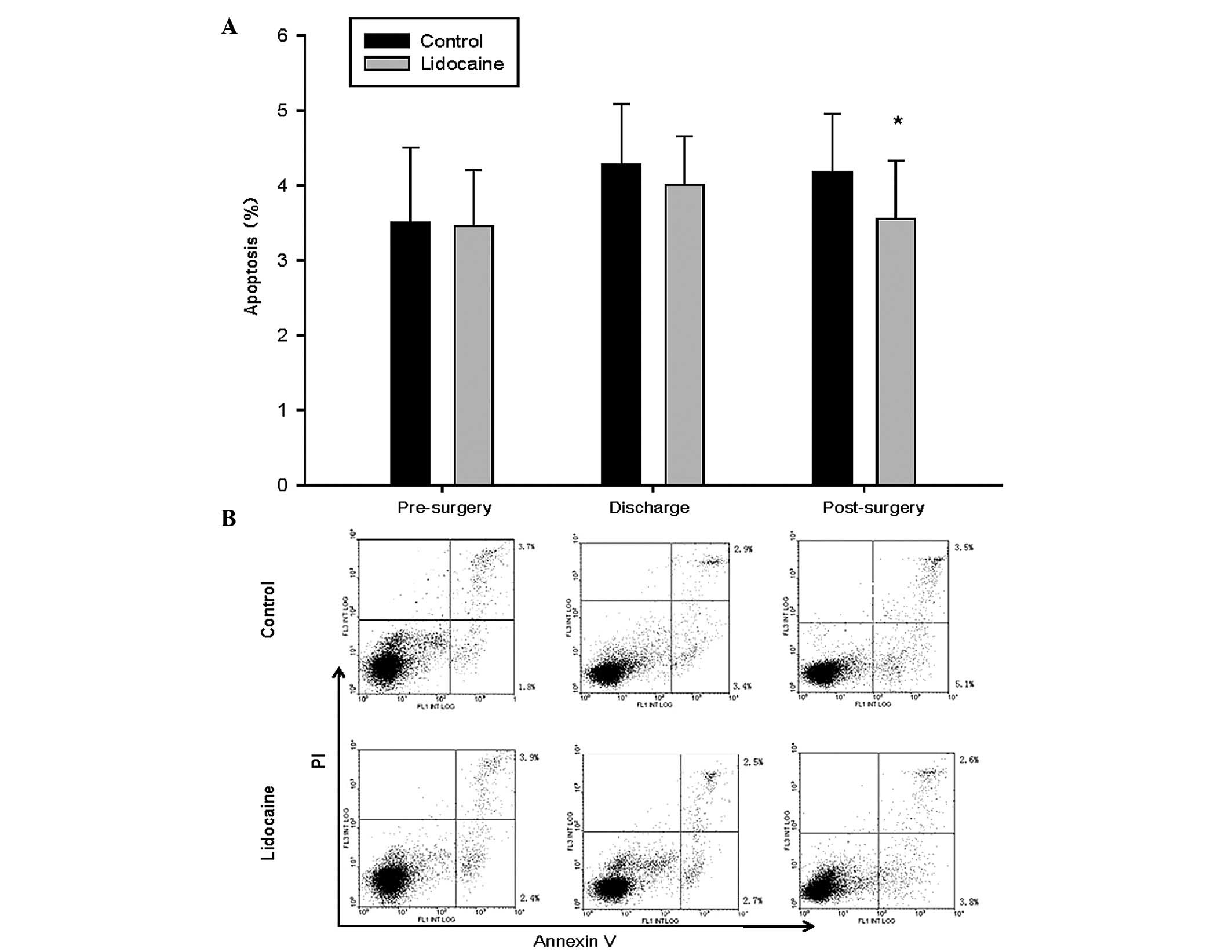
Lidocaine attenuates surgery-induced
apoptosis of PBLs
Surgical stressors not only reduce lymphocyte
proliferation, but they increase apoptosis of immune cells. To
determine whether lidocaine exerted a protective effect on
surgery-induced apoptosis of lymphocytes, the PBLs in the present
study were stained with Annexin V-FITC/PI and assessed using flow
cytometry. As shown in Fig. 2,
intraoperative systemic lidocaine attenuated the surgery-induced
apoptosis of the PBLs.
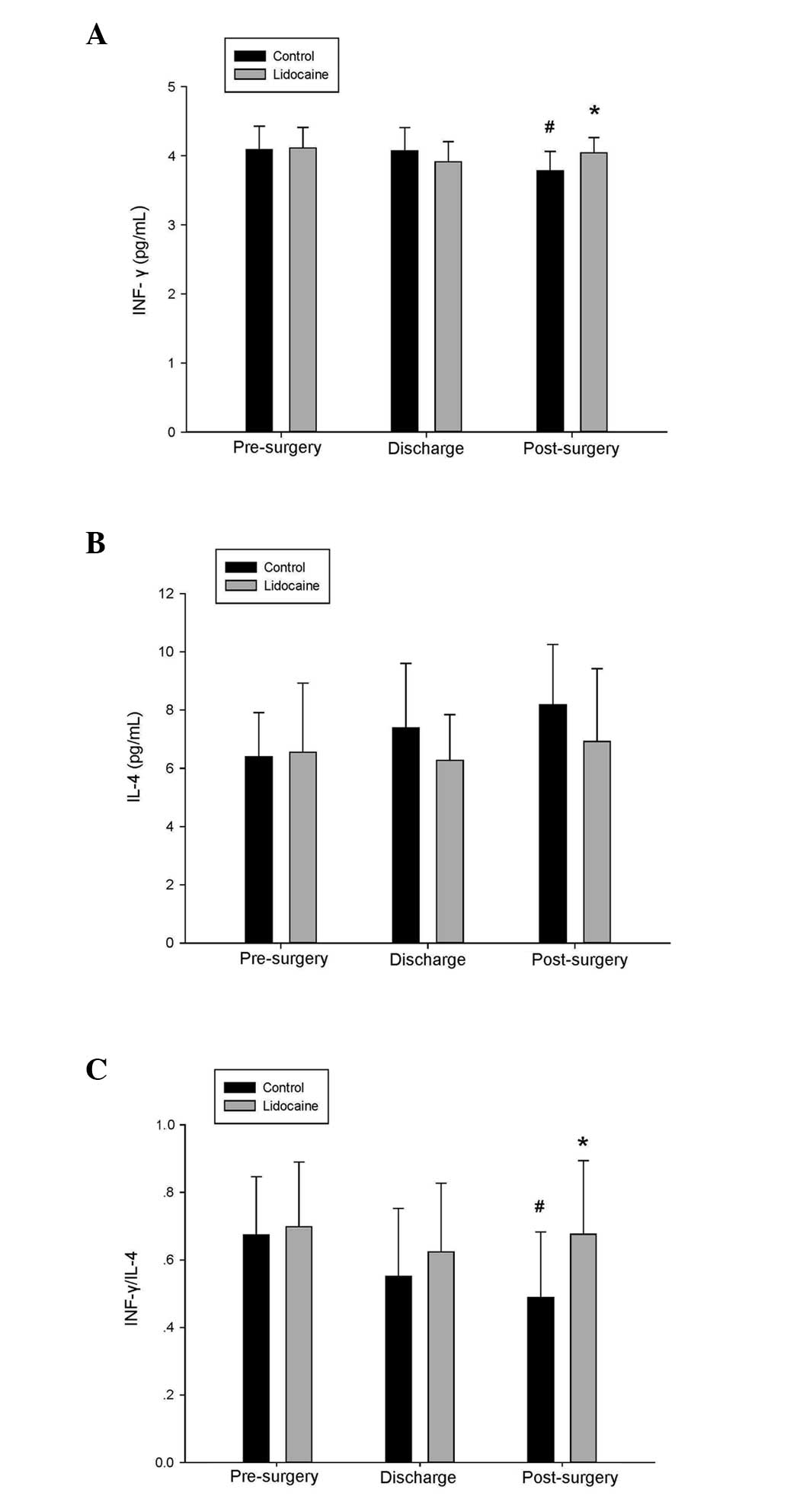
Lidocaine preserves levels of IFN-γ and
IL-4
The serum level of IFN-γ at 48 h post-surgery in the
control group was decreased significantly, compared with the
pre-surgical level (3.782±0.282, vs. 4.089±0.339, pg/ml
respectively). However, no significant differences were observed in
the levels of IFN-γ in the lidocaine group or in the levels of IL-4
in the control and lidocaine group, compared with the pre-surgical
value. As shown in Fig. 3, the
ratio of IFN-γ to IL-4 was well preserved in the lidocaine
group.
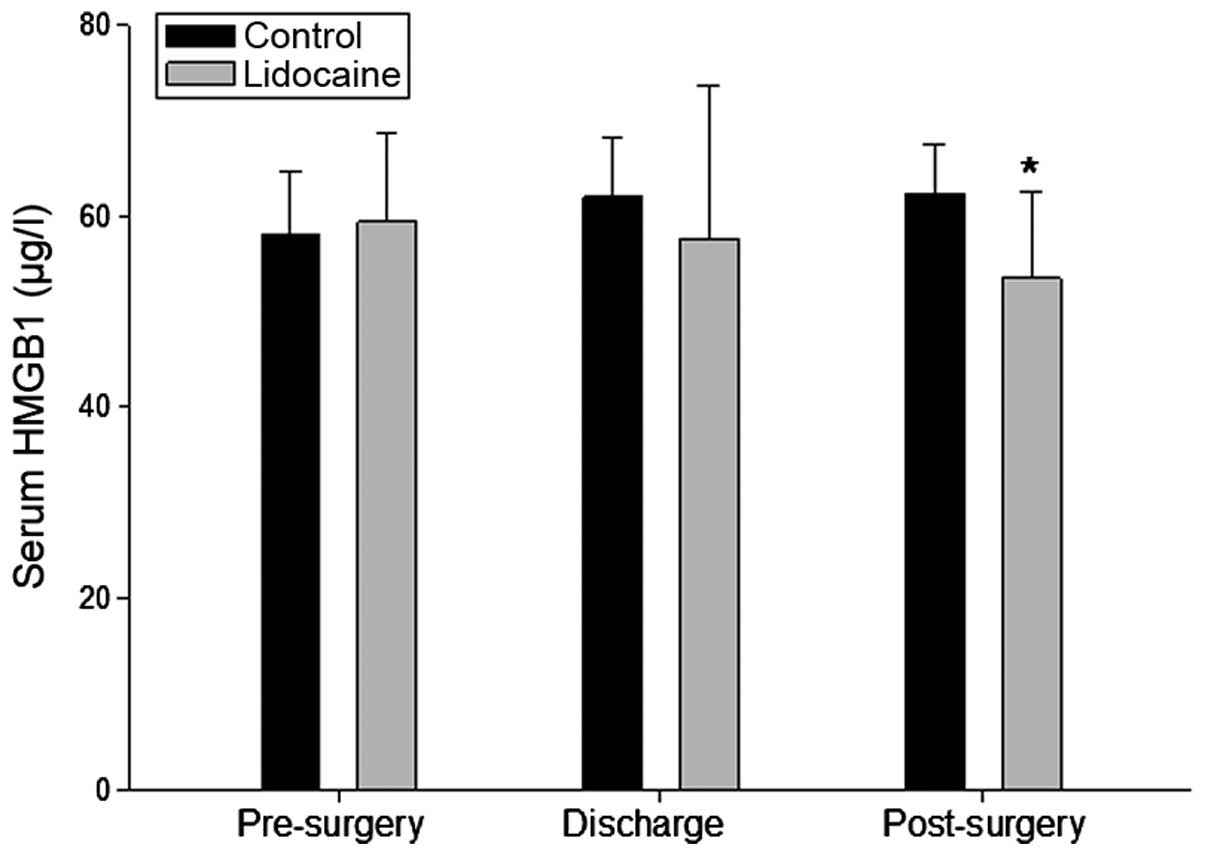
Lidocaine decreases the protein levels of
HMGB1 in the serum
HMGB1, as a critical mediator of several
inflammatory and non-inflammatory diseases, is important in
surgery-associated sepsis and tumor metastasis. To assess whether
lidocaine has an inhibitory effect on its expression in patients
undergoing surgery, the serum level of HMGB1 was determined in the
present study using ELISA. As expected, the serum protein level of
HMGB1 in the lidocaine group was reduced, which was significantly
difference 48 h-post surgery, compared with the control group
(Fig. 4).
Discussion
Surgery-induced stress can increase the
susceptibility to tumor metastasis (14,15),
and it is considered to be one of the causes of the suppression of
CMI secondary to surgical stress (1). The present study indicated that
peri-operative intravenous lido-caine had a beneficial effect on
CMI, and this was associated with the preservation of lymphocyte
proliferation, attenuation of apoptosis, maintainence of the
balance of Th1/Th2 cells and the decreased production of cytokines
in patients undergoing radical hysterectomy.
Lymphocytes are the major cellular components of the
immune response and are important in the recovery of patients
following surgery for the removal of a tumor (16). However, the decrease in lymphocyte
proliferation rate (1) and the
increase in apoptosis (4,5) are common following surgery and are
considered to be the two predominant factors leading to the
decrease of lymphocytes in the circulation. The lymphocyte
mitogenic response to PHA is an important tool, which can mimic the
activity of lymphocytes in response to an exogenous stimulus, and
was well-preserved in the patients in the lidocaine group, compared
with those in the control group in the present study. This result
is similar to that of Yardeni et al (10). In addition, the results of the
present study revealed that intraoperative systemic lidocaine
attenuated the apoptosis of PBL cells induced by surgical
stressors. However, previous in vitro studies have
demonstrated the opposite conclusions regarding cell proliferation
(17,18) and apoptosis (19,20).
This indicates that the protective effects of lidocaine on CMI
in vivo are not directly due to its effects on lymphocytes,
and the underlying mechanisms require further exploration.
Distinct patterns of cytokines are produced by two
types of Th cell. Th1 lymphocytes produce cytokines, including
IFN-γ and IL-2, which favor CMI; whereas Th2 cells secrete
cytokines, including IL-4 and IL-10, which favor humoral immunity
(21). Itis well-established that
surgery reduces the number of Th1 cytokines, including IFN-γ, and
decreases the Th1/Th2 ratio, which is responsible for the
suppression of CMI following surgery (21). This was observed in the patients in
the control group in the present study, however, the serum levels
of IFN-γ did not change significantly in the patients in the
lidocaine group, which maintained the balance of Th1/Th2.
HMGB1, as a critical mediator of several
inflammatory diseases, is important in surgery-associated sepsis
(22). It is also implicated in
non-inflammatory conditions, including cancer, by regulating
tumorigenesis and contributing to metastasis (23). HMGB1 levels increase significantly
and last for a few days following liver resection (24) or cardiac surgery (25). In the present study, although the
increased level of HMGB1 was not significant following surgery
compared with the pre-surgical levels in the control group, there
was a significant decrease at 48 h post-surgery in the lidocaine
group, compared with the control group. It was hypothesized that
lidocaine, used as an anesthetic adjuvant decreases the level of
HMGB1 and may contribute to attenuate the complications of tumor
surgery, including sepsis and metastasis.
There are certain limitations to the present study.
Previous studies have revealed that lidocaine decreases plasma
concentrations of cortisol (26)
and catecholamines (27) in
patients undergoing surgery, whereas glucocorticoids reduce T cell
proliferation and increase the apoptosis of immature T cells
(16). Catecholamines suppress
anti-metastatic immunity and act directly on minimal residual
disease to promote metastatic progression (16). However, the present study did not
further examine the mechanism underlying the protective effect of
lidocaine on CMI. Secondly, investigations are required to
determine the direct association between suppressed CMI and tumor
metastases in patients undergoing surgical removal of a primary
tumor.
In conclusion, the present study demonstrated that
the intraoperative systemic administration of lidocaine exerted a
protective effect on CMI in patients with cervical cancer
undergoing radical hysterectomy. This may be beneficial in reducing
the occurrence of postoperative septic complications and tumor
metastasis formation.
Acknowledgments
This study was supported by the Qilu Hospital
Science Research Foundation (grant no. 26010175616032), and in
part, by Shandong Provincial Natural Science Foundation, China
(grant nos. Y2007C115, ZR2011HM028 and 2009ZRB14031).
References
|
1
|
Hogan BV, Peter MB, Shenoy HG, Horgan K
and Hughes TA: Surgery induced immunosuppression. Surgeon. 9:38–43.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ogawa K, Hirai M, Katsube T, Murayama M,
Hamaguchi K, Shimakawa T, Naritake Y, Hosokawa T and Kajiwara T:
Suppression of cellular immunity by surgical stress. Surgery.
127:329–336. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishikawa M, Nishioka M, Hanaki N, Miyauchi
T, Kashiwagi Y, Ioki H, Kagawa A and Nakamura Y: Perioperative
immune responses in cancer patients undergoing digestive surgeries.
World J Surg Oncol. 7:72009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delogu G, Moretti S, Antonucci A,
Marcellini S, Masciangelo R, Famularo G, Signore L and De Simone C:
Apoptosis and surgical trauma: Dysregulated expression of death and
survival factors on peripheral lymphocytes. Arch Surg.
135:1141–1147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Papadima A, Boutsikou M, Lagoudianakis EE,
Kataki A, Konstadoulakis M, Georgiou L, Katergiannakis V and
Manouras A: Lymphocyte apoptosis after major abdominal surgery is
not influenced by anesthetic technique: A comparative study of
general anesthesia versus combined general and epidural analgesia.
J Clin Anesth. 21:414–421. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hollmann MW and Durieux ME: Local
anesthetics and the inflammatory response: A new therapeutic
indication? Anesthesiology. 93:858–875. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gallos G, Jones DR, Nasr SH, Emala CW and
Lee HT: Local anesthetics reduce mortality and protect against
renal and hepatic dysfunction in murine septic peritonitis.
Anesthesiology. 101:902–911. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang HL, Zhang WH, Lei WF, Zhou CQ and Ye
T: The inhibitory effect of lidocaine on the release of high
mobility group box 1 in lipopolysaccharide-stimulated macrophages.
Anesth Analg. 112:839–844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang HL, Xing YQ, Xu YX, Rong F, Lei WF
and Zhang WH: The protective effect of lidocaine on septic rats via
the inhibition of high mobility group box 1 expression and NF-κB
activation. Mediators Inflamm. 2013:5703702013. View Article : Google Scholar
|
|
10
|
Yardeni IZ, Beilin B, Mayburd E, Levinson
Y and Bessler H: The effect of perioperative intravenous lidocaine
on postoperative pain and immune function. Anesth Analg.
109:1464–1469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farag E, Ghobrial M, Sessler DI, Dalton
JE, Liu J, Lee JH, Zaky S, Benzel E, Bingaman W and Kurz A: Effect
of peri-operative intravenous lidocaine administration on pain,
opioid consumption and quality of life after complex spine surgery.
Anesthesiology. 119:932–940. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yon JH, Choi GJ, Kang H, Park JM and Yang
HS: Intraoperative systemic lidocaine for pre-emptive analgesics in
subtotal gastrectomy: A prospective, randomized, double-blind,
placebo-controlled study. Can J Surg. 57:175–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fitz-Henry J: The ASA classification and
peri-operative risk. Ann R Coll Surg Engl. 93:185–187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldfarb Y, Sorski L, Benish M, Levi B,
Melamed R and Ben-Eliyahu S: Improving postoperative immune status
and resistance to cancer metastasis: A combined perioperative
approach of immunostimulation and prevention of excessive surgical
stress responses. Ann Surg. 253:798–810. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Glasner A, Avraham R, Rosenne E, Benish M,
Zmora O, Shemer S, Meiboom H and Ben-Eliyahu S: Improving survival
rates in two models of spontaneous postoperative metastasis in mice
by combined administration of a beta-adrenergic antagonist and a
cyclooxygenase-2 inhibitor. J Immunol. 184:2449–2457. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neeman E and Ben-Eliyahu S: Surgery and
stress promote cancer metastasis: New outlooks on perioperative
mediating mechanisms and immune involvement. Brain Behav Immun.
30(Suppl): S32–S40. 2013. View Article : Google Scholar
|
|
17
|
Lahat A, Ben-Horin S, Lang A, Fudim E,
Picard O and Chowers Y: Lidocaine down-regulates nuclear
factor-kappaB signalling and inhibits cytokine production and T
cell proliferation. Clin Exp Immunol. 152:320–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawasaki T, Kawasaki C, Sata T and Chaudry
IH: Lidocaine suppresses mouse Peyer's Patch T cell functions and
induces bacterial translocation. Surgery. 149:106–113. 2011.
View Article : Google Scholar
|
|
19
|
Werdehausen R, Braun S, Essmann F,
Schulze-Osthoff K, Walczak H, Lipfert P and Stevens MF: Lidocaine
induces apoptosis via the mitochondrial pathway independently of
death receptor signaling. Anesthesiology. 107:136–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boselli E, Duflo F, Debon R, Allaouchiche
B, Chassard D, Thomas L and Portoukalian J: The induction of
apoptosis by local anesthetics: A comparison between lidocaine and
ropivacaine. Anesth Analg. 96:755–756. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Decker D, Schondorf M, Bidlingmaier F,
Hirner A and von Ruecker AA: Surgical stress induces a shift in the
type-1/type-2 T-helper cell balance, suggesting down-regulation of
cell-mediated and up-regulation of antibody-mediated immunity
commensurate to the trauma. Surgery. 119:316–325. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Ward MF and Sama AE: Targeting
HMGB1 in the treatment of sepsis. Expert Opin Ther Targets.
18:257–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang D, Kang R, Zeh HJ III and Lotze MT:
High-mobility group box 1 and cancer. Biochim Biophys Acta.
1799:131–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsujii S, Okabayashi T, Shiga M, Takezaki
Y, Sugimoto T, Kobayashi M and Hanazaki K: The effect of the
neutrophil elastase inhibitor sivelestat on early injury after
liver resection. World J Surg. 36:1122–1127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hasegawa A, Iwasaka H, Hagiwara S, Koga H,
Hasegawa R, Kudo K, Kusaka J and Noguchi T: Anti-inflammatory
effects of perioperative intensive insulin therapy during cardiac
surgery with cardiopulmonary bypass. Surg Today. 41:1385–1390.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Tahan MR, Warda OM, Diab DG, Ramzy EA
and Matter MK: A randomized study of the effects of perioperative
i.v. lidocaine on hemodynamic and hormonal responses for cesarean
section. J Anesth. 23:215–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palacios R and Sugawara I: Hydrocortisone
abrogates proliferation of T cells in autologous mixed lymphocyte
reaction by rendering the interleukin-2 Producer T cells
unresponsive to interleukin-1 and unable to synthesize the T-cell
growth factor. Scand J Immunol. 15:25–31. 1982. View Article : Google Scholar : PubMed/NCBI
|