Introduction
Tuberculosis (TB) is a major infectious disease
worldwide, from which millions of people are affected each year and
has the second highest mortality rates of infectious diseases
(1). The control of TB infection
requires innate and adaptive immune responses (2,3). The
cellular immunity response of host cells determines whether the
infection becomes a latent TB infection (LTBI) or progresses into
active TB (ATB). Effective cell-mediated immunity can maintain the
TB infection permanently at the LTBI stage, however, if infected
individuals cannot control the initial pulmonary infection, or the
immune system is weakened, Mycobacterium tuberculosis may
cause pulmonary or extrapulmonary tuberculosis (4). The only effective vaccine is the
Bacille Calmette-Guerin vaccine (BCG) vaccine), which has limited
protective effects and is a major contributor to the incidence of
TB increasing over past decades (5). Studies have demonstrated that BCG can
induce apoptosis in THP-1 cells (6). There are ~90% individuals infected
with Mycobacterium tuberculosis, who will stay in
asymptomatic LTBI stage, with only 10% individuals exhibiting
active tuberculosis, indicating that host genetic factors are
important in the regulation of TB infection (7).
MicroRNAs (miRNAs) are evolutionary conserved small
RNAs in eukaryotic organisms, which are involved in
post-transcriptional regulation (8). Various studies have found that the
abnormal expression of miRNAs is associated with numerous human
diseases, such as lung cancer, renal cell carcinoma and
inflammation (9–12). There is evidence to indicate that
certain miRNAs are involved in the regulation of the cellular
immune process, therefore, they can be used as potential immune
disorder biomarkers (13). For
example, miRNA (miR)-146a is expressed largely in human memory T
cells, however, it decreases in naïve T cells and results in
activation of T cell receptors in the initial T lymphocytes
(14). Studies have also
demonstrated that miR-144* is predominantly expressed in
T cells, and its overexpression in patients with ATB suggests that
specific miRNAs may be involved in the biological behavior of T
cells (15,16).
miR-155 has been identified as a multifunctional
miRNA, which is involved in several biological processes, including
tumour growth, infection, inflammation and immunity (17,18).
A previous study found that miR-155 is dysregulated in human
macrophages infected by different Mycobacterium species
(19). The expression of miR-155
can enhance the levels of tumour necrosis factor by increasing mRNA
stability and half-life (20).
Forkhead box O3 (FOXO3) is one of the forkhead
transcription factors, which is involved in regulating cell cycle
and innate immune responses, and resisting oxidation and cell
apoptosis (21–24). Evidence indicates that BCG-mediated
apoptosis of human macrophages is dependent on the activation of
FOXO3 transcription factor, and cell apoptosis induced by BCG is
associated with prosurvival-activated threoninekinase
dephosphorylation and its target FOXO3, with transfer of FOXO3 to
the nucleus of BCG-infected cells leading to increased
transcriptional activity of FOXO3 (25). Investigation involving breast
cancer cell lines demonstrated that FOXO3 is the direct target of
miR-155, and miR-155 is closely associated with the expression of
FOXO3 (26).
Another previous study has confirmed that miR-155
induces cell proliferation by inhibiting apoptosis and promoting
metastasis, as well as the invasion of glioma cells. However,
miR-155 has no effect on cell cycle, but can reduce the expression
of FOXO3a by directly targeting its 3′-untranslated regions
(3′-UTRs) (27).
Materials and methods
Human subjects
A total of 20 patients diagnosed with TB were
recruited from the Department of Tuberculosis, the First Affiliated
Hospital of Xinxiang Medical College (Xinxiang, China). The
diagnosis of tuberculosis was based on serum smear acid-fast
staining or bacterial cultures, chest X-ray, clinical symptoms and
response to anti-TB treatment. In addition, 17 healthy control
individuals were randomly selected from a routine physical
examination of healthy individuals at the First Affiliated Hospital
of Xinxiang Medical College. The inclusion criteria included the
following: A normal physical examination result, no fever, cough or
other active TB symptoms. The study protocols were approved by the
Ethics Committee of the First Affiliated Hospital of Xinxiang
Medical College, and informed written consent was obtained from all
participants prior to commencement.
Flow cytometric analysis
At 8:00 am, peripheral blood samples (5 ml) were
drawn from the antecubital vein of the patients with ATB and the
healthy control individuals, and surface antibody was stained
within 6 h with fluorescein isothiocyanate (FITC)-labeled mouse
anti-human CD14+ monoclonal antibody [HCD14 (cat. no.
301803); BioLegend, Inc., San Diego, CA, USA], incubated for 30 min
on ice, in the dark (dilution, 1:100). The appropriate homeotypic
antibody was used to determine the background level of staining. At
least 100,000 events were collected and analysed using Beckman CXP
(version 2.0) software on a FC-500 Flow Cytometer (Beckman Coulter,
Brea, CA, USA). The content of CD14+ was analysed using
flow cytometric analysis. In addition, samples from the ATB
patients and control individuals were obtained to determine the
levels of apoptosis. The experiments were performed in
triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Density gradient centrifugation was performed, at
1,500 × g at 4°C for 5 min, using Ficoll-Paque (GE Healthcare
Biosciences, Pittsburgh, PA, USA) to purify the peripheral blood
mononuclear cells (PBMCs). Subsequently, RT-qPCR was performed to
analyse the expression of miR-155 in the PBMCs of the ATB patents
and control individuals. Total RNA was extracted using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
according to the manufacturer's instruction. Complementary
deoxyribonucleic acid (cDNA) was synthesised from the total RNA
using a PrimeScript II First Strand cDNA Synthesis kit (Takara
Biotechnology, Co., Ltd., Dalian, China), according to standard
protocol. qPCR was then performed on the cDNA using SYBR Green dye
(Takara Biotechnology, Co., Ltd.) with specific primers as follows:
Forward, TGAACCTCTGCTGCCCATAC and reverse, GCCACCTTCAAGAAGTAGCCTAT
(MGH DNA Core Facility, Cambridge, MA, USA). Briefly, 25 ml SYBR
Green Mix (2X), 0.5 ml cDNA, 2 ml primer pair mix (5 pmol/ml each
primer) and 22.5 ml H2O were used. The thermocycling
conditions were as follows: 50°C for 2 min (1 cycle), 95°C for 10
min (1 cycle), followed by 40 cycles of 95°C for 15 sec, 60°C for
30 sec and 72°C for 30 sec and finally, 72°C for 10 min (1 cycle).
U6 served as an internal control. The median in each triplicate was
used to calculate the relative content using the comparative ΔCt
method (value of 2−cCt) (28). The fold changes in expression were
calculated using 2−ΔΔCt methods. The THP-1 human
monocytic cell line was obtained from Shanghai Institute of Cell
Biology (Shanghai, China). The cells were cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (Shiyi
Biotechnology, Inc., Shanghai, China). The cells were cultured in a
humidified incubator at 37°C with 5% carbon dioxide. The THP-1
cells (density, 10,000/ml) were divided into a treatment group,
which was exposed to BCG (multiplicity of infection=5) for 4 h, and
a control group. The cells were cultured for 24 h at 37°C, and the
expression of miR-155 was analysed using RT-qPCR according to the
above-mentioned method. The cells in the treatment group were
transfected with control miRNA and miR-155 mimics [either small
interfering (si)RNA oligonucleotides or scrambled siRNA controls
with Lipofectamine 2000 (Invitrogen Life technologies) according to
the standard protocol], and apoptosis was assessed using flow
cytometry (FITC Annexin V Apoptosis Detection kit; BD Biosciences,
San Jose, CA, USA). Briefly, camptothecin was added (at a final
concentration of 4–6 µM) to 1×106 cells. The
cells were then incubated for 4–6 h at 37°C, which was followed by
the FITC Annexin V and propidium iodide staining protocol (Beyotime
Institute of Biotechnology, Haimen, China) to measure apoptosis.
The experiments were performed in triplicate.
Western blot analysis and luciferase
assay
The THP-1 cells were cultured for 24 h at 37°C and
then transfected with control miRNA and miR-155 mimics using
Lipofectamine 2000, according to the manufacturer's instructions.
Following incubation of the cells for 48 h, the expression level of
FOXO3 was analysed using western blot analysis, according to the
standard protocols. Briefly, cells were lysed in precipitation
assay buffer (Sigma-Aldrich, St. Louis, MO, USA) and sonicated
(duration, 15 sec; amplitude, 30%) using a Sonics Vibra-Cell
(Sonics Materials, Inc. Newtown, CT, USA) and centrifuged at 16,100
× g for 15 min at 4°C. Protein concentrations in lysates were
measured via BCA assay (Pierce Biotechnology, Inc., Rockford, IL,
USA). Equal quantities of protein were separated in 8% SDS-PAGE
(Shanghai Bogu Biological Technology Co., Ltd., Shanghai, China)
and transferred onto polyvinylidene fluoride membranes (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Membranes were blocked
overnight at 4°C, incubated with the primary antibody (anti-FOXO3A;
cat. no. Ab23683; Abcam, Cambridge, UK; dilution 1:1,000) and the
secondary antibody (HRP-labeled goat anti-rabbit IgG; cat. no.
Ab6720; Abcam; dilution 1:2,500) in 0.1% Tween-20 (Nanjing Sen
Beijia Biological Technology Co., Ltd., Nanjing, China) with 2%
skimmed milk in phosphate-buffered saline or 5% bovine serum
albumin (Shanghai Bogu Biological Technology Co., Ltd.) in
Tris-buffered saline (Shanghai Bogu Biological Technology Co.,
Ltd.). The chemiluminescent signal was detected using a SuperSignal
West Femto Substrate (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and ImageQuant 400 imaging system (GE Healthcare Life
Sciences). Data were normalised to GAPDH. A wild-type and mutant
luciferase reporter plasmid containing FOXO3 3′ end non-coding
region were constructed and cotransfected with the miR-155 mimics
and FOXO3, and the miR-155 inhibitor and FOXO3, respectively, to
observe the expression of luciferase. Briefly, gene expression was
silenced using FOXO3 siRNA (GE Dharmacon, Chicago, IL, USA) and
transfection with siRNAs or plasmid DNAs was performed using
Lipofectamine 2000 according to the manufacturer's instructions.
The transfected cells were then treated with palmitic acid
(Sigma-Aldrich) at the designated concentrations for the indicated
durations. The experiments were performed in triplicate.
Over expression of FOXO3
An overexpression plasmid was constructed from the
3′ end non-coding region of FOXO3, The THP-1 cells were
contransfected with the control miRNA, miR-155 mimic and FOXO3
overexpression plasmid following treatment with BCG. The expression
level of FOXO3 was analysed using western blot analysis, and
changes in apoptosis in the THP-1 cells were determined using flow
cytometric analysis (according to the above-mentioned procedures).
The experiments were performed in triplicate.
Statistical analysis
All the data are presented as the mean ± standard
deviation. The differences between groups were assessed using a
two-tailed non-paired Student's t-test or using one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed on a personal computer with the SPSS statistical package
version 16.0 (SPSS, Inc., Chicago, IL, USA) for Windows.
Results
Apoptosis is reduced in the PBMCs of
patients with ATB
The data regarding the clinical and demographic
characteristics are presented in Table
I. The number of PBMCs of the ATB patients was significantly
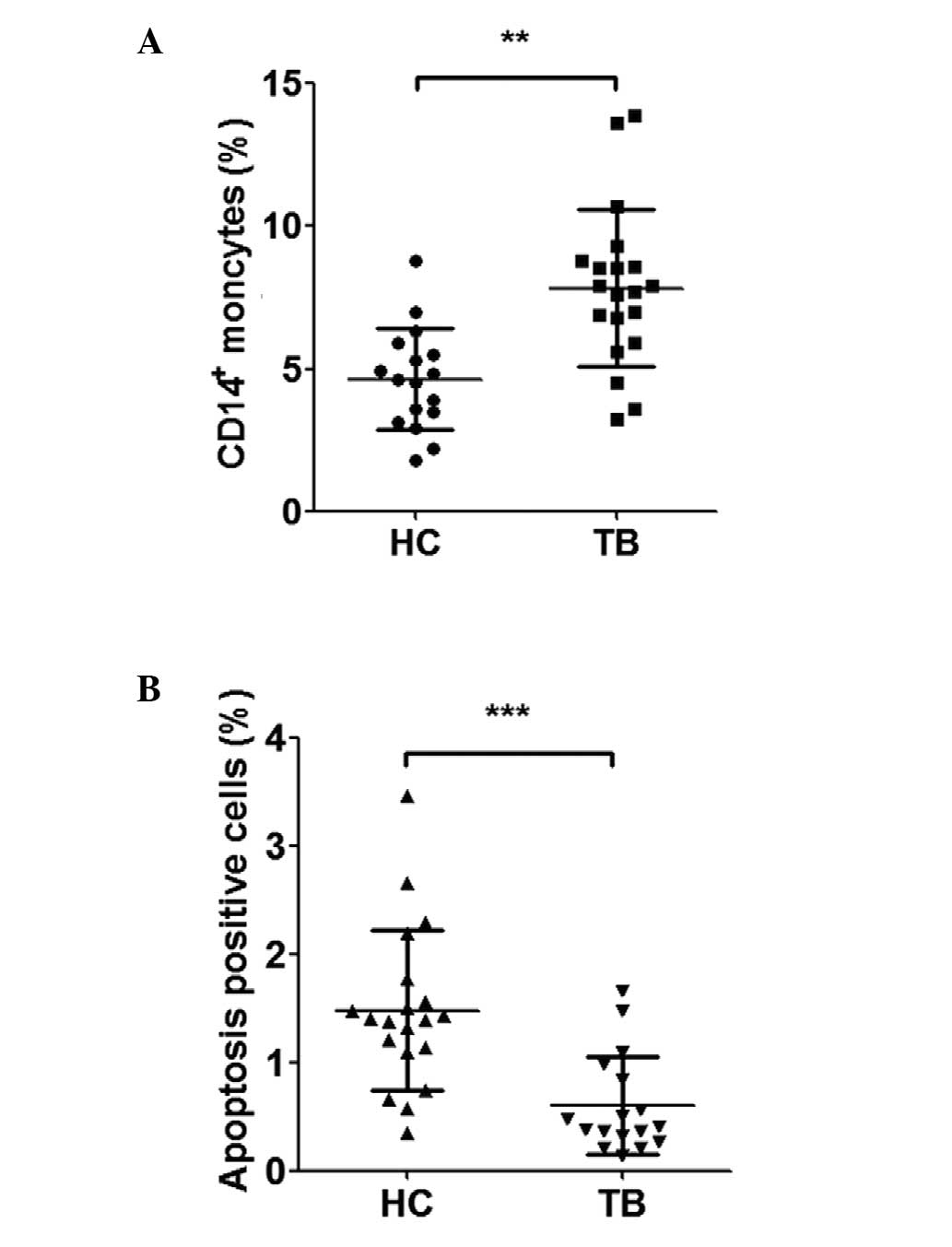
increased, compared with the healthy controls (Fig. 1A). Analysis of the apoptosis of
CD14+ monocytes was performed using a flow cytometric
assay, which demonstrated a significant reduction in the level of
apoptosis in the PBMCs of patients with ATB, compared with the
healthy controls (Fig. 1B).
 | Table IDemographic and clinical
characteristics in patients with ATB and healthy control
individuals. |
Table I
Demographic and clinical
characteristics in patients with ATB and healthy control
individuals.
| Characteristic | TB patients
(n=20) | Healthy controls
(n=17) |
|---|
| Gender
(male/female) | 12/11 | 10/7 |
| Age (years; mean ±
SD) | 54.32±18.17 | 49.17±16.36 |
| Pulmonary TB (n) | 20/20 | – |
| Culture-positive TB
(n) | 20/20 | – |
| Smear-positive TB
(n) | 9/20 | – |
| Tuberculin skin test
(n) |
| >10 cm | 5 | – |
| >5 cm | 6 | – |
| Elevated ESR (n) | 12/20 | – |
| Fever (n) | 7/20 | – |
| MDR/XDR TB (n) | 4/20 | – |
| BCG vaccinated
(n) | 20/20 | 17/17 |
Expression of miR-155
In the PBMCs of patients with ATB, the expression of
miR-155 is increased significantly, compared with that observed in
the PBMCs of the healthy control individuals (Fig. 2A). Following treatment of the THP-1
mononuclear cell line with BCG (MOI=5) for 4 h in solution and
incubation for 24 h, quantitative analysis revealed that the level
of miR-155 was elevated following being infected (Fig. 2B), compared with the control cells
without BCG. The present study also transfected the THP-1 cells
with control miRNA and miR-155 mimics, and subsequent flow
cytometry was performed to observe the levels of apoptosis. The
results of this analysis revealed that the apoptosis of the cells
reduced following transfection with the miR-155 mimics, compared
with those transfected with the control (Fig. 2C and D).
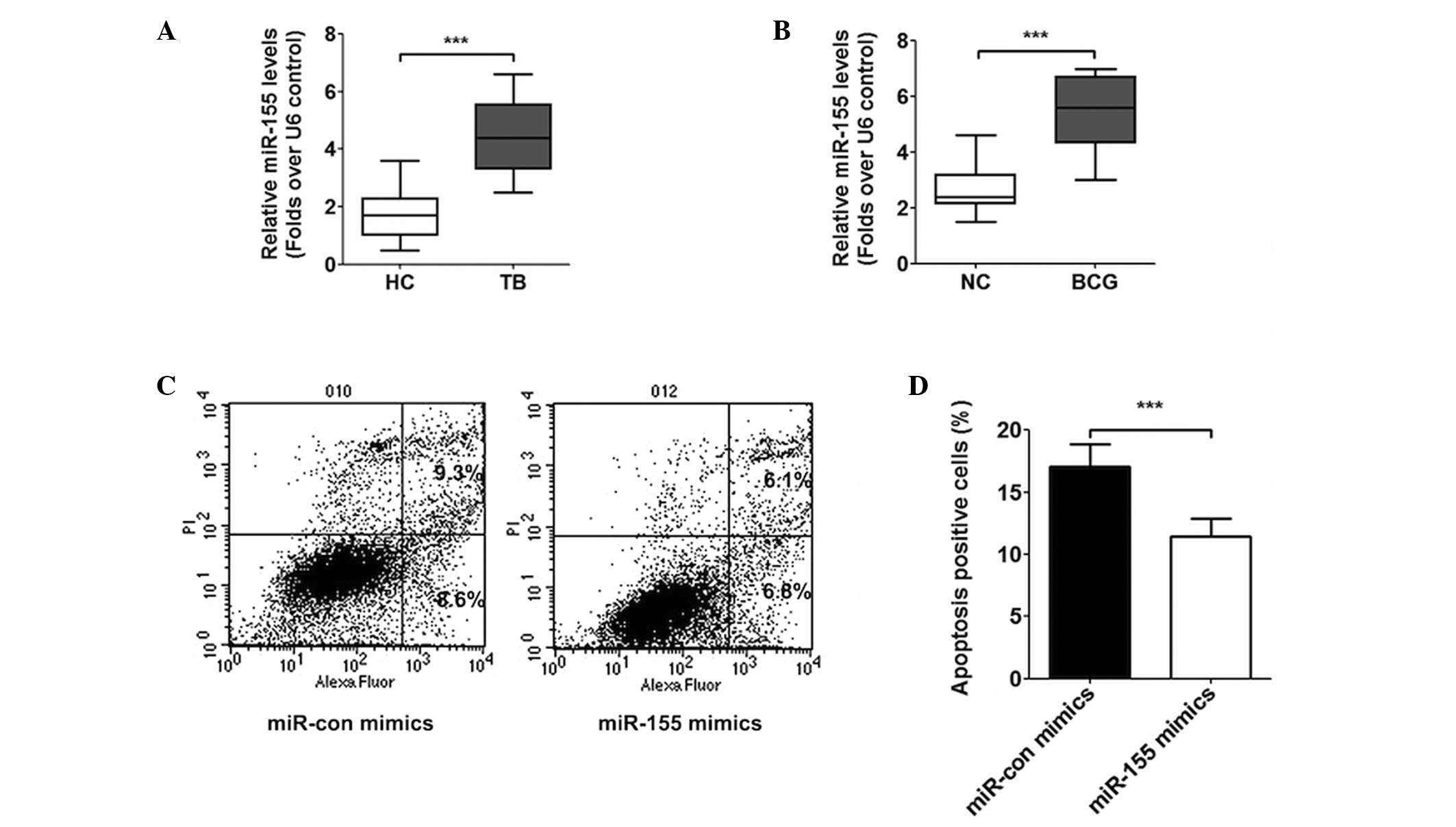 | Figure 2(A) Reverse transcription-quantitative
polymerase chain reaction analysis revealed that the relative
expression level of miR-155 in patients with ATB was significantly
higher than in healthy controls (***P<0.001). (B)
Following infection with BCG, the relative expression level of
miR-155 in THP-1 cells was elevated significantly
(***P<0.001). (C) Apoptosis assay revealed that,
following transfection with miR-155 mimics, the apoptosis of the
THP-1 cells decreased 1.8%, compared to transfection with miR-con
mimics. (D) Apoptosis of THP-1 cells transfected with miR-155
mimics reduced significantly (***P<0.001). The data
are presented as the mean ± standard deviation. HC, healthy
control; NC, normal control; TB, tuberculosis; miR, microRNA; con,
control; PI, propidium iodide. |
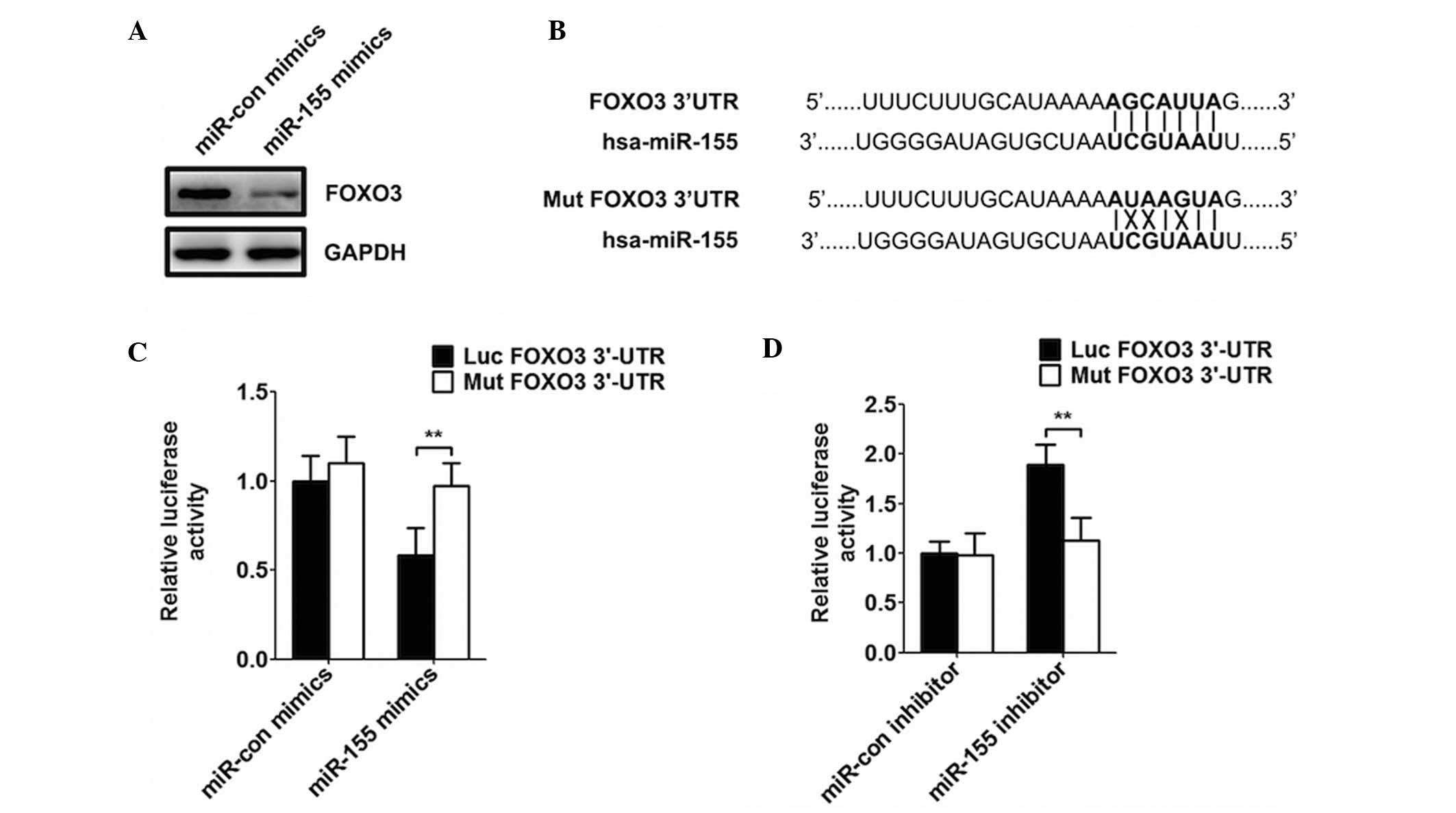
miR-155 decreases the expression of
FOXO3
Following transfection of the cells with miR-155,
the expression of FOXO3 decreased, compared with the miRNA control
cells (Fig. 3A). In addition, the
results of the luciferase assay revealed that cotransfection with
the wild-type luciferase reporter plasmid, containing the FOXO3
3′-end non-coding region, and miR-155 and FOXO3 decreased the
activity of luciferase (Fig. 3B and
C). By contrast, the activity of luciferase was markedly
increased following transfection with miR-155 inhibitor and FOXO3
(Fig. 3D).
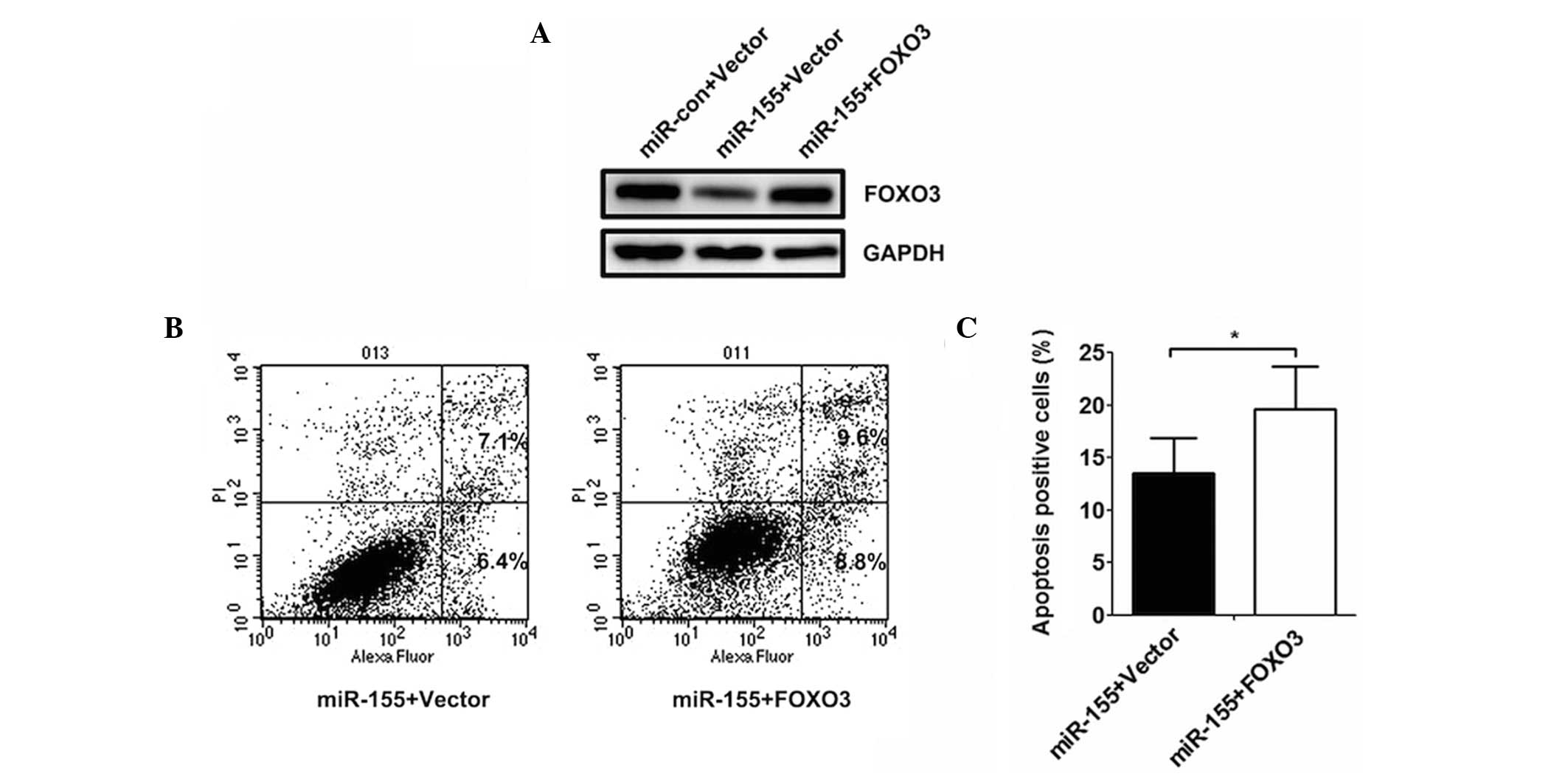
FOXO3 reverses the effect of miR-155 on
THP-1 apoptosis
The expression of FOXO3 in the THP-1 cells
cotransfected with control miRNA, miR-155 mimics and the FOXO3
over-expression plasmid following treating with BCG were also
analysed in the present study. The results revealed that the
expression of FOXO3 decreased following transfection with miR-155
mimics (Fig. 4A), however, there
was no change in expression following transfection with the miRNA
control or miR-155 in combination with FOXO3. The assay to
determine apoptosis demonstrated that the level of apoptosis
increased when the cells were transfected with miR-155 in
combination with FOXO3, which indicated that the overexpression of
FOXO3 significantly reversed the effect of miR-155 on THP-1 cell
apoptosis (Fig. 4B and C).
Discussion
Peripheral blood in the blood of patients with ATB
contains various differences to that of healthy individuals in
terms of miRNAs, and a previous study revealed that monocytes grow
in quantity in patients with ATB (29), with the expression of miR-155
demonstrating the same trend (30). In the present study, the number of
monocytes in patients with ATB were analysed and its association
with the expression level of miR-155 was investigated. First, the
present study confirmed that the number of CD14+
monocytes in the peripheral blood of patients with ATB increased
significantly, whereas the levels of apoptosis markedly decreased.
In addition, the expression of miR-155 in the PMBCs of the patients
with ATB, as well as the expression of miR-155 in THP-1 cells
infected by BCG increased significantly, which demonstrated that
mycobacterial infection may elevate the level of miR-155. A
previous study verified that BCG can induce the apoptosis of THP-1
cells (6). In the present study,
the transfection of THP-1 cells with miRNA and miR-155 mimics was
performed following treatment with BCG. Subsequent analyses led to
the conclusion that miR-155 mimics inhibited the apoptosis of THP-1
cells. Through the assessment of molecular information, the present
study established that FOXO3 is a downstream target gene of
miR-155, and a previous report demonstrated that FOXO3 is involved
in apoptosis caused by infection with mycobacterium (25). In the present study, the THP-1
cells were transfected with miRNA control and miR-155 mimics at the
same time. It was demonstrated that, following the transfection
with miR-155, the expression of FOXO3 decreased. In addition,
cotransfection of a wild-type luciferase reporter plasmid
containing the FOXO3 3′ end non-coding region with miR-155 and
FOXO3 was observed to lessen the expression of luciferase. By
contrast, the expression of luciferase markedly increased when the
cells were transfected with miR-155 inhibitor and FOXO3, which
indicated that miR-155 may inhibit the expression of FOXO3 by
combining with the FOXO3 3′-end non-coding region. Furthermore, the
present study constructed an overexpression plasmid from the 3′-end
non-coding region of FOXO3, and the THP-1 cells were contransfected
with control miRNA, miR-155 mimics and the FOXO3 overexpression
plasmid following treatment with BCG. The results revealed that
overexpression of FOXO3 significantly reversed the effect of
miR-155 on THP-1 cell apoptosis, which demonstrated that the
inhibition of miR-155 on monocyte apoptosis was associated with the
regulation of FOXO3 target genes.
In conclusion, the results of the present study
confirmed that mononuclear cells in the peripheral blood of
patients with ATB increase as a result of apoptosis being
suppressed which is induced by the expression of miR-155. The
results also demonstrated that inhibitory effect of miR-155 on
monocyte apoptosis occurs through the regulation of FOXO3 target
genes.
References
|
1
|
WHO: Global tuberculosis report. World
Health Organization; France: pp. 1–89. 2012
|
|
2
|
Cooper AM: Cell-mediated immune responses
in tuberculosis. Annu Rev Immunol. 27:393–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Natarajan K, Kundu M, Sharma P and Basu J:
Innate immune responses to M. tuberculosis infection. Tuberculosis
(Edinb). 91:427–431. 2011. View Article : Google Scholar
|
|
4
|
Smith I: Mycobacterium tuberculosis
pathogenesis and molecular determinants of virulence. Clin
Microbiol Rev. 16:463–496. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Russell DG, Barry CE III and Flynn JL:
Tuberculosis: What we don't know can and does, hurt us. Science.
328:852–856. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riendeau CJ and Kornfeld H: THP-1 cell
apoptosis in response to Mycobacterial infection. Infect Immun.
71:254–259. 2003. View Article : Google Scholar :
|
|
7
|
Khalilullah SA, Harapan H, Hasan N,
Winardi W, Ichsan I and Mulyadi M: Host genome polymorphisms and
tuberculosis infection: What we have to say? Egyptian Journal of
Chest Diseases and Tuberculosis. 63:173–85. 2014. View Article : Google Scholar
|
|
8
|
Akita T, Takuno S and Innan H: Modeling
evolutionary growth of a microRNA-mediated regulation system. J
Theor Biol. 311:54–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanwar JR, Mahidhara G and Kanwar RK:
MicroRNA in human cancer and chronic inflammatory diseases. Front
Biosci (Schol Ed). 2:1113–1126. 2010.
|
|
10
|
Chen XM: MicroRNA signatures in liver
diseases. World J Gastroenterol. 15:1665–1672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christensen M and Schratt GM: MicroRNA
involvement in developmental and functional aspects of the nervous
system and in neurological diseases. Neurosci Lett. 466:55–62.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pauley KM, Cha S and Chan EK: MicroRNA in
autoimmunity and autoimmune diseases. J Autoimmun. 32:189–194.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rodriguez A, Vigorito E, Clare S, Warren
MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska
EA, et al: Requirement of bic/microRNA-155 for normal immune
function. Science. 316:608–611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Curtale G, Citarella F, Carissimi C,
Goldoni M, Carucci N, Fulci V, Franceschini D, Meloni F, Barnaba V
and Macino G: An emerging player in the adaptive immune response:
MicroRNA-146a is a modulator of IL-2 expression and
activation-induced cell death in T lymphocytes. Blood. 115:265–273.
2010. View Article : Google Scholar
|
|
15
|
Liu Y, Wang X, Jiang J, Cao Z, Yang B and
Cheng X: Modulation of T cell cytokine production by miR-144* with
elevated expression in patients with pulmonary tuberculosis. Mol
Immunol. 48:1084–1090. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marín ND, París SC, Rojas M and García LF:
Reduced frequency of memory T cells and increased Th17 responses in
patients with active tuberculosis. Clin Vaccine Immunol.
19:1667–1676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsitsiou E and Lindsay MA: MicroRNAs and
the immune response. Curr Opin Pharmacol. 9:514–520. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu R, Kagele DA, Huffaker TB, Runtsch MC,
Alexander M, Liu J, Bake E, Su W, Williams MA, Rao DS, et al:
miR-155 promotes T follicular helper cell accumulation during
chronic, low-grade inflammation. Immunity. 41:605–619. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rajaram MV, Ni B, Morris JD, Brooks MN,
Carlson TK, Bakthavachalu B, Schoenberg DR, Torrelles JB and
Schlesinger LS: Mycobacterium tuberculosis lipomannan blocks TNF
biosynthesis by regulating macrophage MAPK-activated protein kinase
2 (MK2) and microRNA miR-125b. Proc Natl Acad Sci USA.
108:17408–17413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bala S, Marcos M, Kodys K, Csak T,
Catalano D, Mandrekar P and Szabo G: Up-regulation of microRNA-155
in macrophages contributes to increased tumor necrosis factor
{alpha} (TNF{alpha}) production via increased mRNA half-life in
alcoholic liver disease. J Biol Chem. 286:1436–1444. 2011.
View Article : Google Scholar :
|
|
21
|
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo
P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arden KC: FOXO animal models reveal a
variety of diverse roles for FOXO transcription factors. Oncogene.
27:2345–2350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peng SL: Foxo in the immune system.
Oncogene. 27:2337–2344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ticchioni M, Essafi M, Jeandel PY, Davi F,
Cassuto JP, Deckert M and Bernard A: Homeostatic chemokines
increase survival of B-chronic lymphocytic leukemia cells through
inactivation of transcription factor FOXO3a. Oncogene.
26:7081–7091. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haoues M, Refai A, Mallavialle A,
Barbouche MR, Laabidi N, Deckert M and Essafi M: Forkhead box O3
(FOXO3) transcription factor mediates apoptosis in BCG-infected
macrophages. Cell Microbiol. 16:1378–1390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harada Y, Harada Y, Elly C, Ying G, Paik
JH, DePinho RA and Liu YC: Transcription factors Foxo3a and Foxo1
couple the E3 ligase Cbl-b to the induction of Foxp3 expression in
induced regulatory T cells. J Exp Med. 207:1381–1391. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ling N, Gu J, Lei Z, Li M, Zhao J, Zhang
HT and Li X: MicroRNA-155 regulates cell proliferation and invasion
by targeting FOXO3a in glioma. Oncol Rep. 30:2111–2118.
2013.PubMed/NCBI
|
|
28
|
Zhang L, Liu S, Zhang L, You H, Huang R,
Sun L, He P, Chen S, Zhang H and Xie P: Real-time qPCR identifies
suitable reference genes for Borna disease virus-infected rat
cortical neurons. Int J Mol Sci. 15:21825–21839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Jiang J, Wang X, Zhai F and Cheng
X: MiR-582-5p is upregulated in patients with active tuberculosis
and inhibits apoptosis of monocytes by targeting FOXO1. PloS One.
8:e783812013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu J, Lu C, Diao N, et al: Analysis of
microRNA expression profiling identifies miR-155 and miR-155* as
potential diagnostic markers for active tuberculosis: A preliminary
study. Hum Immunol. 73:31–37. 2012. View Article : Google Scholar
|