Introduction
Photodynamic therapy (PDT) is a cytotoxic treatment
induced by light interactions, cell or tissue molecular oxygen and
photosensitizing molecules, termed the photosensitizer (PS)
(1). Once irradiated by visible
light, the PS can react through two photo-oxidative pathways,
classified as type I and type II (2). The type I pathway involves the
generation of free radicals through PS or a substrate. These
radicals react twith oxygen, resulting in the generation of
reactive oxygen species (ROS), including superoxide anion, hydrogen
peroxide nd hydroxyl radicals. In the type II pathway, the PS
directly transfers energy to oxygen to produce
1O2, which induces apoptosis. These two
pathways can lead to oxidative damage and ultimately to cell
damage, including apoptotic, autophagic and/or necrotic cell death
(3,4). PDT has been used for treating various
types of malignancy, including bladder cancer, Barrett's esophagus,
unresectable cholangiocarcinoma and skin cancer (4–6).
Compared with conventional cancer treatment modalities, PDT has
several advantages, including high sensitivity to tumor tissues,
minimal side effects and possible repetitive cycles of treatment,
and the potential for combination with other forms of therapy,
including chemotherapy and radiotherapy (7).
Among the factors affecting the efficacy of PDT, PS
is of importance. Hypericin (Hyp), with a typical naphthodianthrone
structure, is the predominant active component of Hypericum
species; commonly known as Hypericumperforatum or St. John's
wort (8). For decades, Hyp has
been used as a drug treatment for depression and viruses. It is
also one of the most potent PSs, which has a maximum absorption
peak of ~599 nm (9) and has
several advantages over other PSs, including substantial quantum
yield, intense absorption spectrum in the visible region, low
photobleaching and a large excitation range (10). At low concentrations, Hyp induces
apoptosis mediated by 1O2; whereas, at high
concentrations, it tends to induce necrosis via other ROS (11). Although, it has not yet been
approved for clinical application, several studies have
demonstrated that Hyp-PDT has high tumor-specific cytotoxicity and
minimal side effects (12,13).
Physiologically, programmed cell death or apoptosis
is important in eliminating aging and damaged cells. It is involved
in development and tumorigenesis; and for the latter, cells lose
their ability to undergo apoptosis due to a variety of
environmental and genetic factors (14). Therefore, in addition to
restricting cancer cell proliferation and metastasis, promoting
cancer cell apoptosis is also a valid approach in cancer therapy.
It has been demonstrated that apoptosis, secondary to the increase
in ROS, is a predominant mechanism of action in Hyp-PDT, and it has
been suggested that the mitochondrial-mediated (intrinsic) pathway
(15) and the death
receptor-mediated (extrinsic) pathway (16) are involved in Hyp-PDT-induced
apoptosis.
However, the efficacy and safety of Hyp-PDT in
leukemia treatment remains to be fully elucidated. Hyp selectively
accumulates in spheroids (17). In
our previous preliminary experiment, it was observed that Hyp-PDT
in the extracorporeal circulation of normal Sprague-Dawley rats
resulted in marked white blood cell reduction, while red blood
cells generally remained intact. Therefore, it was hypothesized
that Hyp-PDT may be developed as a novel therapy for leukemia, if
the efficacy and safety can be demonstrated in clinical relevant
models. In the present study, the K562 cell line was established
from the pleural effusion of patients with chronic myeloid leukemia
(CML) at the blast crisis phase. With a high activity of BCR-ABL
protein tyrosine kinase, the K562 cell has been reported to resist
the induction of apoptosis by several stimuli (18). The present study aimed to
demonstrate the influence that Hyp-PDT had on K562 leukemia cells
and the possible pathway involved, so as to investigate an
effective treatment or complementary therapy for leukemia,
including for patients with CML in blast crisis.
Materials and methods
Chemicals and reagents
Hyp with a purity of 95% and dimethyl sulfoxide
(DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The
cell culture medium (RPMI 1640) and penicillin-streptomycin were
purchased from Gibco Life Technologies (Carlsbad, CA, USA). Fetal
bovine serum (FBS) was purchased from GE Healthcare Life Sciences
(Logan, UT, USA). The Cell-Counting Kit-8 (CCK-8) was purchased
from Dojindo Laboratories (Kyushu, Japan). Rabbit anti-human
antibodies against C-Jun N terminal kinase (JNK) (cat. no. 9252,
1:1,000), phosphorylated (p) JNK (cat. no. 4668, 1:1,000), caspase
9 (cat. no. 9508, 1:1,000), caspase 3 (cat. no. 9665, 1:1,000), and
cleaved caspase 3 (cat. no. 9664, 1:1,000) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Mouse anti-human
anti-GAPDH monoclonal antibody (cat. no. E021010, 1:1,000) was
supplied by EarthOX Company (San Francisco, CA, USA). Goat
anti-rabbit secondary antibody (cat. no. 103349, 1:12,000) was
purchased from Jackson ImmunoResearch Laboratories, Inc (West
Grove, PA, USA). RIPA buffer was purchased from Sigma-Aldrich.
SDS-PAGE gel and PVDF membrane were from Bio-Rad Laboratories, Inc.
(Hercules, CA, USA). Glutaraldehyde, osmium tetroxide, lead citrate
and uranyl acetate were purchased from Rongbai Biological
Technology Co., Ltd. (Shanghai, China). All other reagents were of
analytical grade.
Cell culture
The K562 human CML cell line K562 was purchased from
the Cell Resource Center, Institute of Chinese Academy of Medical
Sciences (Shanghai, China). The cells were cultured in RPMI 1640
medium containing 10% FBS, penicillin (100 U/ml) and streptomycin
(100 µg/ml) at 37°C, in a 5% CO2 humidified
atmosphere. Exponentially growing cells were used for the following
experiments, with a cell density of ~4×105 cells/ml. The
stock solution of Hyp (0.8 µg/ml) was prepared by dissolving
the compound in DMSO, which was then maintained in the dark at
−4°C. The Hyp working solution for each experiment was freshly
diluted with DMSO priot to its addition to the K562 suspension; and
the final DMSO concentration in all cultures was 0.1%.
PDT procedure
A BHY-Y50 W matrix LED lamp (Baihuixiang Electric
Co., Ltd., Shenzhen, China), with a maximum power of 50 W, was used
as a light source, having a maximum peak value of 595.18 nm
(measured by the Department of Physics, Wenzhou University,
Wenzhou, Zhejiang, China). The light intensity of the led lamp was
adjustable between 0.1 and 40 mW/cm2. The K562
suspensions were incubated with Hyp or DMSO (as a control) for 5 h;
and were then exposed and irradiated under a matrix led lamp for 4
min.
Cell survival assay
Cell viability was measured using a CCK8 assay. To
examine the effectiveness of Hyp-PDT at moderate light intensity,
the cells were seeded into 96-well plates, cultured overnight and
exposed to a Hyp solution (0.4 µg/ml); using a similar in
vitro Hyp-PDT procedure as that performed previously in the
U937 human leukemic monocyte lymphoma cell line (9). Irradiation was performed for 4 min
with 0.1, 0.3 and 0.5 mW/cm2 light intensities on the
sample surface. Subsequently, 10 µl CCK8 solution was added
to each well prior to irradiation, 5 min following irradiation, and
4, 8 and 16 h following irradiation, respectively. The cells were
incubated for an additional 2 h at 37°C. The optical density (OD)
was then measured at 450 nm using a microplate reader (550; Bio-Rad
Laboratories, Inc.). Cell viability was calculated as follows: Cell
viability = (ODtest − ODblank) / ODcontrol − ODblank) ×
100%.
In order to determine the effects of the different
Hyp concentrations at various irradiation durations on the K562
cells, a CCK8 assay was performed with a series of Hyp concentrated
solutions (0, 0.2, 0.4 and 0.8 µg/ml, respectively) and at
various irradiation durations (0, 2, 4 and 8 min, respectively),
using a light intensity of 0.3 mW/cm2 at different
time-points (pre-irradiation, and 5 min, 4, 8 and 16 h
post-irradiation).
Western blot analysis
PDT with 4 min irradiation at a light intensity of
0.3 mW/cm2 was applied to the Hyp-treated K562 cells
(0.4 µg/ml) for 5 h, and the control cells were treated with
DMSO only for 5 h. Cell lysates were then prepared, and total
proteins were extracted at various time-points (prior to
irradiation, and 4, 8, and 16 h post-irradiation). Western blot
analysis was then performed. In brief, the K562 cells were gently
washed thee times with cold phosphate-buffered saline (PBS) and
then lysed with radioimmunoprecipitation assay lysate buffer
containing 1 mmol/l phenylmethylsulfonyl fluoride and 10 mmol/l of
NaF for 30 min on ice. The cell lysates were then centrifuged at
7,200 × g for 15 min at 4°C and the supernatants were collected.
Equal quantities of protein (40 µg) were separated by
SDS-PAGE (8%) and transferred onto a polyvinylidene difluoride
membrane. The membrane was blocked with 5% skim milk in
Tris-buffered saline with Tween (0.05%; TBST) for 1 h at room
temperature. After washing for 15 min with TBST 3 times, the
membrane was immunoblotted with antibodies against JNK, p-JNK,
caspase 9, caspase 3 and cleaved caspase 3 at 4°C overnight.
Subsequently, the membrane was incubated with the secondary
antibody for 2 h at room temperature after washing for 15 min with
TBST 3 times. The protein of interest was identified using an
enhanced chemiluminescence system (GE Healthcare Life Sciences).
All experiments were repeated thee times.
Observation of cell morphology using
microscopy
Cell morphology was examined under a light
microscope and a transmission electron microscope. The K562 cells
were first observed under a Leica DMR-HC (Leica Microsystems GmbH,
Wetzlar, Germany) upright research microscope, and microscopic
images were captured using Openlab imaging software, version 5.5.2
(PerkinElmer, Inc., Waltham, MA, USA). For transmission electron
microscopic observation, the cells were fixed with 2%
glutaraldehyde and 1% osmium tetroxide; and dehydrated in graded
ethanol solutions. Following embedding in TAAB 812 epoxy resin
(TAAB Laboratories Equipment, Ltd., Aldermaston, UK) ultrathin 80
nm sections were produced using a Reichert Ultracut E
Ultramicrotome (Leica, Vienna, Austria). Following staining with 1%
lead citrate and 2% alcoholic uranyl acetate, the sections were
examined under a Philips CM-12 electron microscope (Philips,
Amsterdam, Netherlands) at 80 kV.
Statistical analysis
Experimental data were analyzed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The data are expressed as
the mean ± standard deviation of three experiments. The differences
between two groups were assessed using Student's t-test;
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of Hyp-PDT on cell viability
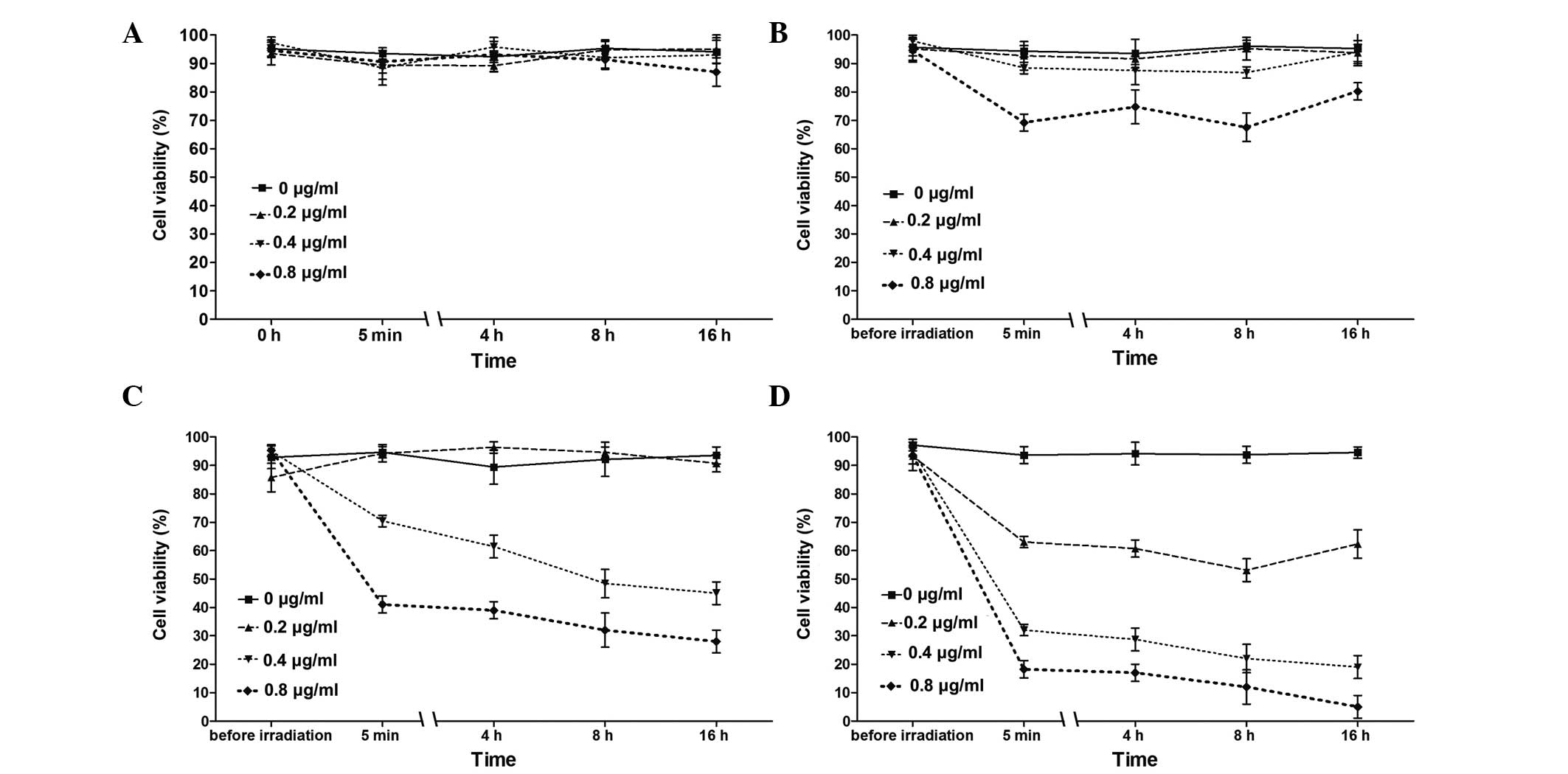
Fig. 1A shows the
results of the cell viability assessment. The cells were incubated
in 0.4 µg/ml Hyp for 5 h, followed by 4 min of irradiation
using an LED lamp (maximum peak value, 595.18 nm) at 0.1, 0.3 and
0.5 mW/cm2 adjusted light intensities at different
time-points (Pre-irradiation; 5 min, and 4, 8 and 16 h
post-irradiation). Hyp-PDT with 0.3 and 0.5 mW/cm2 light
intensities significantly decreased cell viability at different
time-points post-irradiation (all P<0.01). However, the decrease
in cell viability was only observed 4 h post-irradiation with a 0.1
mW/cm2 light intensity (P<0.05). Furthermore, cell
viability at low light intensity (0.1 mW/cm2) was
marginally increased following longer incubation periods (8 and 16
h post-irradiation); however, the differences were not
statistically significant. According to the results above, Hyp-PDT
with 0.3 mW/cm2 light intensity exhibited significant
but moderate cell damage and was selected for the subsequent
experiments. No significant changes were observed in the control
cells at different time-points (Fig.
1B).
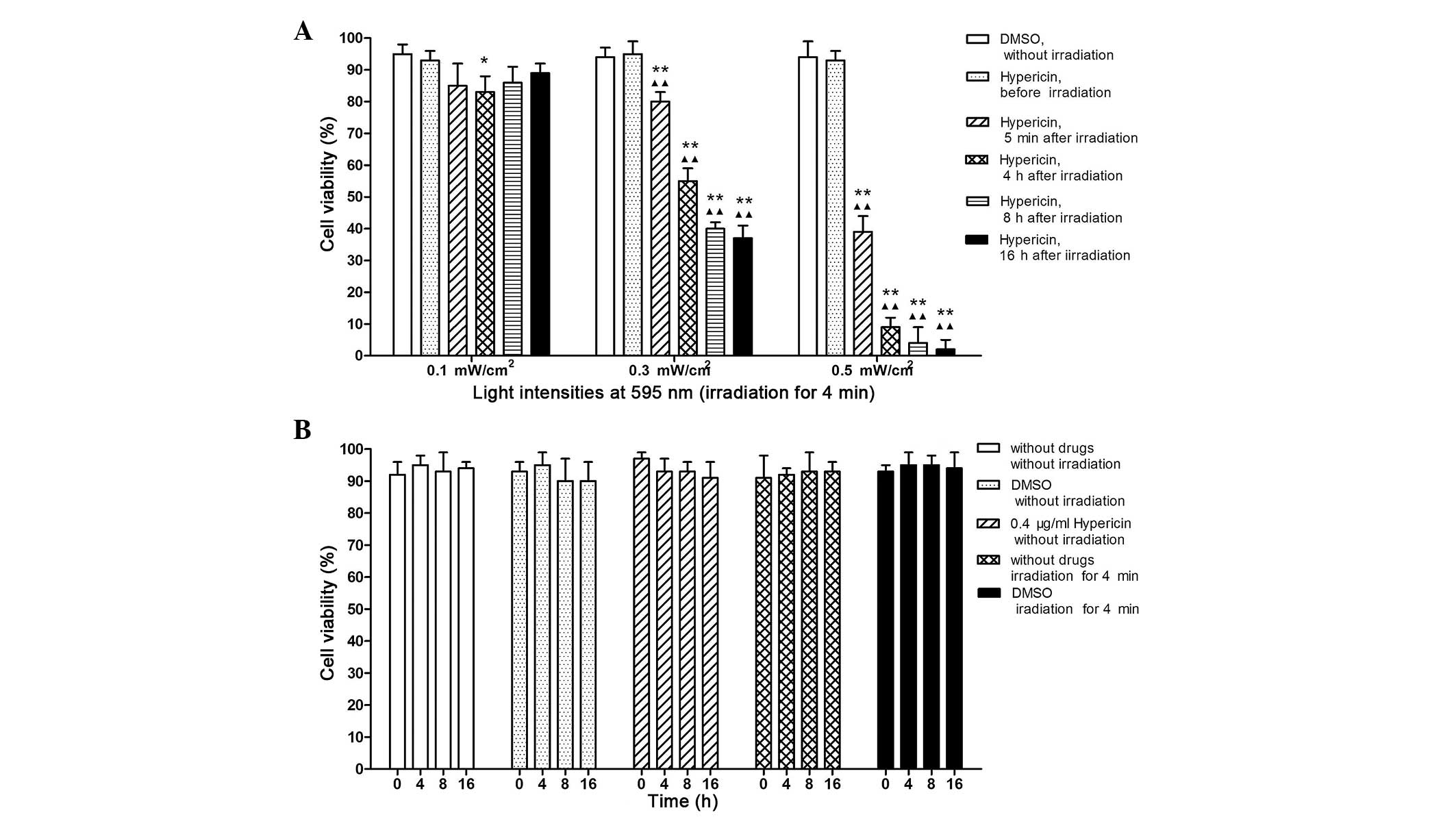 | Figure 1Viability of K562 cells treated with
Hyp-PDT under various conditions. (A) Cell viability of samples
incubated in 0.4 µg/ml Hyp for 5 h prior to irradiating for
4 min using an LED lamp (maximum peak value, 595.18 nm) with 0.1,
0.3 and 0.5 mW/cm2 adjusted light intensities, at
different time-points (5 min-16 h post-irradiation). K562 cells
treated with Hyp or DMSO alone without irradiation were used as
controls. Each experiment was repeated three times and data are
expressed as the mean ± standard deviation. (B) Cell viability of
control cells. The cells were incubated for 5 h with either DMSO
alone, 0.4 µg/ml Hyp or with no treatment. For cells treated
with PTD, the cells were exposed to irradiation for 4 min with 0.3
mW/cm2 light intensity. No significant differences were
observed between the controls at any time-point.
*P<0.05, compared with untreated control;
**P<0.01, compared with untreated control;
ΔΔP<0.01, compared with cells treated with 0.4
µg/ml Hyp, but without irradiation. PTD, photodynamic
therapy; Hyp, hypericin; DMSO, dimethyl sulfoxide. |
Under PDT conditions of 0.3 mW/cm2 light
intensity, the cell viability of the samples was analyzed at a
series of Hyp concentrations (0, 0.2, 0.4 and 0.8 µg/ml)
durations of irradiation (0, 2, 4 and 8 min) and time-points
(pre-irradiation; 5 min post-irradiation; and 4, 8 and 16 h
post-irradiation). As shown in Fig.
2, neither Hyp without irradiation (Fig. 2A) nor irradiation without Hyp, had
any effects on the K562 cells. Overall, Hyp-PDT was observed to
affect cell viability in a dose- and irradiation-time-dependent
manner; with the lowest cell viability (5%) observed in the cells
treated with 0.8 µg/ml Hyp under 8 min of irradiation.
However, the cells treated with 0.2 µg/ml Hyp resulted in
minimal effects on cell viability (Fig. 2B and C); although moderate effects
were observed after 8 min of irradiation (Fig. 2D).
Hyp-PDT-induced cellular morphological
and structural changes
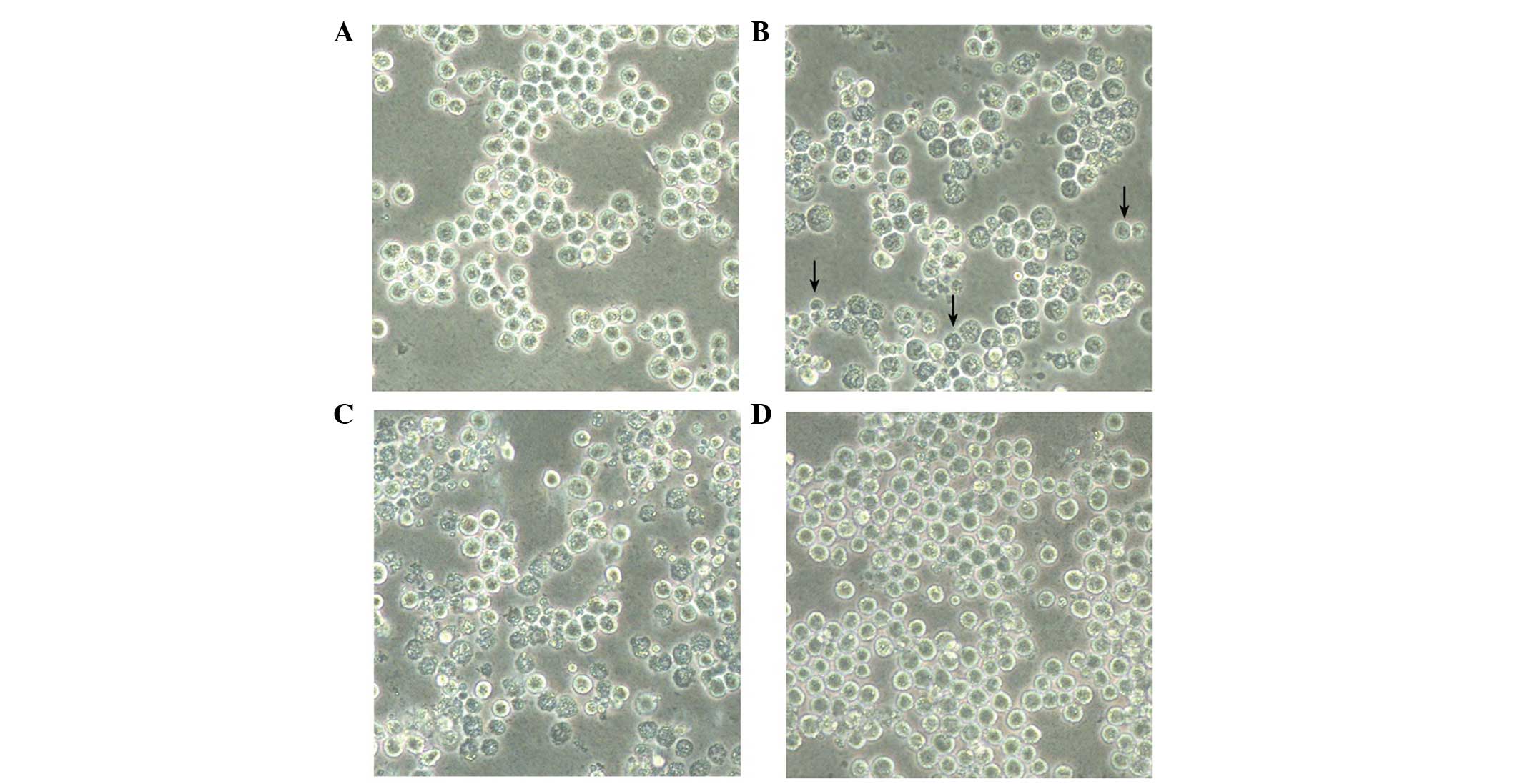
As shown in Fig. 3,
morphological observation under a phase-contrast microscope
confirmed the cytotoxic effect of Hyp-PDT on the K562 cells.
Translucent spheroids with uniform profiles, growing in isolated or
scattered colonies, were observed in the control K562 cells treated
with DMSO alone (Fig. 3A).
Following Hyp-PDT (Fig. 3B and C),
the cells began to float on the culture medium and started to lose
cell-to-cell contact. It also appeared that the cells underwent
cell death and cell intensity decreased, compared with the control
cells (Fig. 3D).
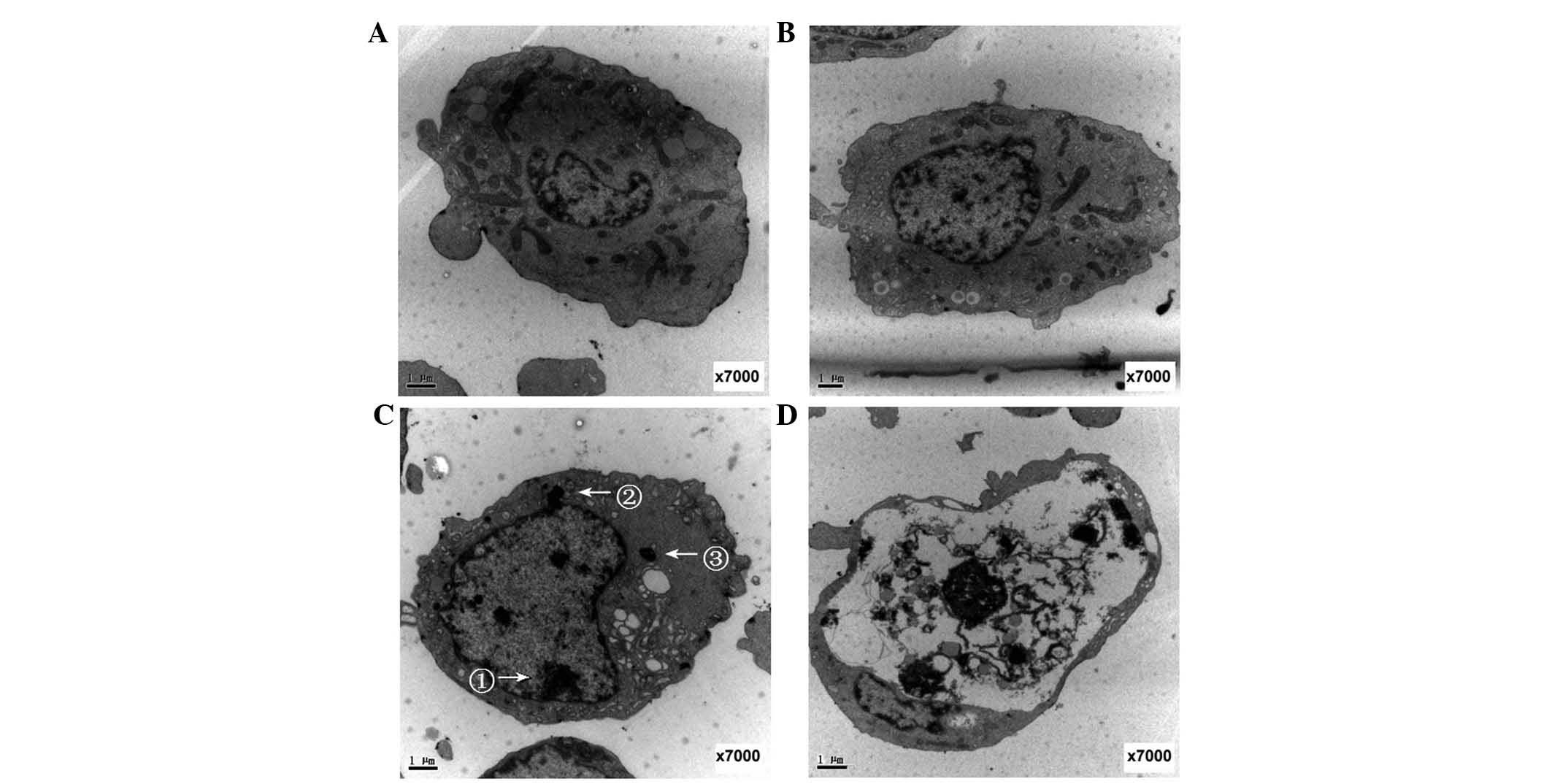
Transmission electron microscopy analysis
demonstrated normal cell morphology in the control K562 cells
treated with DMSO alone (Fig. 4A).
The absence of irradiation predominantly reflected intact cell and
nuclear membranes and an evident nucleus with nuclear material.
Normal organelles were identified in the cytoplasm, consisting of
mitochondria, endoplasmic reticulum and lysosomes (Fig. 4B). Following Hyp-PDT (0.4
µg/ml Hyp; 5 h drug light duration; 4 min irradiation with
0.3 mW/cm2 light intensity), the cells presented typical
apoptotic cell characteristics, including chromatin condensation,
agglomeration at the central nuclear area or gathering at the
periphery to form crescents, a concentrated cytoplasm, bubble-like
protrusions on the cell surface and the formation of apoptotic
bodies (Fig. 4C). Apoptotic
changes were more frequent over time, and numerous cells exhibiting
loss of intracellular detail were clearly observed 16 h after
Hyp-PDT (Fig. 4D).
Hyp-PDT-induced changes in
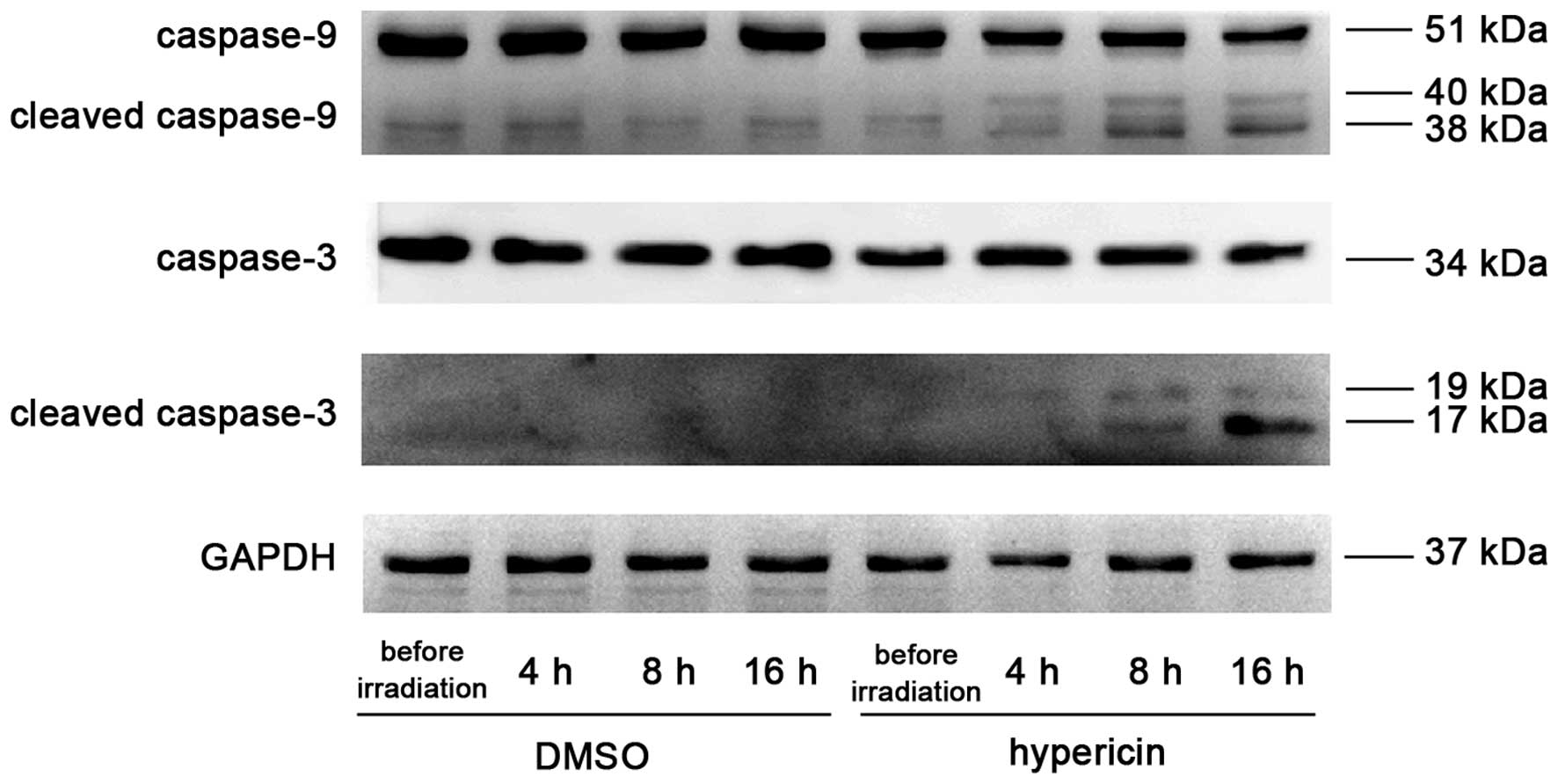
apoptosis-associated proteins
Following incubation for 5 h with 0.4 µg/ml
Hyp and irradiating for 4 min with 0.3 mW/cm2 light
intensity, the expression of cleaved caspase-9 began to appear 4 h
post-irradiation; which significantly increased 8 and 16 h
post-irradiation. The appearance of the processed fragments was
accompanied by a decrease in total capsase-9. Cleaved caspase-3 was
observed 8 h post-irradiation and was significantly upregulated at
16 h, with a corresponding decrease of total caspase-3 at 16 h
post-irradiation (Fig. 5).
Treatment with either Hyp or irradiation alone had no effect on the
expression levels of total or cleaved caspase-9 or caspase-3 in the
control K562 cells.
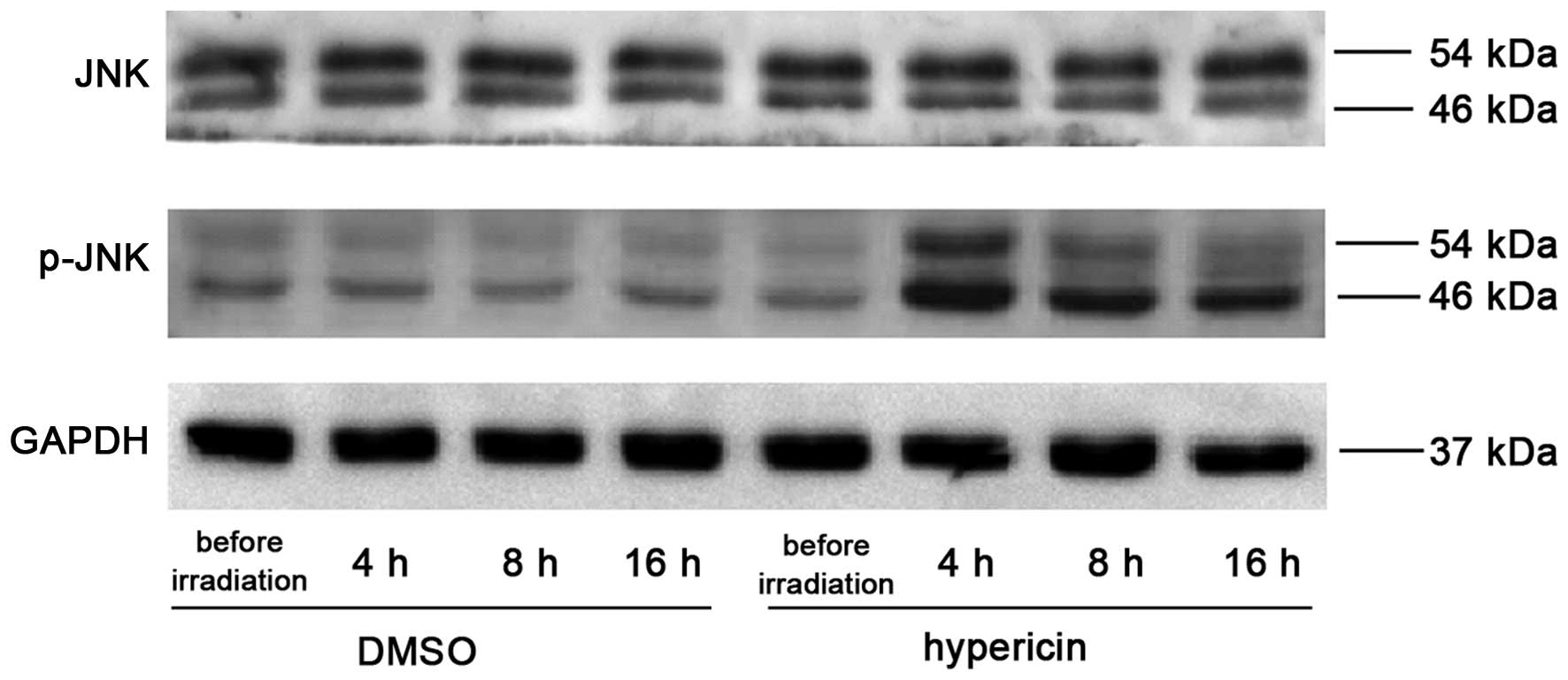
Hyp-PDT-induced upregulation of
P-JNK
In order to investigate the effect of Hyp-PDT on
JNK, the protein expression level of JNK and its phosphorylated
products were determined prior to irradiation, and 4, 8 and 16 h
post-irradiation, respectively. As shown in Fig. 6, Hyp-PDT significantly promoted JNK
phosphorylation, the expression of which peaked at 4 h
post-irradiation with a subsequent marginal decrease. No
significant changes in the protein levels of total JNK were
observed in the Hyp-alone treated and control cells.
Discussion
In previous years, investigations and clinical
trials have revealed that Hyp-PDT exerts potent phototoxicity in
cancer cells (8). Hyp-PDT induces
almost complete apoptosis (94%) in malignant cutaneous T cell
lymphoma (CTCL) cells (19). A
phase II placebo-controlled clinical study performed by Rook et
al (20) revealed that Hyp-PDT
led to the significant improvement of skin lesions, among the
majority of patients with CTCL investigated.
In order to demonstrate the effects of Hyp-PDT on
K562 cells and to develop an optimal PDT protocol, the present
study performed Hyp-PDT experiments using different light
intensities, different concentrations of Hyp and different
durations of irradiation. Briefly, Hyp-PDT with a 5 h
drug-light-interval (10) exerted
substantial phototoxicity in the K562 cells in a light-intensity-,
Hyp-dose- and irradiation-time-dependent manner. Cell viability
decreased to 5% in the presence of 0.8 µg/ml Hyp, following
8 min of irradiation with 0.3 mW/cm2 light intensity
(Figs. 1 and 2). However, the effects were minimal at a
low light intensity (0.1 mW/cm2), low Hyp concentration
(0.2 µg/ml) and short irradiation duration (2 min). A Hyp
concentration of 0.4 µg/ml with a 5 h drug-light interval, 4
min irradiation duration and 0.3 mW/cm2 light intensity
were selected for subsequent experiments; as these conditions
resulted in significant but moderate cell damage, and the side
effects were significantly reduced. The reduction in cell
population reduction and morphological changes observed under a
light microscope confirmed the cell cytotoxicity results.
Furthermore, the condensation and loss of
intracellular details observed in the cells under an electron
microscope clearly demonstrated that apoptosis occurred following
Hyp-PDT. In accordance with these cellular structural changes, the
levels of cleaved caspase-3 and cleaved caspase-9 significantly
increased following Hyp-PDT. Caspases are a family of cysteine
proteases and are key mediators of apoptosis. It has been
demonstrated that Hyp-PDT-induced apoptosis is predominantly
executed via the mitochondrial-mediated apoptotic cascade and can
be triggered directly at the mitochondria (21) or by the endoplasmic
reticulum-stress response (22).
As verified using a cDNA macroarray and reverse
transcription-quantitative polymerase chain reaction analysis,
A-431 human squamous carcinoma cells undergo apoptosis within 1.5–8
h following Hyp-PDT (23). This
was confirmed through the reduction in extracellular signal
transduction, cell detachment changes in cytoskeletal morphology,
and the formation of apoptotic bodies. Following the production of
ROS, mitochondrial-mediated apoptosis is induced by Hyp-PDT
(24). As a key step in endogenous
apoptosis, cytochrome c is released from the mitochondria
through the mitochondrial permeability transition pore, under the
regulation of molecular signals, including members of the B-cell
lymphoma-2 family (25). In the
presence of dATP, cytochrome c combines with apoptosis
protease activating factor-1 (APAF-1) to form an apoptosome with
APAF-1 and pro-caspase-9; and the latter is activated to cleaved
caspase-9 (26). This can
efficiently split the downstream apoptotic executioner caspase-3 to
its active sub-units, and eventually induce apoptosis (21). In the present study, changes in
cleaved caspase-3 and cleaved caspase-9 indicated that Hyp-PDT also
induced potent mitochondrial-mediated apoptosis in the K562 human
leukemia cells.
Apoptosis is a complex process involving various
cellular factors and pathophysiologic pathways. As an indispensable
component of the mitogen-activated protein kinase family, JNK is
important in regulating apoptosis (27). The JNK pathway is activated
predominantly by cell stressors, including ultraviolet radiation,
heat shock and oxidative stress; as well as by proinflammatory
cytokines, including tumor necrosis factor and interleukin-1
(27). Based on different cell
types and stimuli, JNK may antipodally exert a pro-apoptotic or
anti-apoptotic effect in mammals (28). For example, genipin, an aglycone
derived from geniposide, can inhibit proliferation and induce
apoptosis in K562 cells through JNK activation and induction of the
Fas ligand (29); and CMS-9, a
phospholipase A2 isolated from Naja nigricollis venom,
activates the JNK pathway and stimulates mitochondrial apoptosis
(30). The JNK inhibitor,
SP600125, has been reported to prevent the release of cytochrome
c release and attenuate caspase-9 and caspase-3 activity
(31).
Hyp has been reported to exert anti-inflammatory
activities through attenuating the phosphorylation of JNK in rat
peritoneal macrophages (32).
Hyperoside, another active extract from Hypericumperforatum,
can promote proliferation in ECV304 cells via the JNK pathway
(33). However, the association
between JNK and Hyp-PDT remains to be fully elucidated. The present
study demonstrated for the first time, to the best of our
knowledge, that the levels of p-JNK were upregulated 4 h following
Hyp-PDT, prior to any increases in the levels of cleaved caspase-9
and cleaved caspase-3 were detected. The levels of p-JNK exhibited
a marginal decrease at 8 and 16 h post-Hyp-PDT; however, the levels
remained significantly higher than that of the control cells. It
was hypothesized that the early increase in p-JNK, prior to changes
in cleaved caspase-9 and cleaved caspase-3, indicated that
activation of the JNK pathway is the upstream regulator of
Hyp-PDT-induced apoptosis. Further mechanistic investigations are
required in order to confirm this hypothesis.
In conclusion, the results from the present study
demonstrated that Hyp-PDT decreased cell viability in a
light-intensity-, Hyp dose-, and irradiation-time-dependent manner.
Mitochondria-mediated apoptosis was responsible for cell death, and
activating the JNK pathway may be an important regulator. With the
development of fiber-optic technology, laser medicine and
extracorporeal circulation techniques, Hyp-PDT may be used as a
novel therapeutic approach for leukemia.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81200513), the Qianjiang
Talents Project of Technology Office of Zhejiang Province (grant
no. 2011R10049) and Wenzhou Science & Technology Public Welfare
Project (grant no. Y20140482).
References
|
1
|
Panzarini E, Inguscio V, Fimia GM and Dini
L: Rose Bengal acetate photodynamic therapy (RBAc-PDT) induces
exposure and release of damage-associated molecular patterns
(DAMPs) in human HeLa cells. PLoS One. 9:e1057782014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martirosyan AS, Vardapetyan HR, Tiratsuyan
SG and Hovhannisyan AA: Biphasic dose-response of antioxidants in
hypericin-induced photohemolysis. Photodiagnosis Photodyn Ther.
8:282–287. 2011.PubMed/NCBI
|
|
3
|
Plaetzer K, Krammer B, Berlanda J, Berr F
and Kiesslich T: Photophysics and photochemistry of photodynamic
therapy: Fundamental aspects. Lasers Med Sci. 24:259–268. 2009.
View Article : Google Scholar
|
|
4
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JY, Diaz RR, Cho KS, Lim MS, Chung JS,
Kim WT, Ham WS and Choi YD: Efficacy and safety of photodynamic
therapy for recurrent, high grade nonmuscle invasive bladder
cancerrefractory or intolerant to bacille Calmette-Guérin
immunotherapy. J Urol. 190:1192–1199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gray J and Fullarton GM: Long term
efficacy of photodynamic therapy (PDT) as an ablative therapy of
high grade dysplasia in Barrett's oesophagus. Photodiagnosis
Photodyn Ther. 10:561–565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lo VC, Akens MK, Wise-Milestone L, Yee AJ,
Wilson BC and Whyne CM: The benefits of photodynamic therapy on
vertebral bone are maintained and enhanced by combination treatment
with bisphosphonates and radiation therapy. J Orthop Res.
31:1398–1405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krammer B and Verwanger T: Molecular
response to hypericin-induced photodamage. Curr Med Chem.
19:793–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakajima N and Kawashima N: A basic study
on hypericin-PDT in vitro. Photodiagnosis Photodyn Ther. 9:196–203.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lima AM, Pizzol CD, Monteiro FB,
Creczynski-Pasa TB, Andrade GP, Ribeiro AO and Perussi JR:
Hypericin encapsulated in solid lipid nanoparticles: Phototoxicity
and photodynamic efficiency. J Photochem Photobiol B. 125:146–154.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ehrenberg B, Anderson JL and Foote CS:
Kinetics and yield of singlet oxygen photosensitized by hypericin
in organic and biological media. Photochem Photobiol. 68:135–140.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kleemann B, Loos B, Scriba TJ, Lang D and
Davids LM: St John's Wort (Hypericum perforatum L.) photomedicine:
Hypericin-photodynamic therapy induces metastatic melanoma cell
death. PLoS One. 9:e1037622014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng Y, Le V, Cheng Z, Xie S, Li H, Tian
J and Liu J: Development of rapid and highly sensitive HSPA1A
promoter-driven luciferase reporter system for assessing oxidative
stress associated with low-dose photodynamic therapy. Cell Stress
Chaperones. 18:203–213. 2013. View Article : Google Scholar :
|
|
14
|
Tan B, Anaka M, Deb S, Freyer C, Ebert LM,
Chueh AC, Al-Obaidi S, Behren A, Jayachandran A, Cebon J, Chen W
and Mariadason JM: FOXP3 over-expression inhibits melanoma
tumorigenesis via effects on proliferation and apoptosis.
Oncotarget. 15:264–276. 2014. View Article : Google Scholar
|
|
15
|
Miccoli L, Beurdeley-Thomas A, De Pinieux
G, Sureau F, Oudard S, Dutrillaux B and Poupon MF: Light-induced
photo-activation of hypericin affects the energy metabolism of
human glioma cells by inhibiting hexokinase bound to mitochondria.
Cancer Res. 58:5777–5786. 1998.PubMed/NCBI
|
|
16
|
Chen B, Roskams T, Xu Y, Agostinis P and
de Witte PA: Photodynamic therapy with hypericin induces vascular
damage and apoptosis in the RIF-1 mouse tumor model. Int J Cancer.
98:284–290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roelants M, Van Cleynenbreugel B, Lerut E,
Van Poppel H and de Witte PA: Human serum albumin as key mediator
of the differential accumulation of hypericin in normal urothelial
cell spheroids versus urothelial cell carcinoma spheroids.
Photochem Photobiol Sci. 10:151–159. 2011. View Article : Google Scholar
|
|
18
|
Kim HB, Kim MJ, Lee SH, Lee JW, Bae JH,
Kim DW, Dao TT, Oh WK, Kang CD and Kim SH: Amurensin G, a novel
SIRT1 inhibitor, sensitizes TRAIL-resistant human leukemic K562
cells to TRAIL-induced apoptosis. Biochem Pharmacol. 84:402–410.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fox FE, Niu Z, Tobia A and Rook AH:
Photoactivated hypericin is an anti-proliferative agent that
induces a high rate of apoptotic death of normal, transformed and
malignant T lymphocytes: Implications for the treatment of
cutaneous lymphoproliferative and inflammatory disorders. J Invest
Dermatol. 111:327–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rook AH, Wood GS, Duvic M, Vonderheid EC,
Tobia A and Cabana B: A phase II placebo-controlled study of
photodynamic therapy with topical hypericin and visible light
irradiation in the treatment of cutaneous T-cell lymphoma and
psoriasis. J Am Acad Dermatol. 63:984–990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berlanda J, Kiesslich T, Oberdanner CB,
Obermair FJ, Krammer B and Plaetzer K: Characterization of
apoptosis induced by photodynamic treatment with hypericin in A431
human epidermoid carcinoma cells. J Environ Pathol Toxicol Oncol.
25:173–188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buytaert E, Callewaert G, Hendrickx N,
Scorrano L, Hartmann D, Missiaen L, Vandenheede JR, Heirman I,
Grooten J and Agostinis P: Role of endoplasmic reticulum depletion
and multidomain proapoptotic BAX and BAK proteins in shaping cell
death after hypericin-mediated photodynamic therapy. FASEB J.
20:756–758. 2006.PubMed/NCBI
|
|
23
|
Sanovic R, Krammer B, Grumboeck S and
Verwanger T: Time-resolved gene expression profiling of human
squamous cell carcinoma cells during the apoptosis process induced
by photo-dynamic treatment with hypericin. Int J Oncol. 35:921–939.
2009.PubMed/NCBI
|
|
24
|
Ali SM, Chee SK, Yuen GY and Olivo M:
Hypocrellins and Hypericin induced apoptosis in human tumor cells:
A possible role of hydrogen peroxide. Int J Mol Med. 9:461–472.
2002.PubMed/NCBI
|
|
25
|
Koval J, Mikes J, Jendzelovský R, Kello M,
Solár P and Fedorocko P: Degradation of HER2 receptor through
hypericin-mediated photodynamic therapy. Photochem Photobiol.
86:200–205. 2010. View Article : Google Scholar
|
|
26
|
Skulachev VP: The programmed death
phenomena, aging and the Samurai law of biology. Exp Gerontol.
36:995–1024. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 27:6245–6251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Varfolomeev EE and Ashkenazi A: Tumor
necrosis factor: An apoptosis JuNKie? Cell. 116:491–497. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feng Q, Cao HL, Xu W, Li XR, Ren YQ and Du
LF: Apoptosis induced by genipin in human leukemia K562 cells:
Involvement of c-Jun N-terminal kinase in G2/M arrest.
Acta Pharmacol Sin. 32:519–527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen YJ, Liu WH, Kao PH, Wang JJ and Chang
LS: Involvement of p38 MAPK- and JNK-modulated expression of Bcl-2
and Bax in Naja nigricollis CMS-9-induced apoptosis of human
leukemia K562 cells. Toxicon. 55:1306–1316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takamura M, Matsuda Y, Yamagiwa S, Tamura
Y, Honda Y, Suzuki K, Ichida T and Aoyagi Y: An inhibitor of c-Jun
NH2-terminal kinase, SP600125, protects mice from
D-galactosamine/lipopolysaccharide-induced hepatic failure by
modulating BH3-only proteins. Life Sci. 80:1335–1344. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee S, Park HS, Notsu Y, Ban HS, Kim YP,
Ishihara K, Hirasawa N, Jung SH, Lee YS, Lim SS, et al: Effects of
hyperin, isoquercitrin and quercetin on lipopolysaccharide-induced
nitrite production in rat peritoneal macrophages. Phytother Res.
22:1552–1556. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Z, Sethiel MS, Shen W, Liao S and
Zou Y: Hyperoside downregulates the receptor for advanced glycation
end products (RAGE) and promotes proliferation in ECV304 cells via
the c-Jun N-terminal kinases (JNK) pathway following stimulation by
advanced glycation end-products in vitro. Int J Mol Sci.
14:22697–22707. 2013. View Article : Google Scholar : PubMed/NCBI
|