Introduction
Nuclear technology has been widely used in a number
of fields, including industry, agriculture, military and medicine.
Since the Chernobyl nuclear accident (1) and Fukushima nuclear leakage, acute
radiation injury has gained increased attention (2). Skin as a barrier coating on the
surface of human body is easily damaged by radiation. In the
process of clinical radiotherapy, ~95% of the patients show a
degree of skin damage (3).
Radioactive skin damage usually takes a long time to heal, is
characterized by recurrent episodes of necrotic ulcers, intense
pain and potential cancer development in the long-term. It brings
great physical and mental suffering to the patient. Thus, effective
treatment methods are urgently required (4).
Bone mesenchymal stem cells (BMSCs) are a type of
somatic stem cell with self-renewal and multi-directional
differentiation potential. Owing to the progress in cell
engineering and tissue engineering, the application of BMSCs has
gained increasing attention (5).
Studies have confirmed that BMSCs are effective in radioactive
damage. François et al (6)
found that human BMSCs infused into irradiated mice migrate towards
the damaged skin. It was also verified that injection of BMSCs into
rats with radioactively damaged skin improved the repair of the
wounds (7). Lataillade et
al (8) treated several
patients with serious hip skin injuries caused by radiation with
surgery and cell therapy, which obtained positive results. The
mechanism underlying BMSCs promoting repair of
radioactivity-induced damage remains unclear. It is reported that
BMSCs differentiate into the cell type that has been damaged and
thus promote the repair of wounds (9). However, another study demonstrated
that BMSCs promote skin wound healing by paracrine mechanisms, such
as the production of cytokines (10).
Transforming growth factor (TGF)-β1, stromal
cell-derived factor (SDF)-1 and prostaglandin E2 (PGE2) are
important factors involved in inflammatory and anti-inflammatory
responses (11–13). The aim of the present study was to
observe the effects of BMSCs on the repair of radioactivity-induced
acute skin injury in rats caused by a linear accelerator, and to
investigate the underlying mechanism by detecting the expression of
TGF-β1, SDF-1 and PGE2.
Materials and methods
Preparation of rat BMSCs
Healthy male Sprague-Dawley rats (n=43; weight,
60–80 g) from Shanghai Laboratory Animal Center (Shanghai, China),
were sacrificed by neck dislocation. Under aseptic conditions, bone
marrow from the femur and tibia was collected by flushing the
marrow cavity with physiological saline and was isolated using
Ficoll separation solution (Hyclone, Logan, UT, USA). Bone marrow
mononuclear cells prepared were then grown in complete Dulbecco's
modified Eagle's medium (DMEM; HyClone Laboratories, Inc., Logan,
UT, USA) with low glucose supplemented with 10% fetal bovine serum
(FBS; Sigma-Aldrich, St. Louis, MO, USA), 100 U/ml penicillin
(Sigma-Aldrich) and 100 U/ml streptomycin (Fuzhou Maixin
Biotechnology Development Co., Ltd., Fuzhou, China) at 37°C in an
atmosphere of 5% CO2/95% air/100% humidity. The medium
was changed every three days. As the cells grew to 80–90%
confluence, they were subcultured at a ratio of 1:3. This study was
conducted in strict accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health. The animal use protocol was reviewed and
approved by the Institutional Animal Care and Use Committee (IACUC)
of Fuzhou General Hospital (Fuzhou, China).
Osteogenic and adipogenic differentiation
of rat BMSCs
Third passage BMSCs were seeded in 6-well plates at
a density of 3×105 cells/well and were grown in
osteoinduction medium (DMEM with high glucose supplemented with 10%
FBS, 10−7 M dexamethasone, 10 mM β-sodium
glycerophosphate and 50 mg/l vitamin C) or adipoinduction medium
(DMEM with high glucose supplemented with 10% FBS, 10−6
M dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 10
µg/ml insulin and 0.2 mM indomethacin) at 37°C in an
atmosphere of 5% CO2/95% air/100% humidity. The medium
was changed every three days. Cells induced to osteogenesis were
stained with Von Kossa stain three weeks later. Cells induced to
adipogenesis were stained with Oil Red O (Sigma-Aldrich) two weeks
later. The cells were observed under a Leica DVM6 optical
microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Identification of the surface antigens of
rat BMSCs
Third passage BMSCs were harvested and adjusted to a
concentration of 1×106/ml. The cell suspension (100
µl) was stained with PE-labeled CD29, CD34, CD45 and CD90
monoclonal antibodies (Biolegend, San Diego, CA, USA) at room
temperature for 40 min in the dark, respectively. Then the cells
were detected using a flow cytometer (EPICS XL, Beckman Coulter,
Miami, FL, USA).
Establishment of the rat
radioactivity-induced acute skin injury model
Sprague-Dawley rats (n=40) were anesthetized by
intraperitoneal injection of 1% sodium pentobarbital. Then their
right hip skin was irradiated using a linear accelerator
(ZL00C-SN428, Varian Medical Systems, Inc., Palo Alto, CA, USA)
with a total dose of 45 Gy (999 cGy/min). The irradiation area was
2×2 cm. After irradiation, 40 rats were randomly divided into two
groups: BMSC group and control group. Each rat in the BMSC group
received tail vein injection of 2×106 BMSCs immediately
after irradiation and local multipoint injection of
2×106 BMSCs around the damaged skin 2 weeks after
irradiation. Rats in the control group received a tail vein
injection of 1 ml physiological saline.
Gross observation and wound score of
damaged skin
After irradiation, the skin wounds of each rat were
observed every day for a total of 8 weeks. The damaged skin was
scored according to the acute radioactive skin reaction criteria of
the International Union Against Cancer (14). Degree 0, the skin shows no change;
degree I, the skin shows mild erythema, dry desquamation and
reduced sweat; degree II, the skin shows obvious erythema,
porphyritic moist dermatitis and moderate edema; degree III, the
skin shows confluent moist dermatitis and pitting edema; degree IV,
the skin shows necrosis, ulceration and bleeding.
Histopathologic examination of wounded
skin
At 2, 4, 6 and 8 weeks after irradiation, the
wounded skin was sampled from 3 rats of each group after the rats
were anesthetized with 1% pentobarbital sodium by intraperitoneal
injection. Then the skin tissues were fixed in 4% paraformaldehyde
solution and embedded into paraffin. Thereafter, the tissues were
cut into slices, mounted on slides and stained with hematoxylin and
eosin or Masson's trichrome stain.
Determination of cytokine concentrations
in the radioactively damaged skin by immunohistochemistry
At two, four, six and eight weeks after irradiation,
the wounded skin tissue samples were obtained from three rats from
each group under anesthesia by intraperitoneal injection of 0.4
ml/100 g 1% pentobarbital sodium. The tissues were sliced and
mounted on 3-aminopropyltriethoxysilane-coated slides (Fuzhou
Maixin Biotechnology Development Co., Ltd.). Following normal
deparaffinization, enzyme closure with 3% hydrogen peroxide was
performed, followed by antigen retrieval with citrate buffer
(Sigma-Aldrich). The immunohistochemistry for PGE2, TGF-β1 and
SDF-1 was performed according to the manufacturer's instructions.
The primary antibodies, including mouse anti-PGE2 monoclonal
antibody (cat. no. mx18829; dilution, 1:100), mouse anti-TGF-β1
monoclonal antibody (cat. no. mx36721; dilution, 1:200) and mouse
anti-SDF-1 monoclonal antibody (cat. no. mx22617; dilution, 1:200)
were provided by Fuzhou Maixin Biotechnology Development Co., Ltd.
The secondary antibody goat anti-mouse immunoglobulin G-horseradish
peroxidase (cat. no. sc-2031; dilution 1:500) was provided by Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA).
Statistical analysis
All statistical processes were performed using SPSS
13.0 statistical software (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. Comparisons between two
groups were conducted using a t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Morphology and differentiation
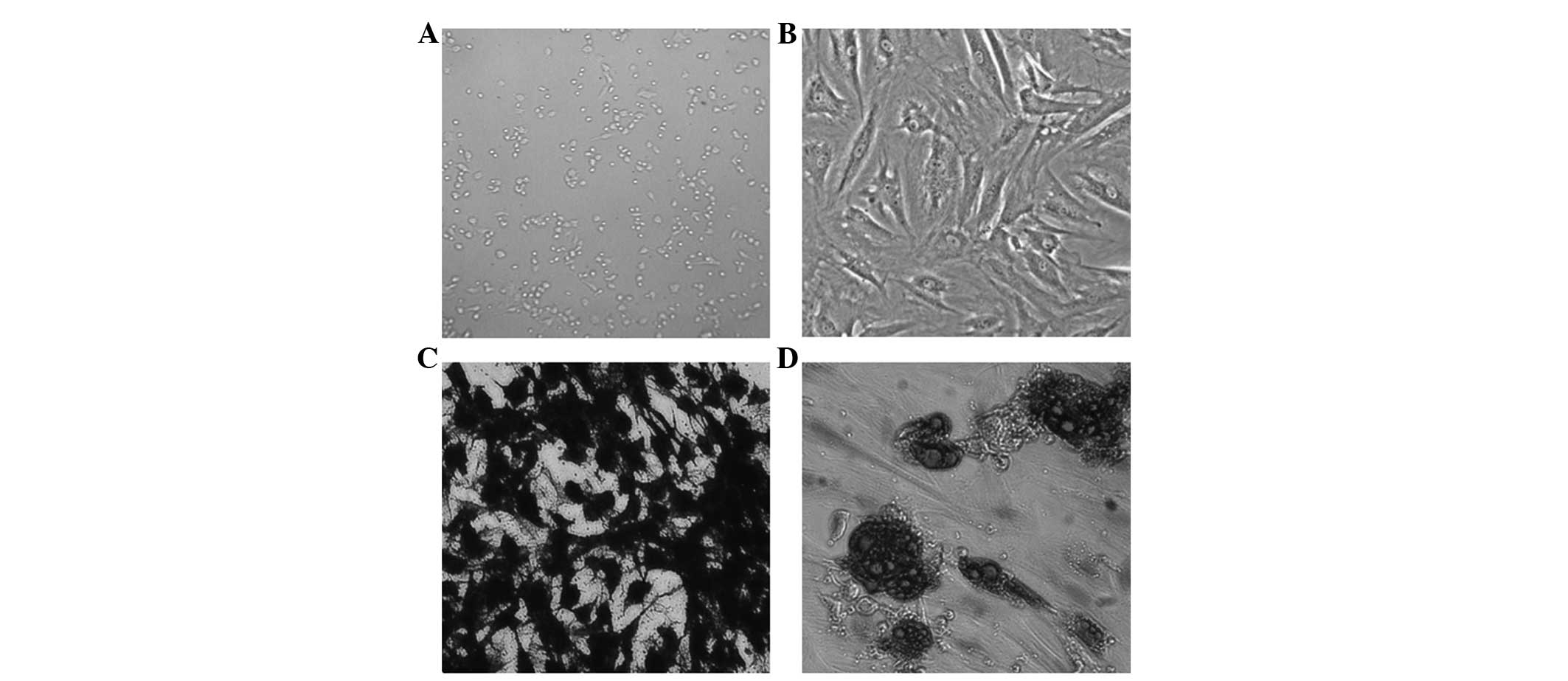
Microscopically, the primary rat BMSCs were round
and of slightly different sizes. They were suspended in the medium
with strong refractivity (Fig.
1A). After 24 h, a number of the BMSCs adhered to the flask
wall. After 2 or 3 days, the number of adherent cells increased
rapidly and extended obvious pseudopodia. In addition, the cells
became spindle-shaped. Within 3 to 4 days, cells grew in whirlpool
colonies. Upon subculture to the third passage, the cells grew in
clusters in relatively uniform, long fusiform shapes (Fig. 1B).
As the rat BMSCs grew in the osteoinduction medium,
they gradually fused. With the extension of induction time, cells
overlapped and the matrix gradually accumulated. In addition,
mineral salt was deposited to form multiple nodules, which
gradually merged. After 3 weeks of cultivation, the small-flake
calcium nodules formed, which were stained black using Von Kossa
stain (Fig. 1C). BMSCs grown in
adipoinduction medium presented with small lipid droplets in the
cytoplasm after 1 week and the cells were arranged in a disorderly
manner. After 2 weeks, highly refractive lipid droplets were
observed in the cytoplasm, which could be stained with Oil Red O
staining (Fig. 1D).
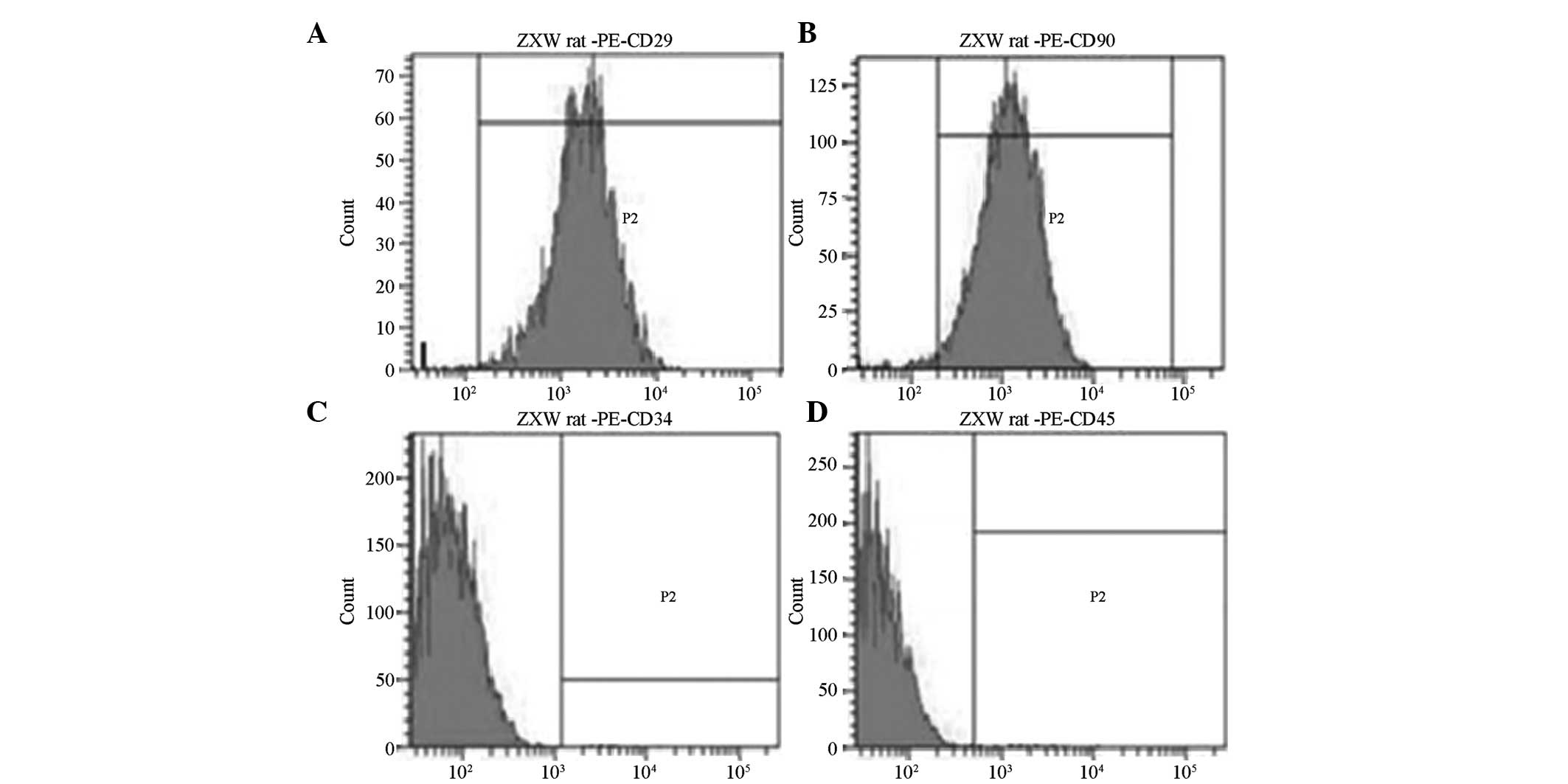
Surface markers
Third passage BMSCs showed positive expression of
CD29 and CD90, but almost no expression of CD34 and CD45, as
determined by flow cytometry. CD29-positive cells accounted for
99.25% and CD90-positive cells accounted for 98.37%, while
CD34-positive and CD45-positive cells only accounted for 1.12 and
1.03%, respectively (Fig. 2).
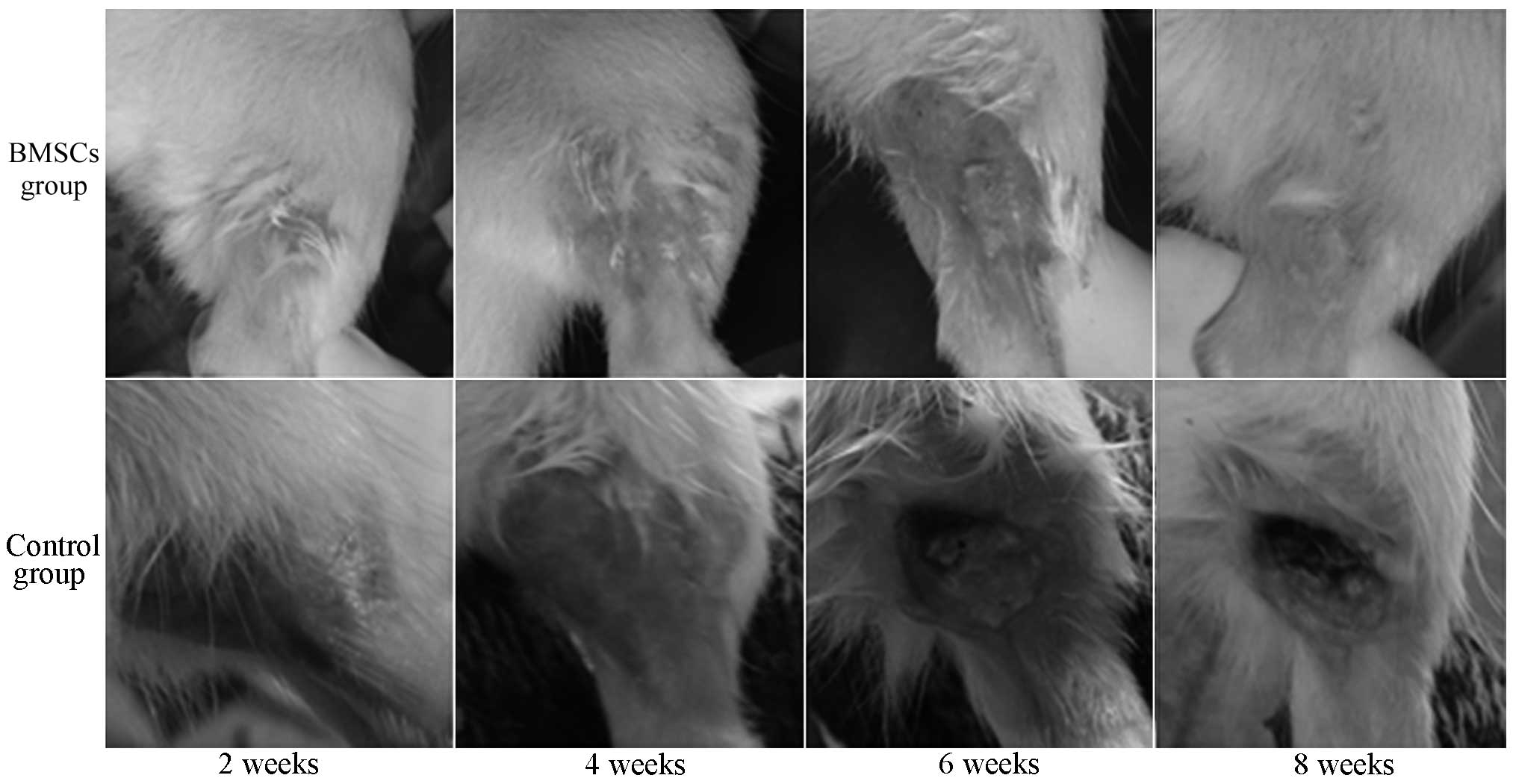
Gross observation and the ratings of
radioactively damaged skin
There were two fatalities in the control group
following irradiation. The other rats presented with depilation,
red swelling, mild erosion and slight seepage on the skin at 2
weeks after irradiation. At 4 weeks after irradiation, the wounded
skin was erosive and formed into ulcers. After 6 weeks, the wounded
skin began to dry and heal gradually, but the healing was slow. At
8 weeks after irradiation, new epithelium could be observed around
the ulcer, but the wound healing was still incomplete (Fig. 3).
No rats in the BMSC group died following
irradiation. At 4 weeks after irradiation, rats presented with
exudation and erosion of the skin, but the depth and area of ulcers
was significantly milder than that in the control group. At 6 weeks
after irradiation, new epithelial growth could be observed at the
injured site. After 8 weeks, the wound was almost healed and sparse
hairs grew on the new skin (Fig.
3). At 4, 6 and 8 weeks after irradiation, the wound scores in
the BMSC group were significantly lower than those in the control
group, respectively (P<0.05, Table
I).
 | Table IWound scores of radioactively damaged
skin. |
Table I
Wound scores of radioactively damaged
skin.
| Group | n | Time after radiation
injury (weeks)
|
|---|
| 2 | 4 | 6 | 8 |
|---|
| Control | 20 | 2.6±0.5 | 3.0±0.0 | 2.3±0.5 | 1.2±0.4 |
| MSCs | 20 | 2.1±1.0 | 2.4±0.7a | 1.5±0.5a | 0.3±0.5a |
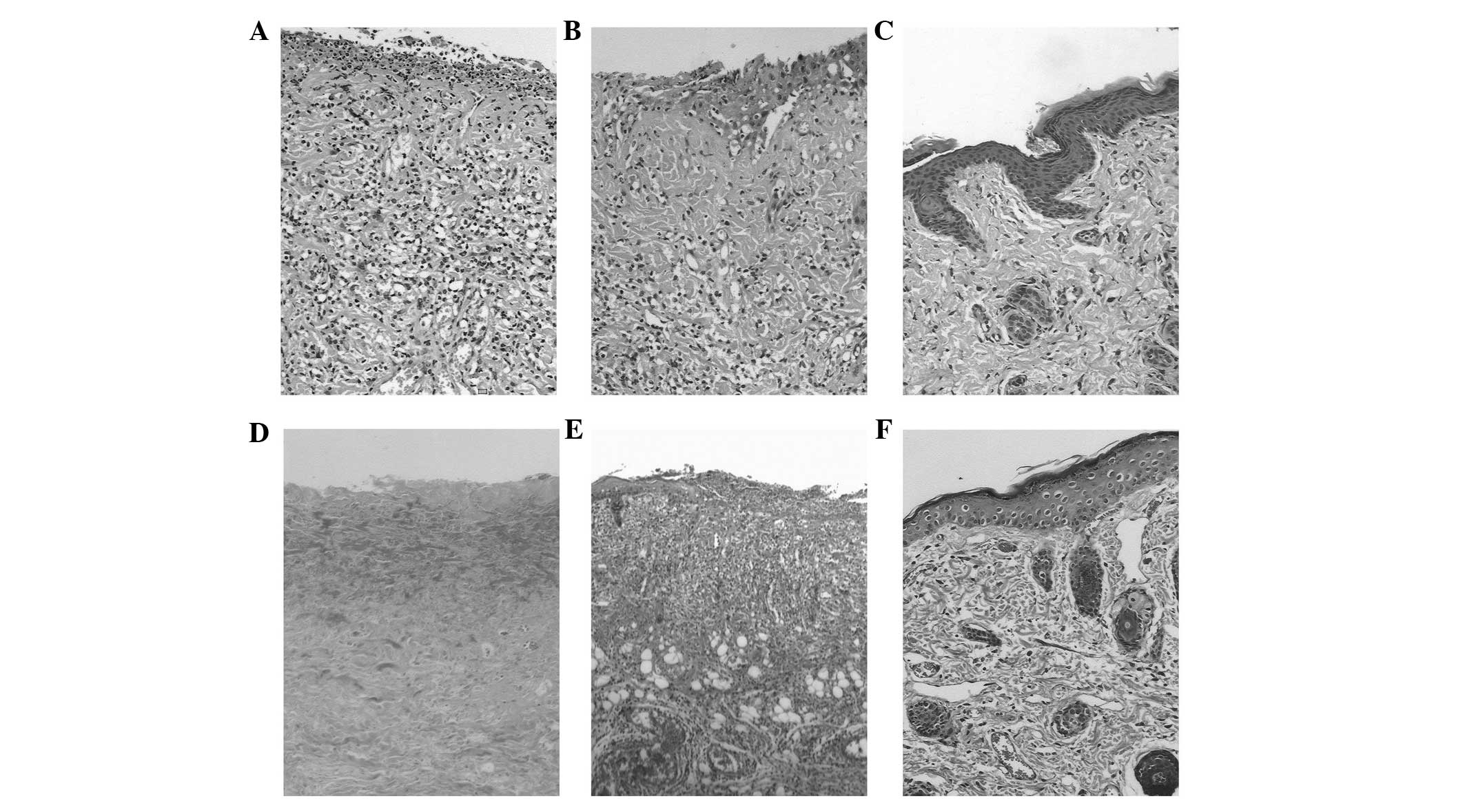
Histopathological results
The wounded skin in the control group revealed
epidermal shedding and ulcer formation and a large number of
inflammatory cells infiltrated 2 weeks after irradiation. The
surface layer was made up of necrotic tissue, in which the hair
follicle, sebaceous glands and other adnexa disappeared (Fig. 4A). Collagen fiber degeneration,
decomposition and breakage could also be seen (Fig. 4B). At 6 weeks after irradiation,
the proliferated epidermis gradually moved towards the ulcerated
area. Combined with the migration and aggregation of subcutaneous
fibroblasts, novel granulation tissue formed (Fig. 4C). Collagen fibers proliferated and
arranged in a disordered manner (Fig.
4D). The degree of necrosis and the extent of inflammatory cell
infiltration in the BMSC group was milder than that in the control
group. New granulation tissue and epidermal hyperplasia could be
observed 4 weeks after irradiation. Gradually, complete coverage of
epithelium, production of hair follicles and sebaceous glands, a
small degree of inflammatory cell infiltration and orderly
arrangement of collagen fibers was observed 8 weeks after
irradiation (Fig. 4E and F).
Expression of cytokines in the wounded
skin
Prostaglandin E2 (PGE2) is important in
inflammation. As detected by immunohistochemistry (IHC), PGE2 was
predominantly expressed in fibroblasts and inflammatory cells
(Fig. 5A and B). At 2 and 4 weeks
after irradiation, the positive expression of PGE2 was prominently
higher in the BMSC group than in the control group, respectively
(P<0.05).
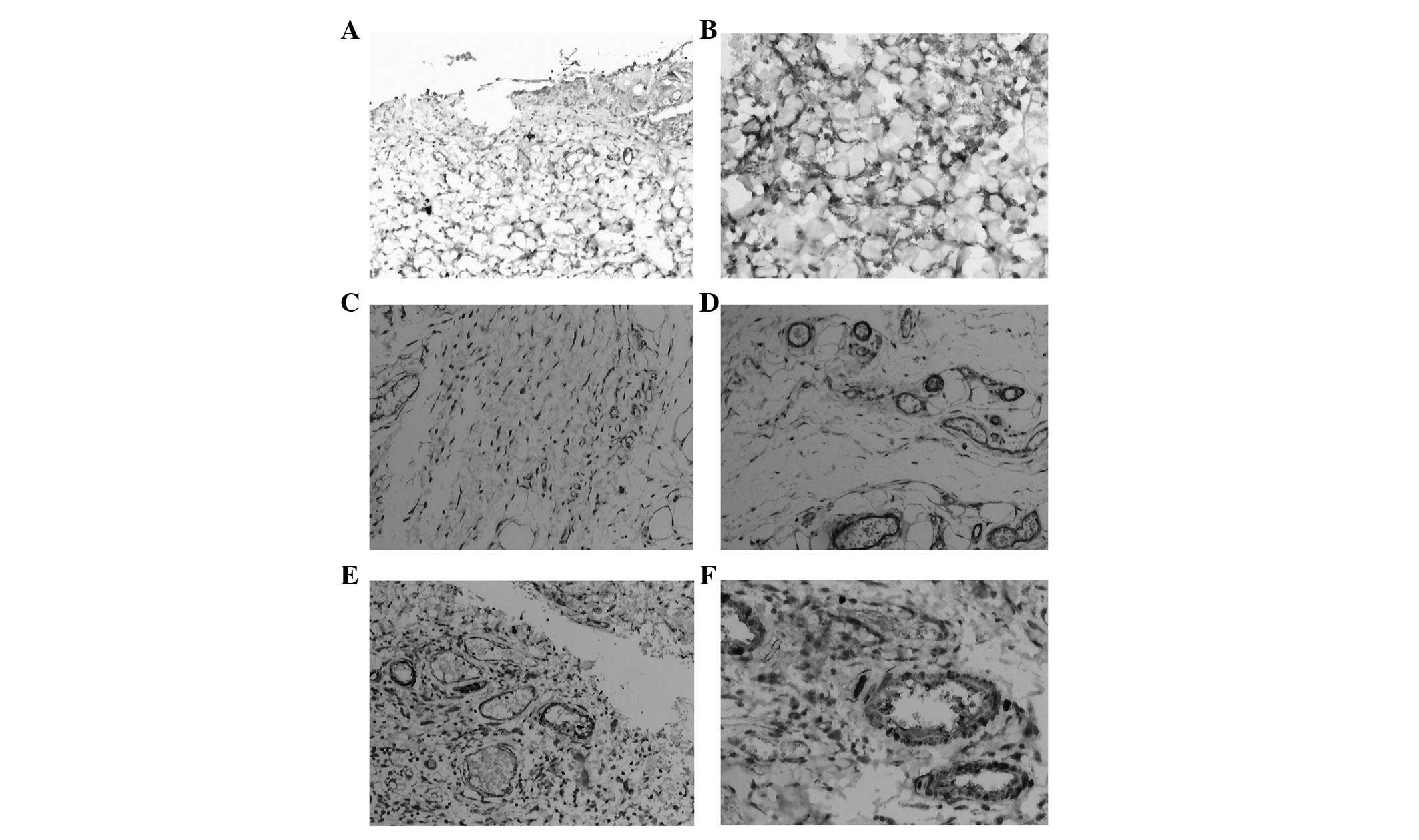 | Figure 5Expression of cytokines in wounded
skin in the BMSC group (immunohistochemical staining, A, C and E,
×100; B, D and F, ×200). (A and B) PGE2 mainly expressed in
fibroblasts and inflammatory cells; (C and D) TGF-β1 mainly
expressed in the hair follicle cells, vascular endothelial cells,
fibroblasts and inflammatory cells; (E and F), SDF-1 was mainly
distributed in new skin cells, fibroblasts and capillary
endothelial cells. PGE2, prostaglandin E2; TGF-β1, transforming
growth factor-β1; SDF-1, stromal cell-derived factor-1. |
TGF-β1 is involved in the inflammatory response at
the early stages of damage and the tissue fibrosis process. The
immunohistochemical staining of TGF-β1 revealed that TGF-β1 was
predominantly distributed in the epithelium, hair follicle cells,
basal vascular endothelial cells and fibroblasts around the ulcer
(Fig. 5C and D). TGF-β1 was
expressed at 2, 4 and 6 weeks after irradiation and its expression
in the BMSC group was significantly lower than its expression in
the control group 4 and 6 weeks after irradiation, respectively
(P<0.05).
SDF-1 is one of the predominant chemotactic factors
in vivo, which contributes to the proliferation and
migration of endothelial cells and accelerates neovascularization.
The IHC results showed that SDF-1 is mainly expressed in the hair
follicles, new skin cells, fibroblasts and capillary endothelial
cells around the edge of the wounds (Fig. 5E and F). At 2, 4 and 6 weeks, the
positive expression of SDF-1 in the MSCs group was markedly higher
than its expression in the control group (P<0.05).
Discussion
MSCs are characterized by their potential for
self-renewal and multi-directional differentiation, migrating
towards damaged tissues to exert a reparative role and secrete a
variety of growth factors. A large body of studies has shown that
MSCs are involved in tissue repair in intestinal injury caused by
radiation (15,16), lung injury (17,18),
salivary gland damage (19) and
combined radiation burn injury (20). In this study, radioactivity-induced
acute skin damage was treated with BMSCs and it was demonstrated
that BMSCs had protective effects. BMSCs not only reduced the
extent of injury but also promoted repair by secreting cytokines,
such as TGF-β1, SDF-1 and PGE2.
It is reported that the reason that radioactive skin
damage is difficult to heal is that the local vascular injury leads
to micro-circulation disturbance and tissue collagen fiber damage
(21). Hu et al (22) reported that an appropriate quantity
of MSCs promotes the repair of damage in hematopoiesis and immune
organs, exhibiting a protective role in mice with acute radiation
injury. Agay et al (23)
established a minipigs model and found that the application of MSCs
can significantly reduce the extent of radioactive injury and
result in lymphocyte infiltration and angiogenesis in the dermis
and subcutaneous tissue. In this study, the inflammatory reaction
and the depth and area of ulcers in the MSCs group was
significantly milder than that of the control group 2 weeks after
irradiation, indicating that MSCs have a protective effect on
radioactivity-induced skin damage.
Deng et al (24) treated C57BL/6 mice receiving a
lethal dose of irradiation with fluorescence-labeled BMSCs and
found that donor BMSCs migrated to the skin and converted into skin
cells, so as to regenerate skin tissue. Moroz et al
(25) also demonstrated that the
implanted MSCs migrated to the damaged skin and subcutaneous
injection of MSCs surrounding the damaged skin at 8 days after
irradiation accelerated the healing of skin ulcers. It is also
reported that treatment with MSCs can enhance the growth of hair in
mice with radioactivity-induced injuries (26). The present results showed that
BMSCs promoted the growth of granulation tissue and neovessels, as
well as collagen hyperplasia at 6 weeks after irradiation. At 8
weeks after irradiation, epithelialization and increased cutaneous
appendages were observed in the BMSC group, suggesting that BMSCs
can accelerate wound healing, consistent with the above
results.
TGF-β1 is involved in the inflammatory response at
the early stages of injury (27).
It is also involved in fibrosis by promoting the transport of
fibronectin and epithelial cells during the process of
tissue-repair (11). SDF-1 is a
chemo-kine involved in the process of stem cell homing (12). PGE is widely distributed in
vivo, among which PGE2 exhibits an important regulatory role in
physiological processes, such as inflammatory and anti-inflammatory
responses (13). The results of
the present study showed that BMSCs could prominently reduce the
expression of TGF-β1 and PGE2, and significantly increase SDF-1
expression in the irradiated rats as compared with the control
group. It was hypothesized that BMSCs promote wound healing by
inhibiting the expression of PGE2 and TGF-β1, reducing local
inflammatory reactions and inhibiting fibrosis at the inflammatory
reaction stage. During wound repair, BMSCs can promote the
production of SDF-1 by fibroblasts and thereby promote the
migration of BMSCs to the injury site. These results were
consistent with the results from Horton et al (28).
In conclusion, BMSCs effectively promote the repair
of radiation-induced acute skin injury by secreting a variety of
cytokines to communicate with the microenvironment of the wounded
skin and lead to inhibition of the inflammatory response, increased
chemotaxis, proliferation and prevent fibrosis. With the
development of tissue engineering and cell engineering, BMSCs may
become a novel method for the treatment of wounds caused by
radioactivity. However, the appropriate BMSC treatment timing,
treatment pathway and administration quantity requires further
investigation in future studies.
Acknowledgments
The present study was supported by grants from the
key project of the Army 'Twelfth Five-year' Science and Technology
Plan (grant no. BWS11J004), the key project of the Science and
Technology Plan of Nanjing Military Command (grant no. 10z031) and
the Science and Technology Innovation Platform Construction Project
of Fujian province, China.
References
|
1
|
Ogrodnik A, Hudon TW, Nadkarni PM and
Chandawarkar RY: Radiation exposure and breast cancer: Lessons from
Chernobyl. Conn Med. 77:227–234. 2013.PubMed/NCBI
|
|
2
|
Heslet L, Bay C and Nepper-Christensen S:
Acute radiation syndrome (ARS) treatment of the reduced host
defense. Int J Gen Med. 5:105–115. 2012.
|
|
3
|
Porock D, Nikoletti S and Kristjanson L:
Management of radiation skin reactions: Literature review and
clinical application. Plast Surg Nurs. 19:185–192. 1999.
|
|
4
|
Kamiya H: Radiation-induced skin injuries.
Nihon Rinsho. 70:427–430. 2012.In Japanese. PubMed/NCBI
|
|
5
|
Sharma RR, Pollock K, Hubel A and McKenna
D: Mesenchymal stem or stromal cells: A review of clinical
applications and manufacturing practices. Transfusion.
54:1418–1437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
François S, Bensidhoum M, Mouiseddine M,
et al: Local irradiation not only induces homing of human
mesenchymal stem cells at exposed sites but promote their
widespread engraftment to multiple to organs: A study of their
quantitative distribution after irradiation damage. Stem Cells.
24:1020–1029. 2006. View Article : Google Scholar
|
|
7
|
François S, Mouiseddine M, Mathieu N,
Semont A, Monti P, Dudoignon N, Saché A, Boutarfa A, Thierry D,
Gourmelon P and Chapel A: Human mesenchymal stem cells favour
healing of the cutaneous radiation syndrome in a xenogenic
transplant model. Ann Hematol. 86:1–8. 2007. View Article : Google Scholar
|
|
8
|
Lataillade JJ, Doucet C, Bey E, Carsin H,
Huet C, Clairand I, Bottollier-Depois JF, Chapel A, Ernou I,
Gourven M, et al: New approach to radiation burn treatment by
dosimetry-guided surgery combined with autologous mesenchymal stem
cell therapy. Regen Med. 2:785–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Fu X, Ouyang Y, Cai C, Wang J and
Sun T: Adult bone-marrow-derived mesenchymal stem cells contribute
to wound healing of skin appendages. Cell Tissue Res. 326:725–736.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galeano M, Altavilla D, Cucinotta D, Russo
GT, Calò M, Bitto A, Marini H, Marini R, Adamo EB, Seminara P, et
al: Recombinant human erythropoietin stimulates angiogenesis and
wound healing in the genetically diabetic mouse. Diabetes.
53:2509–2517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rolfe KJ, Irvine LM, Grobbelaar AO and
Linge C: Differential gene expressionin response to transforming
growth factor-beta 1 by fetal and posnata1 dermal fibroblasts.
Wound Repair Regen. 15:897–906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Q, Zhang A, Tao C, Li X and Jin P: The
role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose
tissue-derived mesenchymal stem cells in vitro. Biochem Biophys Res
Commun. 441:675–680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao X, Liu L, Liu D, Fan H, Wang Y, Hu Y
and Hou Y: Progesterone enhances immunoregulatory activity of human
mesenchymal stem cells via PGE2 and IL-6. Am J Reprod Immunol.
68:290–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei R and Jiang W, Su J, He L, Yang Z and
Jiang W: Intensity modulated radiation therapy for 90 untreated
nasopharyngeal carcinoma. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
37:173–178. 2012.In Chinese. PubMed/NCBI
|
|
15
|
Chang P, Qu Y, Liu Y, Cui S, Zhu D, Wang H
and Jin X: Multi-therapeutic effects of human adipose-derived
mesenchymal stem cells on radiation-induced intestinal injury. Cell
Death Dis. 4:e6852013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Linard C, Busson E, Holler V, Strup-Perrot
C, Lacave-Lapalun JV, Lhomme B, Prat M, Devauchelle P, Sabourin JC,
Simon JM, et al: Repeated autologous bone marrow-derived
mesenchymal stem cell injections improve radiation-induced
proctitis in pigs. Stem Cells Transl Med. 2:916–927. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuova LV, Konoplyannikov AG, Pasov VV,
Ivanova IN, Poluektova MV and Konoplyannikova OA: Possibilities for
the use of autologous mesenchymal stem cells in the therapy of
radiation-induced lung injuries. Bull Exp Biol Med. 147:542–546.
2009. View Article : Google Scholar
|
|
18
|
Wang H, Yang YF, Zhao L, Xiao FJ, Zhang
QW, Wen ML, Wu CT, Peng RY and Wang LS: Hepatocyte growth factor
gene-modified mesenchymal stem cells reduce radiation-induced lung
injury. Hum Gene Ther. 24:343–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim JY, Ra JC, Shin IS, Jang YH, An HY,
Choi JS, Kim WC and Kim YM: Systemic transplantation of human
adipose tissue-derived mesenchymal stem cells for the regeneration
of irradiation-induced salivary gland damage. PLoS One.
8:e711672013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hao L, Wang J, Zou Z, Yan G, Dong S, Deng
J, Ran X, Feng Y, Luo C, Wang Y and Cheng T: Transplantation of
BMSCs expressing hPDGF-A/hBD2 promotes wound healing in rats with
combined radiation-wound injury. Gene Ther. 16:34–42. 2009.
View Article : Google Scholar
|
|
21
|
Millar WT, Van Den Aardweg GJ, Hopewell JW
and Canney PA: Repair kinetics in pig epidermis: An analysis based
on two separate rates of repair. Int J Radiat Biol. 69:123–140.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu KX, Sun QY, Guo M and Ai HS: The
radiation protection and therapy effects of mesenchymal stem cells
in mice with acute radiation injury. British J Radiol. 83:52–58.
2010. View Article : Google Scholar
|
|
23
|
Agay D, Scherthan H, Forcheron F, Grenier
N, Hérodin F, Meineke V and Drouet M: Multipotent mesenchymal stem
cell grafting to treat cutaneous radiation syndrome: Development of
a new minipig model. Exp Hematol. 38:945–956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z,
You S, Deng H, Murad F and Zhao RC: Engrafted bone marrow-derived
flk- (1+) mesenchymal stem cells regenerate skin tissue. Tissue
Eng. 11:110–119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moroz BB, Onizhshenko NA, Lebedev VG,
Deshevoĭ IuB, Sidorovich GI, Lyrshchikova AV, Rasulov MF,
Krasheninnikov ME and Sevast'ianov VI: The influence of
multi-potent mesenchymal stromal cells of bone marrow on process of
local radiation injury in rats after local beta-irradiation.
Radiats Biol Radioecol. 49:688–693. 2009.In Russian.
|
|
26
|
Xie MW, Gorodetsky R, Micewicz ED,
Mackenzie NC, Gaberman E, Levdansky L and McBride WH:
Marrow-derived stromal cell delivery on fibrin microbeads can
correct radiation-induced wound-healing deficits. J Invest
Dermatol. 133:553–561. 2013. View Article : Google Scholar
|
|
27
|
Ueno T, Nakashima A, Doi S, Kawamoto T,
Honda K, Yokoyama Y, Doi T, Higashi Y, Yorioka N, Kato Y, et al:
Mesenchymal stem cells ameliorate experimental peritoneal fibrosis
by suppressing inflammation and inhibiting TGF-β1 signaling. Kidney
Int. 84:297–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Horton JA, Hudak KE, Chung EJ, White AO,
Scroggins BT, Burkeen JF and Citrin DE: Mesenchymal stem cells
inhibit cutaneous radiation-induced fibrosis by suppressing chronic
inflammation. Stem Cells. 31:2231–2241. 2013. View Article : Google Scholar : PubMed/NCBI
|