Introduction
Spinal cord injury (SCI) not only causes damage to
local nerve tissue degeneration and necrosis, cavity formation and
glial scar formation, but also can involve tracts, causing atrophy
of the brain and cardiovascular activities of central nuclei of
neurons, degeneration and necrosis, resulting in secondary damage
and cardiovascular dysfunction (1,2).
Oxidative stress is a series of adaptive reactions caused by the
dysequilibrium between reactive oxygen in the body and the
antioxidant system and, due to its importance in secondary injury
in SCI, it has received increasing attention (3). Lam et al reported that the
potential confounding effects of oxidative stress improved maximize
functional recovery following SCI (4), and Ordonez et al found that
arm-cranking exercises improved chronic spinal cord injury through
the downregulation of oxidative damage (5).
Barriers to the local microcirculation leads to
edema following SCI, and the release of arachidonic acid and its
products, including prostaglandins, leukotrienes and thromboxane
cause secondary damage to local tissue, resulting in severe
inflammation, thereby causing irreversible damage to the spinal
cord (6). Studies have
demonstrated that, following SCI, several factors are involved in
the process of apoptosis, in which inflammatory cytokine are
important role. Zhang et al suggested that plumbagin
protects against SCI-induced oxidative stress and inflammation
through the upregulation of Nrf-2 in rats (7).
The Bcl-2 gene family is an important regulator of
apoptosis in SCI, and Bax and Bcl-2 are the most representative
genes in the Bcl-2 family, which are apoptotic and anti-apoptotic
genes respectively (8,9). Chen et al reported that the
administration of Ad-HIF-1α ameliorates neuronal apoptosis and
promotes angiogenesis through the expression of Bax/Bcl-2 in SCI
rats (10). Ray et al
indicated that E-64-d prevented calpain upregulation and apoptosis
in SCI rats through the Bax/bcl-2 pathway (11).
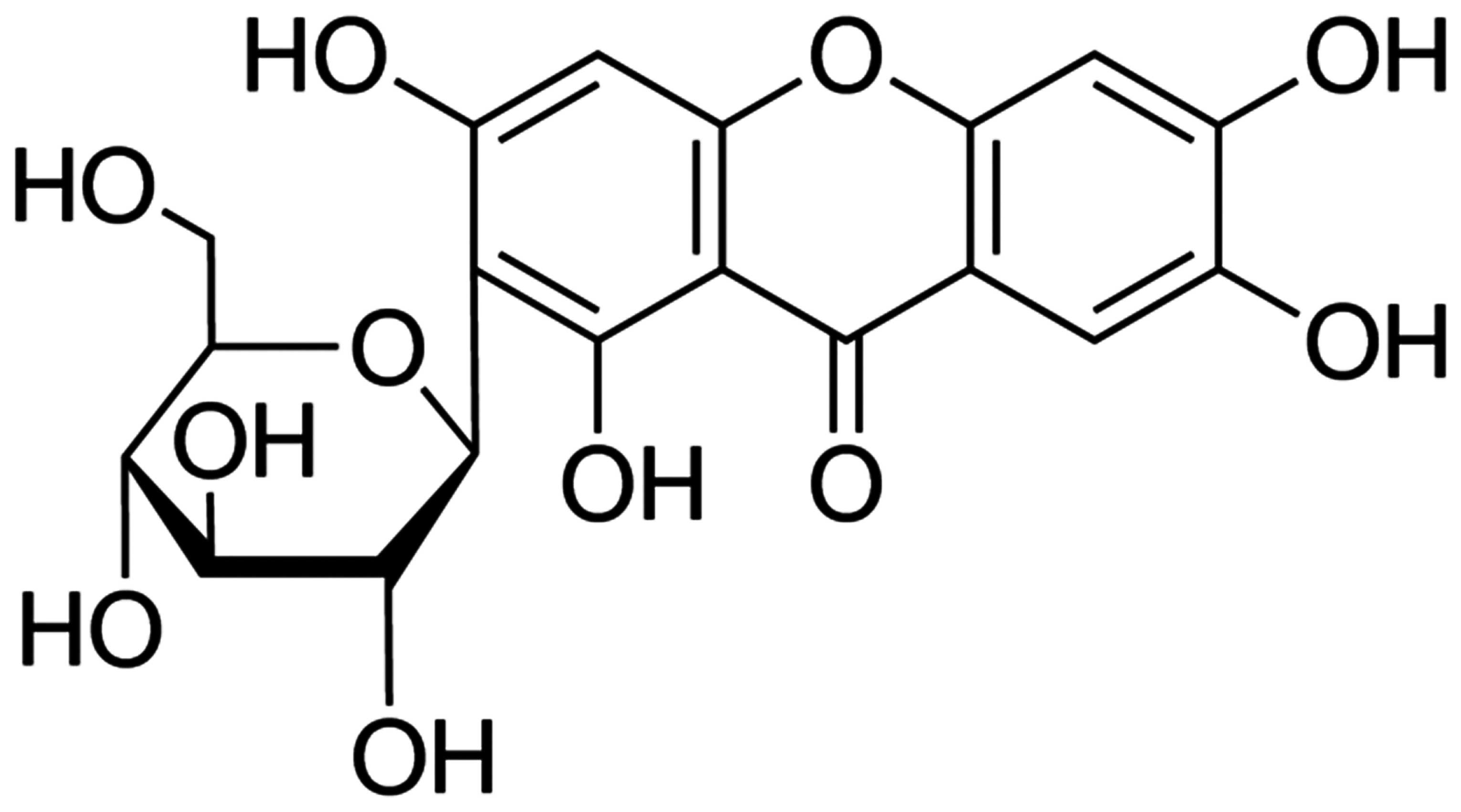
Mangiferin is a four-hydroxypyridine carbon
glycoside, which belongs to double benzene pyridine ketones. Modern
pharmacological and clinical studies have revealed that mangiferin
has several physiological and pharmacological effects, including
anti-oxidation, anti-virus, apoptosis regulating,
anti-inflammatory, anticancer, antidiabetic, osteoclast formation
inhibiting and bone resorption (12–17).
However, to the best of our knowledge, detailed mechanisms
regarding the effect of mangiferin on SCI have not been described.
Therefore, the present study designed experiments to investigate
the mechanisms underlying the protective action of mangiferin in
oxidative stress, inflammation, and induction of the Bcl-2 and Bax
signaling pathway induced by SCI, using rats as the working
model.
Materials and methods
Drugs and chemicals
Mangiferin (purity >98%) was purchased from
Nanjing traditional Chinese medicine Institute of Chinese Material
Medica (Nanjing, China). In accordance with a previous report, the
dosage and dosing frequency of mangiferin were selected. The
chemical structure is indicated in Fig. 1. Methylprednisolone (MPSS) was
supplied by the First Hospital of Jilin University (Jilin, China).
Malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT)
and glutathione peroxidase (GSH-PX) commercial kits were acquired
form Beyotime Institute of Biotechnology, (Nanjing, China). Nuclear
factor (NF)-κB p65 unit, tumor necrosis factor-α (TNF-α),
interleukin (IL)-1β, IL-6, caspase-3 and caspase-9 commercial kits
were acquired from Jiancheng Bioengineering Institute (Nanjing,
China).
Animals and the induction of the SCI rat
model
A total of 48 adult male Sprague-Dawley (SD) rats
(250–270 g) were obtained from the Animal Resource Center of the
First Hospital of Jilin University. The study was approved by the
Medical Ethics Committee of the First Hospital of Jilin University.
The present study was performed in strict accordance with the
institutional guidelines provided by the Committee on Animal
Research at First Hospital of Jilin University. All rats were
housed in individual cages and had free access to food and water
(temperature, 22±1°C; 12-h light-dark cycle). The rat model of SCI
was performed, as described previously (18). In addition, the rats were
anesthetized via intraperitoneal (i.p.) injection of sodium
pentobarbital (50 mg/kg; Sigma-Aldrich, St. Louis, MO, USA),
containing ketamine (45 mg/kg; Sangon Biotech Co., Ltd., Shanghai,
China) and xylazine (5 mg/kg; Sangon Biotech Co., Ltd.) and
atropine (0.02633 mg/kg, Sangon Biotech Co., Ltd.). Subsequently,
the rat model of SCI was generated by performing a laminectomy,
during which the T8 and T9 vertebral peduncles were removed. The
control model rats were subjected to the same laminectomy, but
without compression.
Experimental groups and procedures
All rats were randomly divided into five groups: i)
control group (Con; n=8), in which normal rats that received
physiological saline (0.1 ml/100 g, i.p.) once a day for 30 days;
ii) SCI group (SCI; n=10), in which the SCI rats received
physiological saline (0.1 ml/100 g, i.p.) once a day for 30 days;
iii) MPSS group (n=10), in which SCI rats were treated with 100
mg/kg MPSS (i.p.) once a day for 30 days; iv) mangiferin group (MAN
20; n=10), in which SCI rats were treated with mangiferin at a dose
of 20 mg/kg once a day for 30 days; v) mangiferin group (MAN 40;
n=10), in which SCI rats were treated with mangiferin at a dose of
40 mg/kg once a day for 30 days.
Evaluation of neuronal function
recovery
Following SCI, the locomotor recovery was evaluated
using the Basso, Beattie and Bresnahan (BBB), locomotor rating
scale, between 0 and 20, in which 0 indicates no observable
hind-limb movements), and 21, indicating normal locomotion
(19).
Measurement of the water content of the
spinal cord following SCI
The effect of mangiferin on the SCI was evaluated by
determining the water content of the SCI. Rats were sacrificed by
decollation. For the duration of the investigation, the SCI of all
the rats were dried for 48 h at 80°C for determination of the dry
weight. The water content of the SCI was obtained using the
following calculations: Wet weight - dry weight / wet weight.
Evaluation of oxidative stress
Following treatment with mangiferin for 30
consecutive days, the peripheral blood was collected from the
animals in each group and was centrifuged at 18,600 g for 10 min at
4°C. The supernatant was collected and oxidative stress was
analyzed by determining the levels of MDA, SOD, CAT and GSH-PX in
the SCI rats. According to the manufacturer's instructions
(Beyotime Institute of Biotechnology), the concentrations of MDA,
SOD, CAT and the activity of GSH-PX were analyzed using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Evaluation of inflammatory effects
Following treatment with mangiferin for 30
consecutive days, 300 µl peripheral blood was collected from
the animals in 3 rats of each group and was centrifuged at 18,600 ×
g for 10 min at 4°C. Following centrifugation at 18,600 g for 10
min at 4°C, the serum activities of NF-κB p65 unit, TNF-α, IL-1β
and IL-6 were measured by analyzing enzyme dynamics using
commercial kits, according to the manufacturer's instructions
(Sangon Biotech Co., Ltd.).
Western blot analysis
Samples of the exposed spinal cord tissue (10 mg)
were removed and incubated with 100 µl tissue lysis buffer
(Beyotime Institute of Biotechnology) containing 2 mM EDTA, 10 mM
EGTA, 0.4%NaF, 20 mM Tris-HCl and protease inhibitors (pH 7.5) for
10–15 min on ice. Subsequently, the homogenates were centrifuged at
18,600 g for 10 min at 4°C. The protein concentration of the
soluble materials was determined using a Bicinchoninic Acid protein
assay (Beyotime Institute of Biotechnology). Equal quantities of
protein (50 µg) were fractioned on 12% sodium dodecyl
sulfate-polyacrylamide gels (Invitrogen Life Technologies,
Carlsbad, CA, USA), followed by transfer onto polyvinylidene
fluoride membranes (0.22 mm; EMD Millipore, Bedford, MA, USA). The
membranes were blocked with phosphate-buffered saline (PBS) with 5%
non-fat milk to inhibit nonspecific binding sites. The membranes
were then incubated with anti-Bcl-2 (sc-492; 1:1,500; Santa Cruz
Biotechnology, Inc, Santa Cruz, CA, USA), anti-Bax (sc-493; 1:500;
Santa Cruz Biotechnology, Inc,) and anti-β-actin (sc-130656; 1:500;
Sangon Biotech Co., Ltd.) overnight at 4°C. Following incubation,
the membrane was washed three times with Tris-buffered saline with
Tween 20 (Biosharp, St. Louis, MO, USA) for 2 h, and the proteins
were then detected by incubating the membrane with anti-mouse IgG
(sc-358922; 1:1,000; Santa Cruz Biotechnology, Inc.) conjugated
with horseradish peroxidase for 2 h at room temperature. The
relative band intensity was determined using a gel image analysis
system (GDS8000; UVP, Upland, CA, USA).
Evaluation of caspase-3 and
caspase-9
Following treatment with mangiferin for 30
consecutive days, the peripheral blood was collected from each
group and centrifuged at 18,600 × g for 10 min at 4°C. According to
the manufacturer's instructions (Jiancheng Bioengineering
Institute), the levels of caspase-3 and caspase-9 were analyzed
using commercial kits at A405 nm.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
software package (SPSS, Inc., Chicago, IL, USA. Data are presented
as the mean ± standard deviation. Statistical analysis was
performed using one-way analysis of variance followed by Dunnett's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
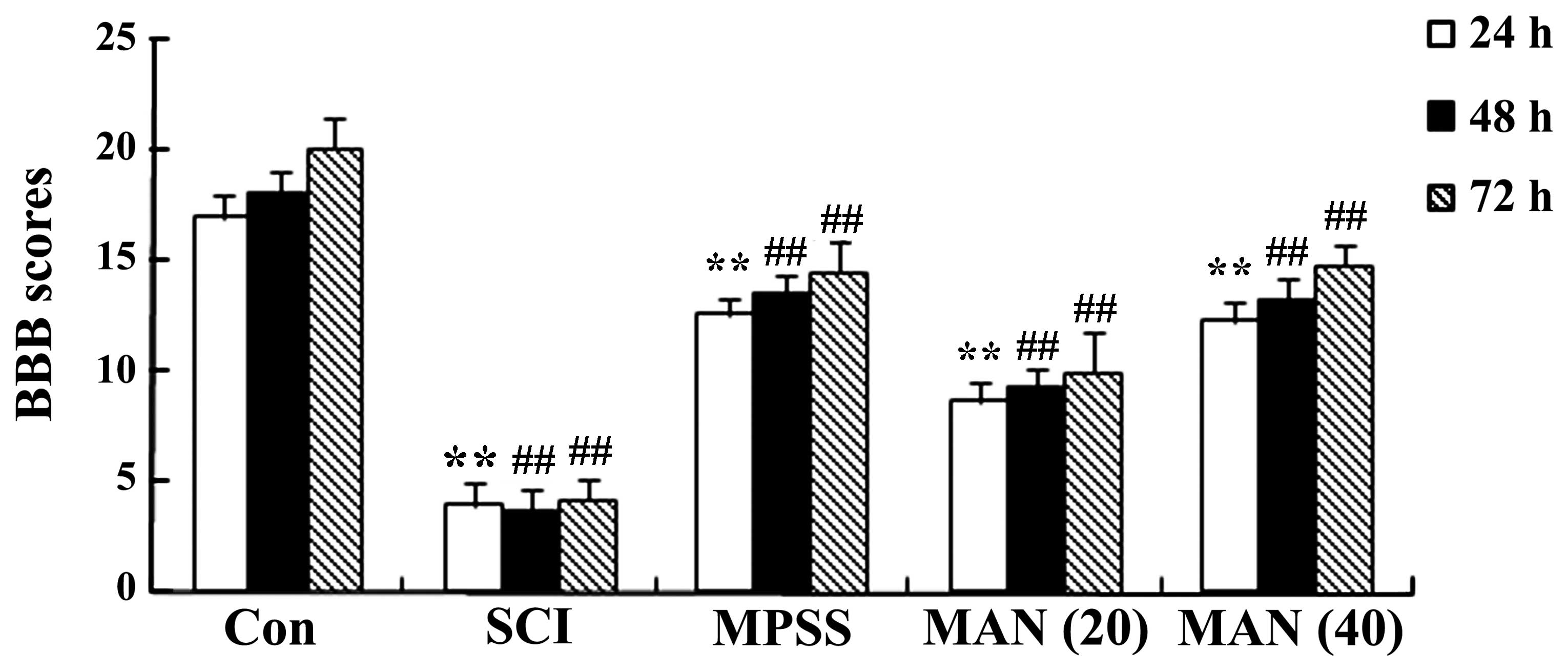
BBB scores for the evaluation of
neurological function
In the present study a model of SCI in rats was
establish, which exhibited persistent changes in neurological
function. The results revealed that the BBB scores of the SCI model
rat were reduced at 24, 48 and 72 h post-surgery respectively,
compared with those of the control group (Fig. 2). However, treatment with
mangiferin (20 and 40 mg/kg) of the rats in the SCI model group
exhibited significantly improved neurological function and
increased BBB scores, compared with the untreated SCI model group
(Fig. 2). In addition, as shown in
Fig. 2, the BBB scores of the rats
treated with mangiferin at a dose of 40 mg/kg were similar to those
obtained in the MPSS group, although not statistically significant
(P>0.05).
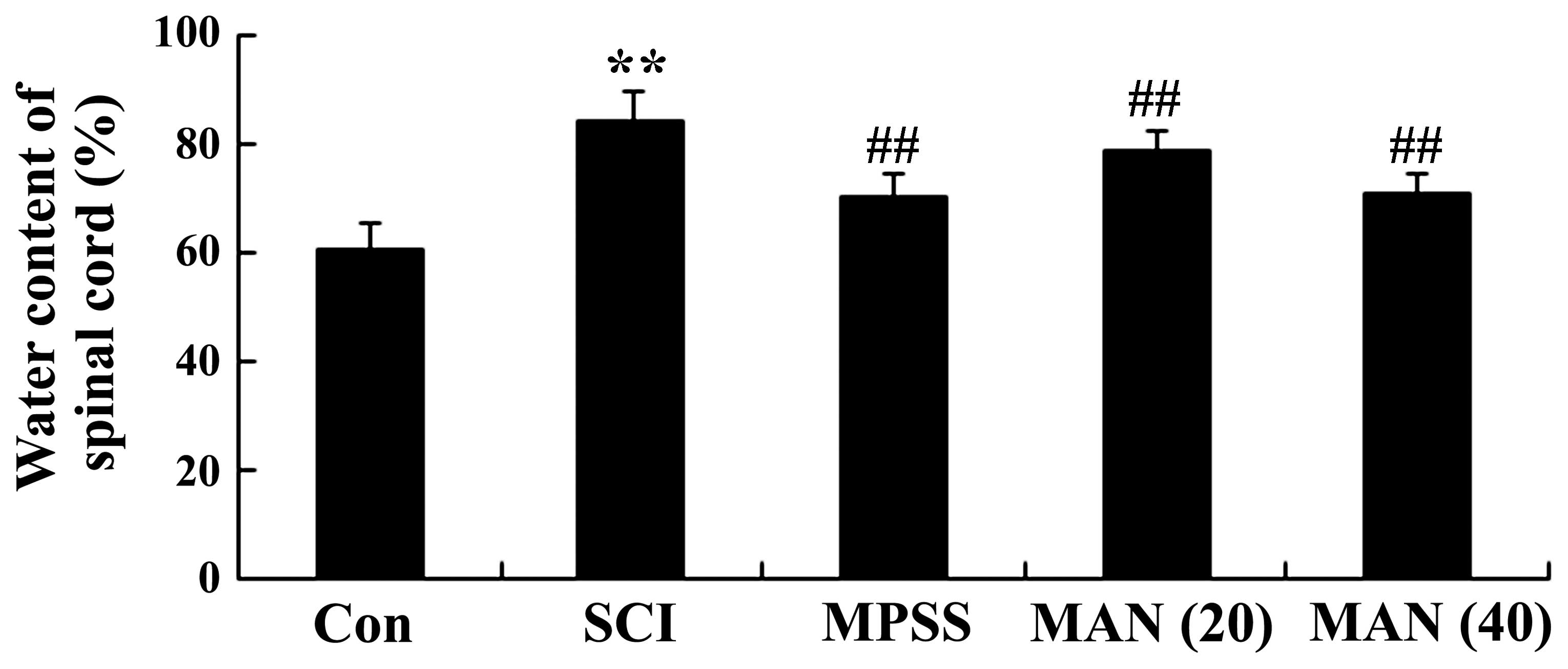
Mangiferin reduces the water content of
the spinal cord following SCI
To determine the effect of mangiferin on SCI, the
water content of the spinal cord tissues were measured in the
present study. As shown in Fig. 3,
the water content of spinal cord was increased in the SCI model
rats, compared with the rats in the control group. However, the
water content of the spinal cords in the mangiferin-treated (20 and
40 mg/kg) groups were significantly lower than that observed in the
SCI model group (Fig. 3). No
significant difference was observed between the MPSS group and the
MAN 40 group (P>0.05).
Anti-oxidative effects of mangiferin
The results of the present study revealed that the
level of MDA in the SCI model rats was enhanced, compared with that
in the control group (Fig. 4A).
Treatment with mangiferin (20 and 40 mg/kg) reduced the
concentrations of MDA, compared with the SCI model group (Fig. 4A). The results also demonstrated
that the concentrations of SOD and CAT, and the activity of GSH-PX
were weak in the SCI model rat group, compared with those observed
in the control group (Fig. 4B–D).
However, the concentrations of SOD and CAT, and the activity of
GSH-PX were increased in the mangiferin-treated (20 and 40 mg/kg)
groups, compared with the SCI model group (Fig. 4D). No significant difference was
observed in the concentrations of MDA, SOD or CAT, or the activity
of GSH-PX between the MPSS group and the MAN 40 group
(P>0.05).
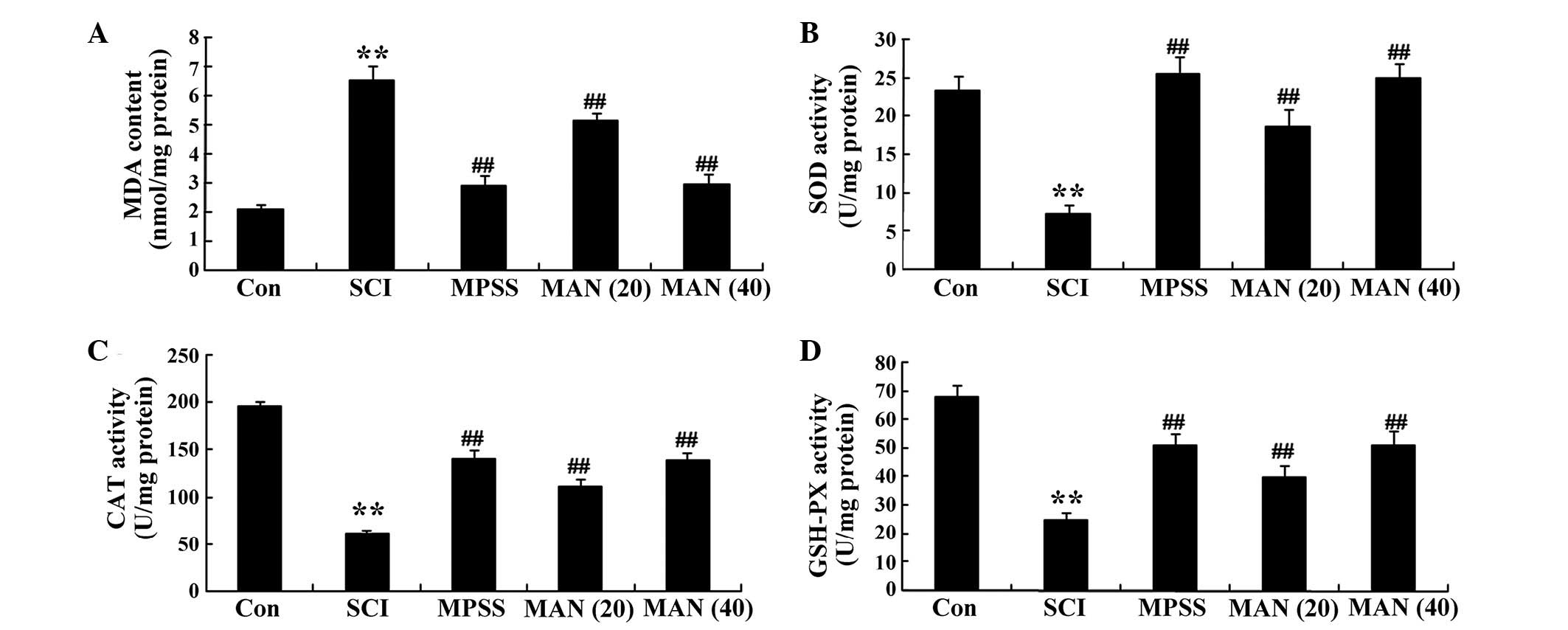 | Figure 4Anti-oxidative effects of mangiferin.
The anti-oxidative effects of mangiferin on the concentrations of
(A) MDA, (B) SOD, (C) CAT and (D) GSH-PX in the SCI model rats.
Data are presented as the mean ± standard deviation.
**P<0.01, compared with the control group;
##P<0.01, compared with the SCI group. Con, control;
SCI, spinal cord injury; MPSS, methylprednisolone; MAN (20), mangiferin (20 mg/kg); MAN (40), mangiferin (40 mg/kg); MDA,
malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GSH-PX,
glutathione peroxidase. |
Anti-inflammatory effects of
mangiferin
To determine the anti-inflammatory effect of
mangiferin on SCI, the serum activities of NF-κB p65 unit, TNF-α,
IL-1β and IL-6 were analyzed in the present study. The results
revealed that SCI induced the inflammatory reaction and increased
the serum activities of NF-κB p65 unit, TNF-α, IL-1β and IL-6 in
the SCI model rat group, compared with those of the control group
(Fig. 5A–D). However, these
inflammatory factors were reduced in the mangiferin-treated (20 and
40 mg/kg) groups, compared with those in the SCI model group
(Fig. 5A–D). No significant
inter-group differences in inflammatory reaction were identified
between the MPSS group and the MAN 40 group in the SCI model rat
(P>0.05).
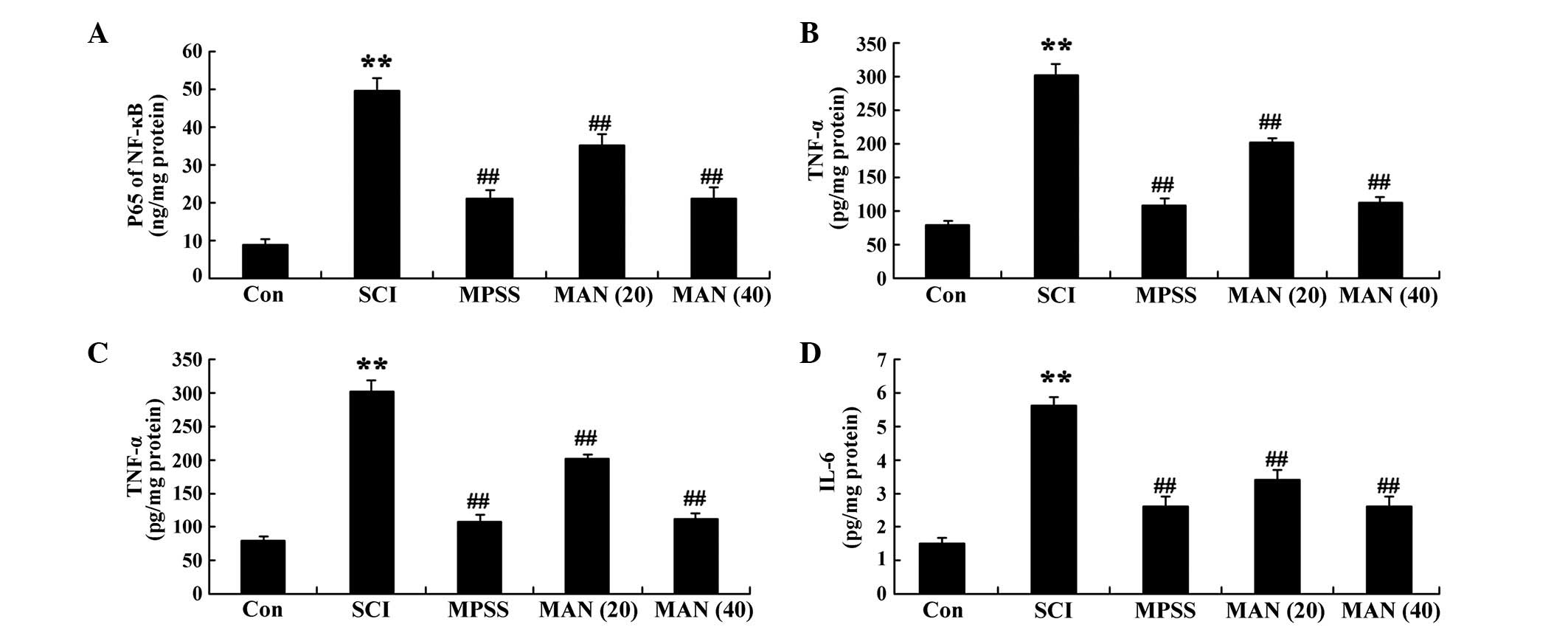 | Figure 5Anti-inflammatory effects of
mangiferin. The anti-inflammatory effects of mangiferin on the
serum activities of (A) NF-κB p65, (B) TNF-α, (C) IL-1β and (D)
IL-6 in SCI model rats. Data are presented as the mean ± standard
deviation. **P<0.01, compared with the control group;
##P<0.01, compared with the SCI group. Con, control;
SCI, spinal cord injury; MPSS, methylprednisolone; MAN (20), mangiferin (20 mg/kg); MAN (40), mangiferin (40 mg/kg); NF-κB,
nuclear factor κB; TNF, tumor necrosis factor; IL, interleukin. |
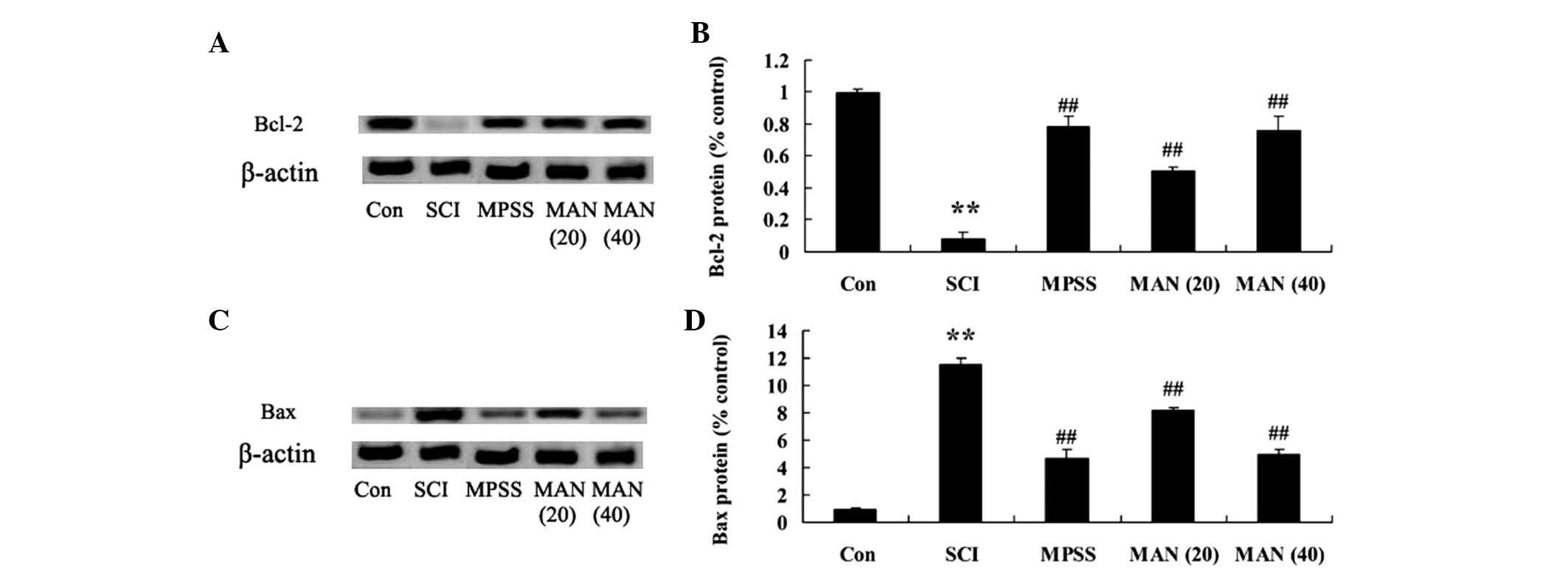
Astaxanthin alters the expression of
Bcl-2 and Bax
A previous study reported that astaxanthin adjusts
the expression levels of Bcl-2 and Bax in the SCI model rat. In the
present study, the expression of Bax in the SCI model group was
significantly increased, compared with that of the control group
(Fig. 6A). Treatment with
mangiferin (20 and 40 mg/kg) reduced the expression of Bax,
compared with the SCI model group (Fig. 6A). The expression of Bcl-2 in the
SCI model group was significantly lower than that of the control
group (Fig. 6B). By contrast, the
expression levels of Bcl-2 in the mangiferin-treated (20 and 40
mg/kg) groups were enhanced compared with that of the SCI model
group (Fig. 6B). However, no
significant changes amongst the expression levels of Bcl-2 and Bax
were observed between the MPSS group and the MAN 40 group
(P>0.05).
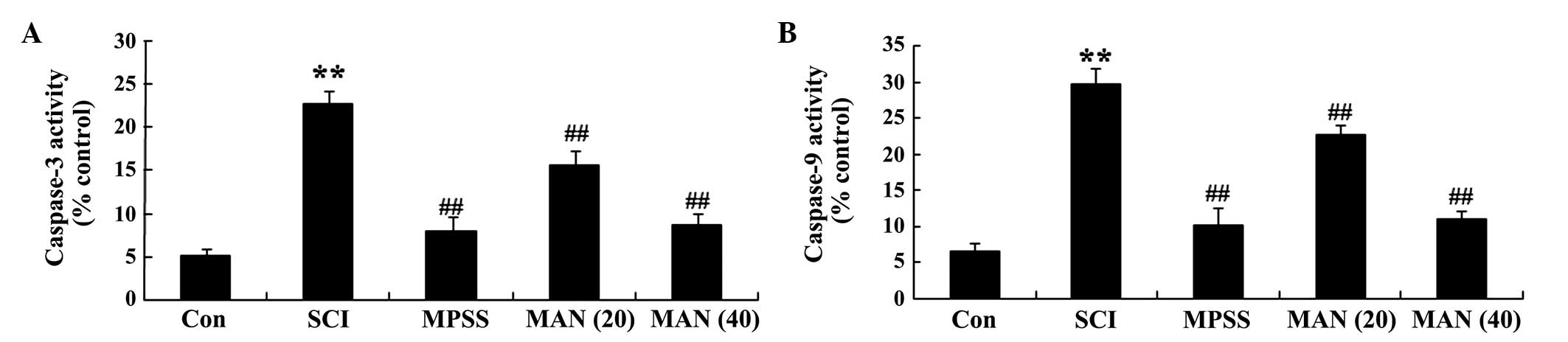
Anti-apoptotic effects of mangiferin
The results of the present study demonstrated that
the levels of caspase-3 and caspase-9 were significantly higher in
the SCI group, compared with the control group (Fig. 7A and B). However, the levels of
caspase-3 and caspase-9 in the mangiferin-treated (20 and 40 mg/kg)
groups were weak, compared with that in the SCI model group
(Fig. 7A and B). No significant
differences were observed between MPSS group and the MAN 40 group
(P>0.05).
Discussion
SCI is characterized by high morbidity rates with
serious complications, and treatment is difficult causing
significant economic and social burdens for individuals, families
and the community (20). In the
present study, mangiferin significantly improved BBB scores and
reduced the water content of the spinal cord in the SCI model rats.
In addition, the protective action of mangiferin on SCI at a dose
of 40 mg/kg was similar to that in the MPSS group.
Oxidative stress is a basic protective mechanism of
the body, which is involved in the regulation of life activities,
including cell signal transduction, cell proliferation and
apoptosis (21). Mitochondrial
dysfunction is an important factor leading to nerve cell death
following SCI, which is directly associated with substantial
accumulation of Ca2+ in the cells following injury
(22). Oxidative stress following
SCI damages ion homeostasis inside and outside the membrane, and a
large quantity of Ca2+ enters into the mitochondria,
accumulating inside and causing damage to mitochondria, which leads
to aerobic energy metabolism, inhibiting the synthesis of ATP
(23). In the present study,
mangiferin effectively decreased the concentrations of MDA and
augmented the concentrations of SOD and CAT, and the activity of
GSH-PX in the SCI model rats. However, no significant differences
were observed in these oxidative stress factors between the MAN40
group and MPSS group. Sellamuthu et al indicated that the
anti-oxidative effects of mangiferin significantly increase the
levels of SOD, CAT, GSH-PX and GSH in diabetic rats (24), and Viswanadh et al revealed
that pretreatment with mangiferin significantly increases GSH,
glutathione-S-transferase (GST), SOD and CAT activity (25).
SCI is a common type of trauma and the
pathophysiological changes in SCI can be divided into primary
mechanical damage and consequent secondary injury. The mechanism of
secondary SCI is complex, in which inflammation is important
(26). Acute SCI can activate
NF-κB in glial cells, neural cells and vascular endothelial cells,
causing the activation of NF-κB. The early activation of NF-κB
regulates the expression levels of a series of immune and
inflammatory-associated genes at the transcriptional level,
inducing a variety of inflam-matory factors (27). Inhibiting the expression of NF-κB
activity is key in inhibiting the inflammatory response and
reducing secondary SCI (28).
TNF-α, as an inflammatory cytokine with a variety of biological
activities in vivo, is important in the inflammatory
response and immune regulation (29). There is evidence to indicate that,
following acute SCI, macrophages, microglial cells, endothelial
cells and neurons can generate active NF-κB, and upregulated NF-κB
can induce the RNA expression of TNF-α (30). The rapid and sustained increased
expression of TNF-α is involved in SCI (31). IL-1β and IL-6 are also typical
inflammatory cytokines following SCI, predominantly secreted by
mononuclear macrophages, neutrophils and endothelial cells
(32). The emergence of IL-1β,
IL-6 and other inflammatory cytokines can increase secondary SCI.
In the SCI model in the present study, mangiferin effectively
reduced the serum activities of NF-κB p65 unit, TNF-α, IL-1β and
IL-6 in the SCI model rats, suggesting the persistent suppression
of inflammatory factors. No significant difference was observed
between the anti-inflammatory effects of mangiferin (40 mg/kg) and
MPSS. Gong et al suggested that the effects of mangiferin on
sepsis-induced lung injury occurred viathe suppression of
inflammatory factors and the upregulation of heme oxygenase-1 in
mice (33). In addition,
García-Rivera et al reported that mangiferin inhibits the
expression levels of NF-κB p65 unit, TNF-α and IL-6 in MDA-MB231
cells (34).
Bax is the major gene involved in determining cell
apoptosis in the Bcl-2 family, and promotes the mechanisms of
apoptosis. Bax can promote the release of cytochrome c,
activating caspase and leading to apoptosis; and Bcl-2 and Bax can
combine to reduce the gene expression of Bcl-2 (35). Homodimers or heterodimers may be
formed between Bcl-2 and Bax by BH1 and BH2. In order to inhibit
apoptosis, Bcl-2 requires combination with Bax to form a
heterodimer, and only when the number of Bcl-2/Bax heterodimers
exceeds the numbers of Bcl-2/Bcl-2 homodimers and Bax/Bax
homodimers, can cell apoptosis be inhibited (36). Therefore, the positive expression
ratio of Bcl-2/Bax in cells directly determines whether cells
undergo apoptosis. In the present study, mangiferin reduced the
protein expression of Bax and promoted the protein expression of
Bcl-2 in the SCI model rats. However, no significant difference was
observed in the in the levels of Bcl-2 and Bax between the MPSS
group and MAN 40 group. Pan et al indicated that the
antiproliferative effects of mangiferin were regulated by Bcl-2 and
Bax (37). In addition, Kavitha
et al concluded that mangiferin attenuates
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced dopaminergic
neurodegeneration and improves motor impairment through
downregulating the expression of Bcl-2 and upregulating the
expression of Bax in diseased mice (38). Pal et al demonstrated that
mangiferin protects the murine liver in Pb (II)-induced hepatic
damage and cell death through regulation of the Bcl-2/Bax pathways
(39).
The present study demonstrated that mangiferin
protected spinal cord cells by suppressing apoptosis and reducing
the levels of caspase-3/9 in the SCI model rats. No significant
changes in antiapoptitic effects were detected between the MPSS
group and MAN 40 group. Similarly, Ghosh et al reported that
mangiferin protects rat kidneys in DGal-induced oxidative stress
and acute nephrotoxicity through caspase-3/9 activities (40). In conclusion, the findings of the
present study established, for the first time, that mangiferin
attenuated contusive SCI in rats and provided effective protection
against oxidative stress, inflammation and apoptosis in the SCI
rats through the Bcl-2/Bax signaling pathway.
References
|
1
|
Cizkova D, Rosocha J, Vanický I, Jergová S
and Cízek M: Transplants of human mesenchymal stem cells improve
functional recovery after spinal cord injury in the rat. Cell Mol
Neurobiol. 26:1167–1180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cui B, Li E, Yang B and Wang B: Human
umbilical cord blood-derived mesenchymal stem cell transplantation
for the treatment of spinal cord injury. Exp Ther Med. 7:1233–1236.
2014.PubMed/NCBI
|
|
3
|
Lee JY, Maeng S, Kang SR, Choi HY, Oh TH,
Ju BG and Yune TY: Valproic acid protects motor neuron death by
inhibiting oxidative stress and endoplasmic reticulum
stress-mediated cytochrome C release after spinal cord injury. J
Neurotrauma. 31:582–594. 2014. View Article : Google Scholar :
|
|
4
|
Lam T, Chen Z, Sayed-Ahmed MM, Krassioukov
A and Al-Yahya AA: Potential role of oxidative stress on the
prescription of rehabilitation interventions in spinal cord injury.
Spinal Cord. 51:656–662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ordonez FJ, Rosety MA, Camacho A, Rosety
I, Diaz AJ, Fornieles G, Bernardi M and Rosety-Rodriguez M:
Arm-cranking exercise reduced oxidative damage in adults with
chronic spinal cord injury. Arch Phys Med Rehabil. 94:2336–2341.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bareyre FM and Schwab ME: Inflammation,
degeneration and regeneration in the injured spinal cord: Insights
from DNA micro-arrays. Trends Neurosci. 26:555–563. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang W, Cheng L, Hou Y, Si M, Zhao YP and
Nie L: Plumbagin protects against spinal cord injury-induced
oxidative stress and inflammation in wistar rats through Nrf-2
upregulation. Drug Res (Stuttg). Sep 22–2014.Epub ahead of
print.
|
|
8
|
Mohammadi E, Ghaedi K, Esmailie A and
Rahgozar S: Gene expression profiling of liver X receptor alpha and
Bcl-2-associated X protein in experimental transection spinal
cord-injured rats. J Spinal Cord Med. 36:66–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, He P, Liu F, Shi L, Zhu H, Cheng X,
Zhao J, Wang Y and Zhang M: Prognostic significance of B-cell
lymphoma 2 expression in acute leukemia: A systematic review and
meta-analysis. Mol Clin Oncol. 2:411–414. 2014.PubMed/NCBI
|
|
10
|
Chen MH, Ren QX, Yang WF, Chen XL, Lu C
and Sun J: Influences of HIF-lα on Bax/Bcl-2 and VEGF expressions
in rats with spinal cord injury. Int J Clin Exp Pathol.
6:2312–2322. 2013.
|
|
11
|
Ray SK, Matzelle DC, Wilford GG, Hogan EL
and Banik NL: E-64-d prevents both calpain upregulation and
apoptosis in the lesion and penumbra following spinal cord injury
in rats. Brain Res. 867:80–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Agarwala S, B NR, Mudholkar K, Bhuwania R
and Satish Rao BS: Mangiferin, a dietary xanthone protects against
mercury-induced toxicity in HepG2 cells. Environ Toxicol.
27:117–127. 2012. View Article : Google Scholar :
|
|
13
|
Yoosook C, Bunyapraphatsara N, Boonyakiat
Y and Kantasuk C: Anti-herpes simplex virus activities of crude
water extracts of thai medicinal plants. Phytomedicine. 6:411–419.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Campos-Esparza MR, Sanchez-Gómez MV and
Matute C: Molecular mechanisms of neuroprotection by two natural
anti-oxidant polyphenols. Cell Calcium. 45:358–368. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garrido-Suárez BB, Garrido G, Delgado R
and Bosch F: A Mangifera indica L. extract could be used to treat
neuropathic pain and implication of mangiferin. Molecules.
15:9035–9045. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rajendran P, Ekambaram G and Sakthisekaran
D: Cytoprotective effect of mangiferin on benzo(a)pyrene-induced
lung carcinogenesis in swiss albino mice. Basic Clin Pharmacol
Toxicol. 103:137–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin H, Chen R, Liu X, Sheng F and Zhang H:
Study on inter-action of mangiferin to insulin and glucagon in
ternary system. Spectrochim Acta A Mol Biomol Spectrosc.
75:1584–1591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ravikumar R, Fugaccia I, Scheff SW, Geddes
JW, Srinivasan C and Toborek M: Nicotine attenuates morphological
deficits in a contusion model of spinal cord injury. J Neurotrauma.
22:240–251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Basso DM, Beattie MS, Bresnahan JC,
Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ,
Nockels R, et al: MASCIS evaluation of open field locomotor scores:
Effects of experience and teamwork on reliability. Multicenter
animal spinal cord injury study. J Neurotrauma. 13:343–359. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao J, Xie J, Lin D, Lu N, Guo L, Li W,
Pu B, Yang Y, Yang Z, Zhang Y and Song Y: Meglumine cyclic
adenylate improves neurological function following acute spinal
cord injury in rats. Mol Med Rep. 10:1225–1230. 2014.PubMed/NCBI
|
|
21
|
Maher P and Schubert D: Signaling by
reactive oxygen species in the nervous system. Cell Mol Life Sci.
57:1287–1305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al Dera H, Habgood MD, Furness JB and
Brock JA: Prominent contribution of L-type Ca2+ channels to
cutaneous neurovascular transmission that is revealed after spinal
cord injury augments vasoconstriction. Am J Physiol Heart Circ
Physiol. 302:H752–H762. 2012. View Article : Google Scholar
|
|
23
|
Xia M and Zhu Y: FOXO3a involvement in the
release of TNF-alpha stimulated by ATP in spinal cord astrocytes. J
Mol Neurosci. 51:792–804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sellamuthu PS, Arulselvan P, Kamalraj S,
Fakurazi S and Kandasamy M: Protective nature of mangiferin on
oxidative stress and antioxidant status in tissues of
streptozotocin-induced diabetic rats. ISRN Pharmacol.
2013:7501092013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Viswanadh EK, Rao BN and Rao BS:
Antigenotoxic effect of mangiferin and changes in antioxidant
enzyme levels of Swiss albino mice treated with cadmium chloride.
Hum Exp Toxicol. 29:409–418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alexander JK and Popovich PG:
Neuroinflammation in spinal cord injury: Therapeutic targets for
neuroprotection and regeneration. Prog Brain Res. 175:125–137.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bethea JR, Castro M, Keane RW, Lee TT,
Dietrich WD and Yezierski RP: Traumatic spinal cord injury induces
nuclear factor-kappaB activation. J Neurosci. 18:3251–3260.
1998.PubMed/NCBI
|
|
28
|
Ni H, Jin W, Zhu T, et al: Curcumin
modulates TLR4/NF-kappaB inflammatory signaling pathway following
traumatic spinal cord injury in rats. J Spinal Cord Med.
38:199–206. 2015. View Article : Google Scholar
|
|
29
|
Vidal PM, Lemmens E, Geboes L,
Vangansewinkel T, Nelissen S and Hendrix S: Late blocking of
peripheral TNF-alpha is ineffective after spinal cord injury in
mice. Immunobiology. 218:281–284. 2013. View Article : Google Scholar
|
|
30
|
Yuan B, Liu D and Liu X: Spinal cord
stimulation exerts analgesia effects in chronic constriction injury
rats via suppression of the TLR4/NF-kappaB pathway. Neurosci Lett.
581:63–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yune TY, Lee SM, Kim SJ, Park HK, Oh YJ,
Kim YC, Markelonis GJ and Oh TH: Manganese superoxide dismutase
induced by TNF-beta is regulated transcriptionally by NF-kappaB
after spinal cord injury in rats. J Neurotrauma. 21:1778–1794.
2004.
|
|
32
|
Liu YL, Zhou LJ, Hu NW, et al: Tumor
necrosis factor-alpha induces long-term potentiation of C-fiber
evoked field potentials in spinal dorsal horn in rats with nerve
injury: the role of NF-kappa B, JNK and p38 MAPK.
Neuropharmacology. 52:708–715. 2007. View Article : Google Scholar
|
|
33
|
Gong X, Zhang L, Jiang R, Ye M, Yin X and
Wan J: Anti-inflammatory effects of mangiferin on sepsis-induced
lung injury in mice via up-regulation of heme oxygenase-1. J Nutr
Biochem. 24:1173–1181. 2013. View Article : Google Scholar
|
|
34
|
García-Rivera D, Delgado R, Bougarne N,
Haegeman G and Berghe WV: Gallic acid indanone and mangiferin
xanthone are strong determinants of immunosuppressive anti-tumour
effects of Mangifera indica L. Bark in MDA-MB231 breast cancer
cells. Cancer Lett. 305:21–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tarantino G, Scopacasa F, Colao A, Capone
D, Tarantino M, Grimaldi E and Savastano S: Serum Bcl-2
concentrations in overweight-obese subjects with nonalcoholic fatty
liver disease. World J Gastroenterol. 17:5280–5288. 2011.
View Article : Google Scholar
|
|
36
|
Qiao WL, Wang GM, Shi Y, Wu JX, Qi YJ,
Zhang JF, Sun H and Yan CD: Differential expression of Bcl-2 and
Bax during gastric ischemia-reperfusion of rats. World J
Gastroenterol. 17:1718–1724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan LL, Wang AY, Huang YQ, Luo Y and Ling
M: Mangiferin induces apoptosis by regulating Bcl-2 and bax
expression in the CNE2 nasopharyngeal carcinoma cell line. Asian
Pac J Cancer Prev. 15:7065–7068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kavitha M, Nataraj J, Essa MM, Memon MA
and Manivasagam T: Mangiferin attenuates MPTP induced dopaminergic
neurode-generation and improves motor impairment, redox balance and
Bcl-2/Bax expression in experimental Parkinson's disease mice. Chem
Biol Interact. 206:239–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pal PB, Sinha K and Sil PC: Mangiferin, a
natural xanthone, protects murine liver in Pb(II) induced hepatic
damage and cell death via MAP kinase, NF-kB and mitochondria
dependent pathways. PLoS One. 8:e568942013. View Article : Google Scholar
|
|
40
|
Ghosh M, Das J and Sil PC: D(+)
galactosamine induced oxidative and nitrosative stress-mediated
renal damage in rats via NF-kB and inducible nitric oxide synthase
(iNOS) pathways is ameliorated by a polyphenol xanthone,
mangiferin. Free Radic Res. 46:116–132. 2012. View Article : Google Scholar
|