Introduction
Mesenchymal stem cells (MSCs) were initially
isolated from bone marrow (BM) but are now known to reside in
almost every type of connective tissue. MSCs are a heterogeneous
subset of cells which can proliferate in vitro as
plastic-adherent cells to develop as fibroblast colony-forming
units (1). The most important
characteristics of MSCs are their self-renewal and differentiation
ability (2). Other important
features of MSCs include the lack of co-stimulator molecules [CD80,
CD86 and human leukocyte antigen (HLA)-E] (3) and immunosuppressive effects (4). All of these properties render MSCs as
the best candidate for use in cell-based therapy. MSCs have now
been widely evaluated in clinical trials on the treatment of
conditions including left ventricular ejection fraction, graft
versus host disease, pancreatic regeneration and rheumatoid
arthritis (5). However, the
results of clinical trials have not met their primary aims, and the
current understanding of the fate of MSC following systemic
infusion is limited (6). Previous
studies indicated an enhanced immunogenicity of MSCs under specific
conditions or upon specific stimulation, as indicated by changes in
their differentiation status (7),
as well as the activation of the memory T cell response (8) and the complement response (9).
Toll-like receptors (TLRs) are type I integral
membrane glycoproteins known as pathogen-associated molecular
patterns (PAMPs). The human TLR family has 11 members, which all
have important roles in recognizing microbial components (10). It has also been indicated that TLRs
are important in regulating the biological functions of MSCs
isolated from BM and adipose tissues (11). However, to date, no study has
elucidated the role of TLRs in influencing the immunogenicity of
MSCs isolated from umbilical cords (UCs). Our group has performed
systematic investigations to confirm the role of TLRs in promoting
the immunogenicity of MSCs from UCs, which indicated that the
activation of TLR7 enhances the immunogenicity of UCMSCs (12). In the present study, the TLR5
pathway was stimulated in UCMSCs by flagellin treatment and the
effects of the activation of TLR5 on the immunogenicity of UCMSCs
were investigated.
Materials and methods
Isolation and culture of UCMSCs
The UCMSCs (Sichuan Umbilical Cord Blood Stem Cell
Bank, Sichuan, China) were isolated from two UCs as previously
reported (13). After disinfection
in 75% ethanol for 20 min, the UC was dissected into cubical-shaped
pieces, which were planted on 25 mm2 plates. Complete
Dulbecco's modified Eagle's medium (DMEM; Invitrogen Life
Technologies, Carlsbad, CA, USA) with 10% fetal bovine serum
(Invitrogen Life Technologies) was added to the plates with the
small UC tissues tightly attached to the bottom. The tissues were
removed when dissociated MSCs were observed following culture for
almost eight days. The UCMSCs were then digested with trypsin
(Invitrogen Life Technologies) and either stored in liquid nitrogen
for future use or subjected to further culturing. The present study
was approved by the ethics committee of the West China Hospital,
Sichuan University (Chengdu, China), and written informed consent
was obtained from the patients.
TLR5 stimulation
TLR5 agonist flagellin (no. AG-40B-0025; Adipogen,
San Diego, CA, USA) was dissolved in sterile water at a
concentration of 0.1 mg/ml. UCMSCs were seeded into a six-well
plate at a density of 1.5×105 in 2 ml medium and
flagellin was added to the medium at a final concentration of 50
ng/ml.
Assessment of leukocyte
proliferation
Peripheral blood mononuclear cells (PBMCs) were
obtained from two healthy student volunteers were recruited from
the Laboratory of Pathology, West China Hospital, Sichuan
University, and isolated by density centrifugation gradient (300 ×
g, 20 min) and labeled by carboxyfluorescein diacetate succinimidyl
ester (CFSE; eBioscience, San Diego, CA, USA) at a final
concentration of 10 µM. Pre-cooled complete DMEM was added
following 10 min to terminate the reaction. The CFSE-labeled PBMCs
were then washed three times with cold phosphate-buffered saline
(1,200 xg, 5-min). PBMCs were cultured alone or co-cultured with
UCMSCs (10:1) in the absence or presence of flagellin for 72 h and
collected for fluorescence-associated cell sorting (FACS) detection
(FACScan flow cytometer; Beckman Coulter, Brea, CA, USA).
Lactate dehydrogenase (LDH) assay
Supernatants from the PBMC-UCMSC co-culture system
were collected at 24, 48 and 72 h post-stimulation and assessed
using the cytotoxicity detection LDH kit (cat. no. G1780; Promega
Corporation, Madison, WI, USA). The release of LDH from damaged
cells was assayed according to the manufacturer's instructions.
Colorimetric evaluation was conducted using a MQX200 plate reader,
BioTek, Winooski, VT, USA. Percentage of lysed cells was calculated
by the following formula: (E-M)/(T-M) ×100%, where E is
experimental release, M is the spontaneous release in the presence
of media alone, and T is the maximum release in the presence of 5%
Triton X-100 (Invitrogen Life Technologies).
Flow cytometric analysis of surface
markers
Flagellin-treated and untreated UCMSCs were
harvested 72 h post-stimulation and stained with antibodies
(eBioscience; 1:100 dilution; incubated for 30 min at room
temperature) against surface marker molecules (Table II). Flow cytometric results were
analyzed using CXP flow cytometry software (Beckman Coulter). The
positive standard of cells was gated according to the fluorescence
intensity of the negative control group.
 | Table IIMonoclonal antibodies used for
fluorescence-assisted cell sorting analysis. |
Table II
Monoclonal antibodies used for
fluorescence-assisted cell sorting analysis.
| Name | Catalog number |
|---|
| CD80 | 11-0809 |
| CD86 | 12-0869 |
| HLA-E | 17-9953 |
| CD90 | 45-0909 |
| CD59 | 11-0596 |
| CD29 | 17-0299 |
UCMSC differentiation
UCMSCs were seeded into six-well plates at
1.5×105 cells per well. Differentiation medium specific
for chondrocytes (no. A10071-01; Gibco-BRL, Invitrogen Life
Technologies), osteocytes (no. A10072-01; Gibco-BRL) and adipocytes
(no. A10070-01; Gibco-BRL) was added to the respective wells
together with 10 µg/ml imiquimod (Novus Biologicals, LLC,
Littleton, CO, USA). Oil-red O (eBioscience) was used for staining
of adipocytes, alizarin red (eBioscience) for osteocytes and
safranine (eBio-science) for chondrocytes at 7, 14 and 21 days of
culture.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
The RNA extraction, cDNA synthesis and quantitative
PCR were performed according to a protocol of our previous study
(12). After treatment with
flagellin (50 ng/ml) for 4, 12, 24, 72 or 120 h, total RNA was
isolated from the UCMSCs using an RNeasy mini kit (Qiagen, Hilden,
Germany). cDNA was synthesized from 50 ng RNA using the ReverTra
Ace qPCR kit (FSQ-101; Toyobo, Osaka, Japan). The RT conditions
were 65°C for 5 min, followed by 37°C for 15 min and 98°C for 5
min. qPCR was performed using RealMaster mix (SYBR Green; FP202;
Tiangen, Beijing, China) with 50 ng cDNA. qPCR was performed in an
iCycler iQTM Optical Module (Beckman Coulter) under the following
conditions: One cycle at 95°C for 30 sec, then 40 cycles at 95°C
for 30 sec, 58°C for 30 sec and 72°C for 30 sec, followed by a
melting curve from 55–95°C in 0.5°C increments and 10-sec
intervals. The primers used (Chengdu Branch Zi Qing Xi
Biotechnology Co., Ltd., Chengdu, China) are listed in Table I. All experiments were performed
three times.
 | Table IPrimers used for real-time polymerase
chain reaction analysis. |
Table I
Primers used for real-time polymerase
chain reaction analysis.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | GenBank number |
|---|
| IL-1β |
ACGAATCTCCGACCACCACT |
CCATGGCCACAACAACTGAC | M15330 |
| IL-6 |
GACCCAACCACAAATGCCA |
GTCATGTCCTGCAGCCACTG | M14584 |
| IL-8 |
CTGGCCGTGGCTCTCTTG |
CCTTGGCAAAACTGCACCTT | NM_000584 |
| IL-10 |
GGTGATGCCCCAAGCTGA |
TCCCCCAGGGAGTTCACA | U16720 |
| IL-12 |
CGGTCATCTGCCGCAAA |
CAAGATGAGCTATAGTAGCGGTCCT | M65272 |
| IFN-β |
CAGCAATTTTCAGTGTCAGAAGCT |
TCATCCTGTCCTTGAGGCAGT | M28622 |
| IP-10 |
TGAAATTATTCCTGCAAGCCAA |
CAGACATCTCTTCTCACCCTTCTTT | NM_001565 |
| NF-κB |
AGAGTGCTGGAGTTCAGGATA |
AAGGTGGATGATTGCTAAGTGT | AJ271718 |
| TGF-β |
TATCGACATGGAGCTGGTGAAG |
CAGCTTGGACAGGATCTGGC | X02812 |
| TNF-α |
GGTGCTTGTTCCTCAGCCTC |
CAGGCAGAAGAGCGTGGTG | M10988 |
| CCL5 |
AGCAGAGGCTGGAGAGCTACA |
GGGTCAGCACAGATCTCCTTGT | NM_006273 |
| CCL24 |
CCTCTCCTGCCTCATGCTTATT |
CTCTGTCTCTGCATCATTTGTGAA | U58914 |
| GAPDH |
GAAGGTGAAGGTCGGAGTC |
GAAGATGGTGATGGGATTTC | J04038 |
Statistical analysis
The qPCR data were analyzed using Bio-Rad iQ5
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). GAPDH was
used as an internal control. Normal peripheral blood lymphocytes
were used as a negative control. Values are expressed as the mean ±
standard error of the mean. Statistical analyses were performed
using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Values of P<0.05
and P<0.001 were considered to indicate a significant difference
compared with the control group. Graphs were prepared using
GraphPad Prism5 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
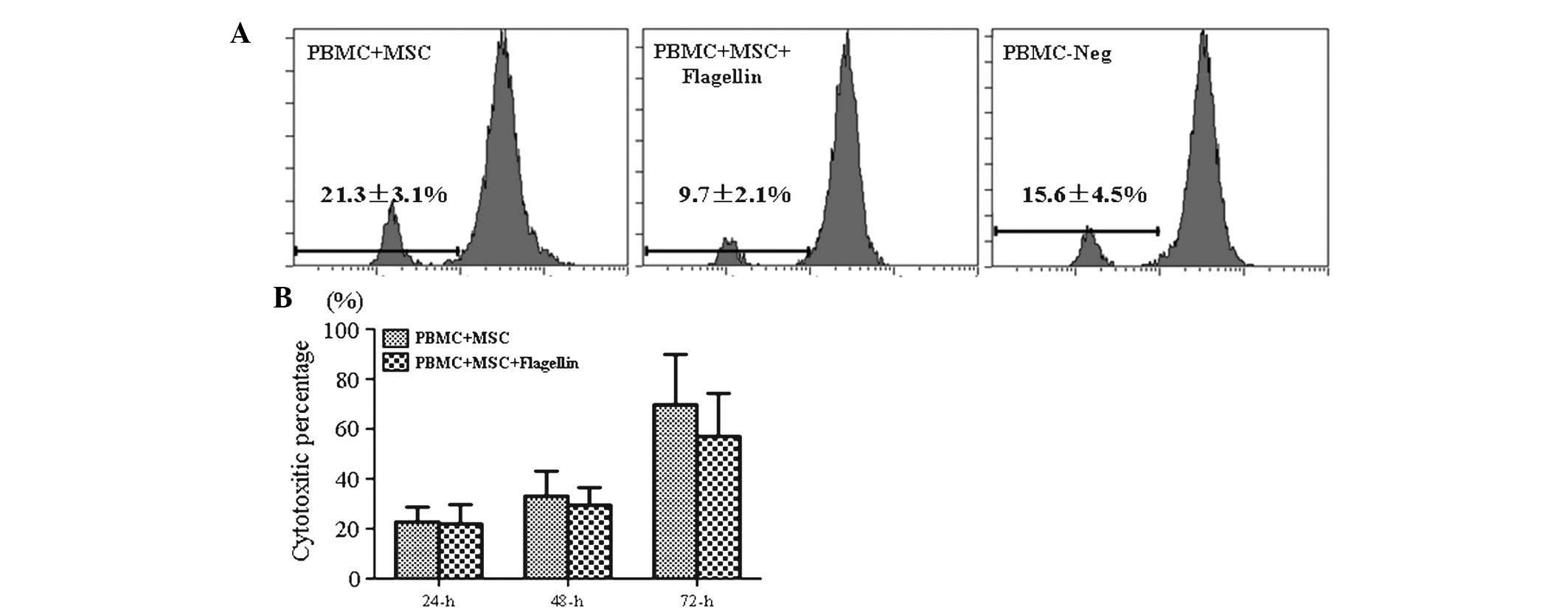
TLR5 activation did not affect the
proliferation of PBMCs or leukocyte-mediated cytotoxicity
PBMCs from human volunteers were labeled with CFSE
and then co-cultured with UCMSCs in the presence of flagellin.
PBMCs were collected and their proliferation was assessed by flow
cytometric analysis following 72 h of co-culture. The results
showed that the proliferation of PBMCs in the PBMC-UCMSC co-culture
system in the presence of TLR5 agonist (9.7%) was not obviously
different from that in the control groups (21.3% for PBMC-MSCs and
15.6% for PBMCs only), which suggested that TLR5 activation did not
stimulate the immune response (Fig.
1A).
Leukocyte-mediated cytotoxicity was determined by
measuring the LDH release by damaged cells in the culture
supernatant. The results indicated that LDH levels were not
significantly different between the experimental group (PBMC-UCMSCs
co-culture in the presence of flagellin) and control group (PBMCs +
MSCs) following 24, 48 and 72 h of incubation (Fig. 1B). This result indicated that the
activation of the TLR5 pathway did not stimulate the in
vitro immune response in the PBMC-UCMSC co-culture system.
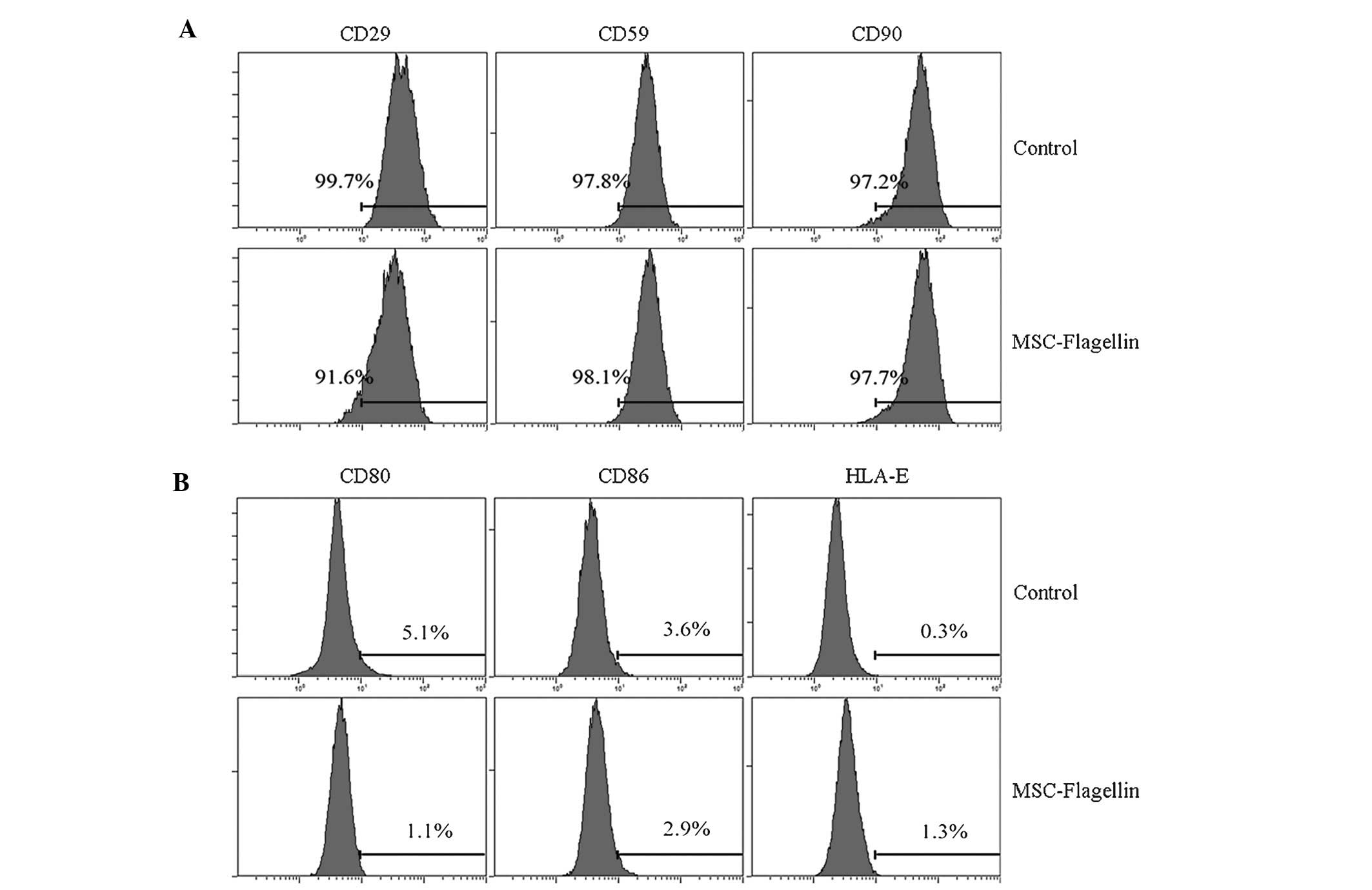
TLR5 activation does not affect the
expression of co-stimulatory molecules and stem cell markers
The observation that flagellin did not change the
immune status motivated us to investigate whether the activation of
TLR5 influences the expression of surface molecules on UCMSCs,
particularly that of co-stimulatory molecules and stem cell
markers. UCMSCs were incubated with or without flagellin, and the
co-stimulatory molecules CD80, CD86 and HLA-E, as well as the
surface stem cell markers CD29, CD59 and CD90 were assessed by
RT-qPCR. The results showed that stimulation with flagellin for
five days did not significantly alter the expression of the
co-stimulatory factors (P>0.05; CD80, 5.1 vs. 1.1%; CD86, 3.6
vs. 2.9%; HLA-E, 0.3 vs. 1.3; control vs. flagellin group,
respectively) (Fig. 2A). The
expression of stem cell markers was also not significantly changed
(CD29, 99.7 vs. 91.6%; CD59, 97.8 vs. 98.1%; CD90, 97.2 vs. 97.7%;
control vs. flagellin group, respectively) (Fig. 2B).
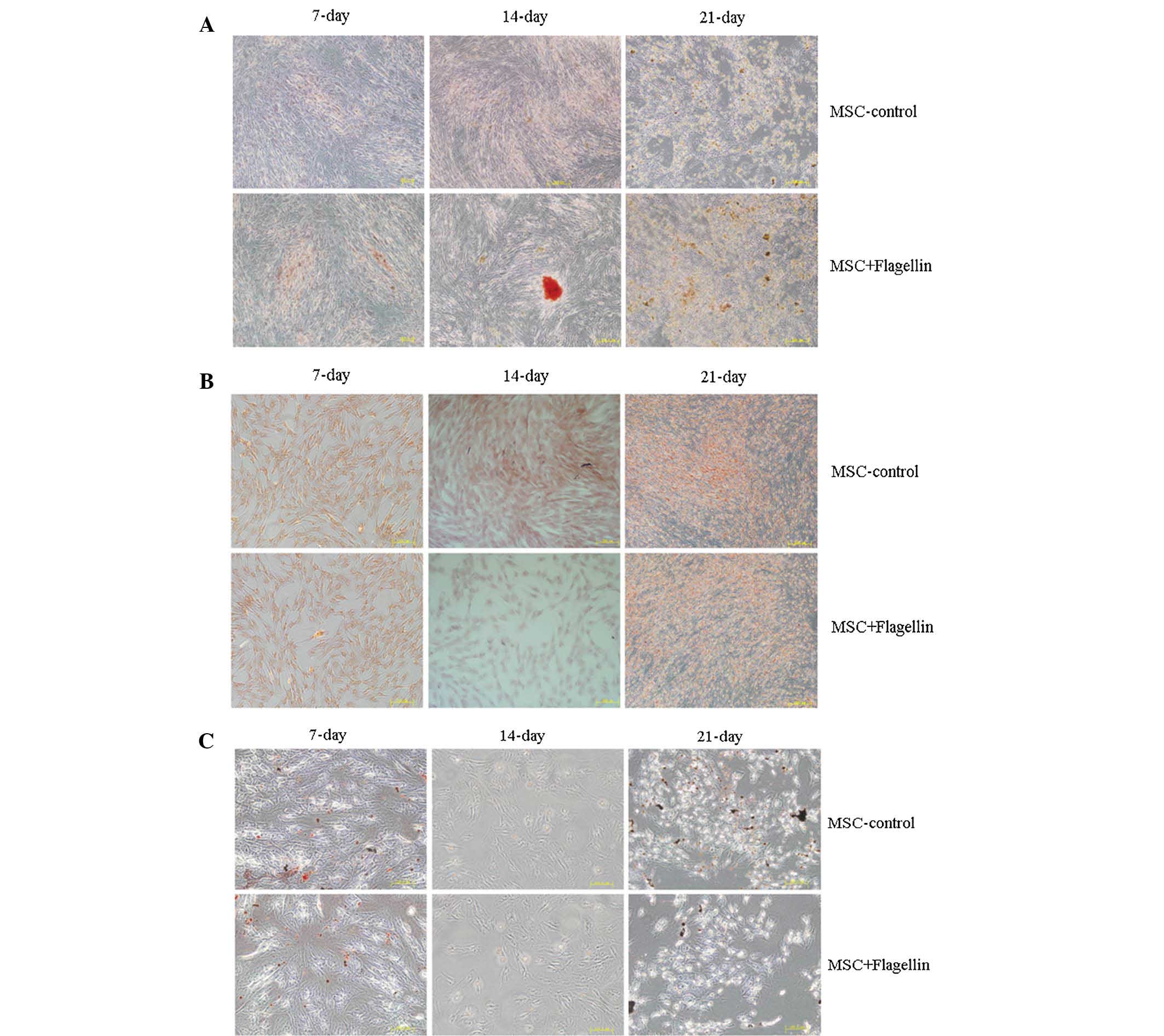
Activation of the TLR5 pathway does not
affect the differentiation ability of UCMSCs
Next, the present study examined the role of the
TLR5 pathway in regulating the differentiation of UCMSCs. Culture
was performed in differentiation media specific for adipocytes,
osteoblasts and chondrocytes to induce UCMSC differentiation
(Fig. 3). The cell phenotypes were
identified by staining with alizarin red for osteoblasts, safranine
for chondrocytes and Oil red O for adipocytes in order to assess
the differentiation levels of UCMSCs at 7, 14 and 21 days of
culture in the presence of flagellin. The results clearly showed
that there were no differences between the control and
flagellin-treated groups, indicating that the TLR5 pathway was not
involved in the differentiation of UCMSCs (Fig. 3A–C).
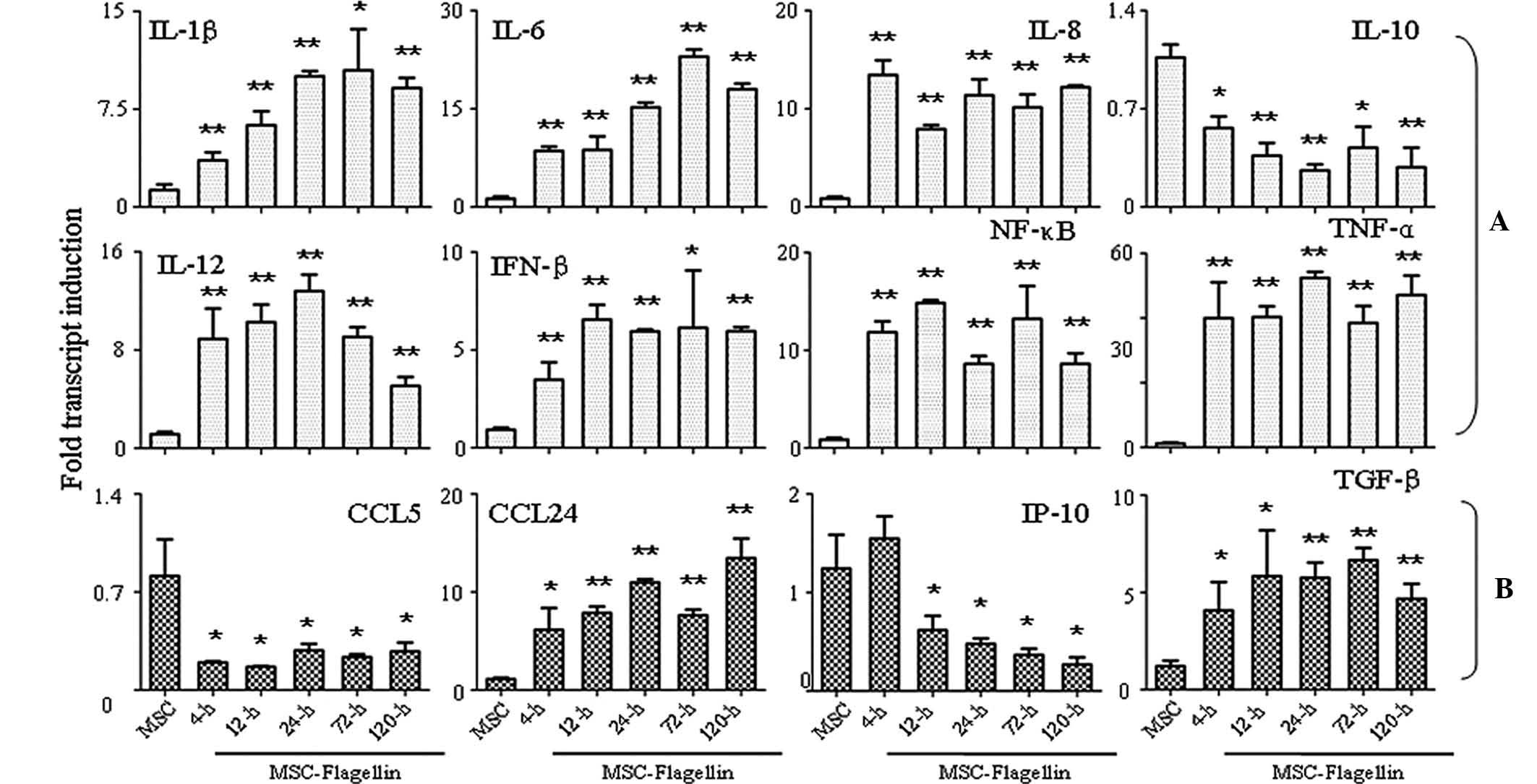
Activation of TLR5 enhances the
expression of cytokines and chemokines
Finally, the present study performed an RT-qPCR
analysis to assess the expression of several important cytokines
and chemokines, which have significant roles in biological
functions of UCMSCs. Eight important pro-inflammatory molecules
(IL-1β, IL-6, IL-8, IL-10, IL-12, IFN-β, NF-κB and TNF-α) were
analyzed, which showed that the expression of all tested factors
except IL-10 was markedly enhanced at 4, 12, 24, 72 and 120 h
post-stimulation (all P<0.05 or P<0.001) (Fig. 4A). IL-10 expression was
significantly inhibited following 4-h treatment with flagellin,
which persisted for 120 h until the end of the experiment
(P<0.05 or P<0.001) (Fig.
4A). With regard to chemokine expression, CCL5 was inhibited in
the presence of flagellin stimulation for 4–120 h (P<0.05), and
IP-10 was also found inhibited following treatment for 12–120 h
(P<0.05) (Fig. 4B). The
expression of the other two chemokines, CCL24 and TGF-β, was
significantly induced upon flagellin stimulation (P<0.05 and
P<0.001) (Fig. 4B).
Discussion
MSCs can differentiate into mesodermal cells,
including bone, cartilage, adipocyte and connective stromal cells.
Furthermore, it was evidenced that MSCs differentiate into not only
ectodermal lineages, including neurons and epithelial cells, but
also endodermal lineages, including hepatocytes and muscle cells
(14). The immune phenotypes of
MSCs are positive expression of CD29, CD59, CD90 and CD105, but
lack of expression of CD14, CD19, CD31 and CD34, and specifically,
negative expression of the co-stimulators CD80, CD86 and HLA-E
(15). MSCs also possess
immunomodulatory properties, which enables them to suppress the
activation and proliferation of T- and B-lymphocyte-mediated immune
responses, as well as to interfere with the maturation and function
of dendritic cells and natural killer cells (16). However, accumulating evidence
indicated that the in vivo microenvironment is able to
modify the low immune status of MSCs to finally result in an
enhanced immune response (7,8,9).
The TLR family is the most important class of
signaling molecules associated with pathogen-associated molecular
patterns and has a critical function in bridging innate and
adaptive immune responses. In addition, TLR ligands have been
linked with the perpetuation of inflammation in a number of chronic
inflammatory diseases due to the permanent presentation of the
immune system with TLR-specific stimuli (17). A previous study indicated that the
activation of numerous TLR-associated pathways did not alter the
immune status of MSCs (11);
however, the study used MSCs isolated from BM. In recent years, the
human UC has attracted increasing attention due to its increased
efficiency with regard to the expansion, proliferation and
differentiation potential of its MSCs. UCMSCs and BMMSCs show
marked similarities in metabolic, biological regulatory and
multicellular processes, although there are also differences
between BMMSCs and UCMSCs, with the predominant genes in UCMSCs
including neurogenesis transcriptional factors such as sex
determining region Y-box 11 and paired-like homeodomain 1 (18).
Our group has investigated the role of TLRs in
influencing the immune status of UCMSCs and has shown that a TLR7
agonist enhanced the immunogenicity of UCMSCs (19). The present study assessed the role
of TLR5 in regulating the immune status and differentiation ability
of UCMSCs. Among all TLR members, TLR5 locates on the cell surface,
is responsible for the detection of flagellin and specifically
recognizes the constant domain D1. The results of the present study
indicated that activation of the TLR5 pathway did not increase the
proliferation of PBMCs or the release of LDH in a PBMC-UCMSC
co-culture system in the presence of flagellin. Assessment of
co-stimulatory molecules also showed that these were not affected
by TLR5 activation. Furthermore, TLR5 had no influence or inductive
effects on the differentiation ability of UCMSCs into adipocytes,
osteoblasts or chondrocytes. These results indicated that
activation of TLR5 did not affect the immune status and
differentiation ability of UCMSCs. However, the present study
detected an increased expression of pro-inflammatory molecules in
UCMSCs upon flagellin treatment, which may indicate that the
induced immune-associated factors have biological functions in
UCMSCs other than those assessed in the present study, which will
be addressed in a future study.
Acknowledgments
The present study was supported by grants from the
National Natural Scientific Foundation of China (no. 81200315) and
the China Postdoctoral Science Foundation (nos. 2011M501413 and
2013T60855).
References
|
1
|
Bordignon C, Carlo-Stella C, Colombo MP,
De Vincentiis A, Lanata L, Lemoli RM, Locatelli F, Olivieri A,
Rondelli D, Zanon P and Tura S: Cell therapy: Achievements and
perspectives. Haematologica. 84:1110–1149. 1999.PubMed/NCBI
|
|
2
|
Phinney DG and Prockop DJ: Concise review:
Mesenchymal stem/multipotent stromal cells: The state of
transdifferentiation and modes of tissue repair-current views. Stem
Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bassi E, Aita CA and Câmara NO: Immune
regulatory properties of multipotent mesenchymal stromal cells:
Where do we stand? World J Stem Cells. 3:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi M, Liu ZW and Wang FS:
Immunomodulatory properties and therapeutic application of
mesenchymal stem cells. Clin Exp Immunol. 164:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui J, Wahl RL, Shen T, Fisher SJ, Recker
E, Ginsburg D and Long MW: Bone marrow cell trafficking following
intraveneous administration. Br J Haematol. 107:895–902
|
|
6
|
Ankrum J and Karp JM: Mesenchymal stem
cell therapy: Two steps forward, one step back. Trends Mol Med.
16:203–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang XP, Sun Z, Miyagi Y, McDonald
Kinkaid H, Zhang L, Weisel RD and Li RK: Differentiation of
allogeneic mesenchymal stem cells induces immunogenicity and limits
their long-term benefits for myocardial repair. Circulation.
122:2419–2429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nauta AJ, Westerhuis G, Kruisselbrink AB,
Lurvink EG, Willemze R and Fibbe WE: Donor-derived mesenchymal stem
cells are immunogenic in an allogeneic host and stimulate donor
graft rejection in a nonmyeloablative setting. Blood.
108:2114–2120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y and Lin F: Mesenchymal stem cells are
injured by complement after their contact with serum. Blood.
120:3436–3443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blasius AL and Beutler B: Intracellular
toll-like receptors. Immunity. 32:305–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DelaRosa O and Lombardo E: Modulation of
adult mesenchymal stem cells activity by toll-like receptors:
Implications on therapeutic potential. Mediators Inflamm.
2010:8656012010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng Y and Zhang L: Activation of TLR1/2
pathway induced the shaping of immune response status of peripheral
blood leukocytes. Exp Ther Med. 7:1708–1712. 2014.PubMed/NCBI
|
|
13
|
Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM,
Chou SC, Shih YH, Ko MH and Sung MS: Conversion of human umbilical
cord mesenchymal stem cells in Wharton's jelly to dopaminergic
neurons in vitro: Potential therapeutic application for
parkinsonism. Stem Cells. 24:115–124. 2006. View Article : Google Scholar
|
|
14
|
Tolar J, LeBlanc K, Keating A and Blazar
BR: Concise review: Hitting the right spot with mesenchymal stromal
cells. Stem cells. 28:1446–1455. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Augello A, Kurth TB and DeBari C:
Mesenchymal stem cells: A perspective from in vitro cultures to in
vivo migration and niches. Eur Cell Mater. 20:121–133. 2010.
|
|
16
|
Han KH, Ro H, Hong JH, Lee EM, Cho B, Yeom
HJ, Kim MG, Oh KH, Ahn C and Yang J: Immunosuppressive mechanisms
of embryonic stem cells and mesenchymal stem cells in alloimmune
response. Transpl Immunol. 25:7–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tomchuck SL, Zwezdaryk KJ, Coffel SB,
Waterman RS, Danka ES and Scandurro AB: Toll-like receptors on
human mesenchymal stem cells drive their migration and
immuno-modulating responses. Stem Cells. 26:99–107. 2008.
View Article : Google Scholar
|
|
19
|
Zhang L, Liu D, Pu D, Wang Y, Li L, He Y,
Li Y, Li L and Li W: The TLR7 agonist Imiquimod promote the
immunogenicity of mesenchymal stem cells. Biol Res. 48:62015.
View Article : Google Scholar : PubMed/NCBI
|