Introduction
Glioma is the most common type of malignancy in the
central nervous system (1),
accounting for more than half of all intracranial tumors, with an
incidence rate of 6.5/100,000 individuals (2) and an average survival duration of 14
months (3). The five-year survival
rate of malignant glioma is <5% (4). Glioma is characterized by rapid
growth, high levels of invasiveness, frequent postoperative
recurrence and high mortality rates. Although the range of
comprehensive treatments for glioma, including surgical resection,
radiotherapy and chemotherapy, have been improving, the therapeutic
effect remains inadequate (5,6).
Therefore, clarifying the mechanisms of glioma invasion and
metastasis, and determining effective treatment methods, remains a
focus of investigation.
Translationally controlled tumor protein (TCTP) is a
highly conserved hydrophilic protein, which was identified in 1989
(7). The expression levels of TCTP
in a variety of tumor cells are markedly higher, compared with
those in normal tissues (8–11).
Previous studies have indicated that TCTP is important in the
regulation of cell proliferation, cell cycle, malignant metastasis
and anti-apoptotic effects (12–16).
Additionally, TCTP is associated with the development and
progression of glioma (17). In
vitro and in vivo experiments have demonstrated that
abnormally high expression levels of TCTP in glioma cells can
promote cell proliferation, and that this promotion of
proliferation can be eliminated by downregulation of the expression
of TCTP expression (18). The
expression of TCTP is also closely associated with tumor
deterioration and the sensitivity of tumor cells to drugs (19). The over-expression of TCTP in cells
has been observed to significantly inhibit 5-fluorouracil
(5-Fu)-induced apoptosis of ovarian cancer and osteosarcoma cells.
Following silencing of the expression of TCTP using an antisense
oligonucleotide, the sensitivity of U2OS osteosarcoma cells to 5-Fu
is enhanced, and the apoptotic rate is significantly increased
(20,21). However, the role of TCTP in the
occurrence and development of glioma remains to be fully elucidated
and further investigation is required.
In the present study, the expression of TCTP in
glioma cells was downregulated using RNAi to investigate its
effects on the proliferation, apoptosis, metastasis and invasion of
the glioma cells, and to examine the associated mechanisms. This
investigation suggested that TCTP may be a potential target for the
treatment of glioma.
Materials and methods
Cell lines
The U251, A172, U87-MG and SHG-44 human glioma cell
lines were purchased from the Shanghai Institute of Biological
Sciences, Chinese Academy of Sciences (Shanghai, China). The U373
cells were purchased from American Type Culture Collection (ATCC,
Manassas, VA, USA). The U251, U373, A172 and U87-MG cells were
cultured in Dulbecco's Modified Eagle Medium (DMEM, Gibco Life
Technologies, Grand Island, NY, USA) containing 10% fetal bovine
serum (FBS, GE Healthcare, Logan, UT, USA) at 37°C and 5%
CO2. The SHG-44 cells were cultured in RPMI-1640 medium
(Gibco Life Technologies) containing 15% FBS (GE Healthcare) at
37°C and 5% CO2.
Construction of a TCTP short hairpin
(sh)RNA eukaryotic expression plasmid and screening for a stably
transfected cell line
The TCTP interfering sequences were designed,
according to the TCTP mRNA sequence in GenBank using shRNA
designing software, as shown in Table
1. The obtained interfering sequences (Wanleibio, Shenyang,
China) were ligated into the pRNA-H1.1 eukaryotic expression vector
(GenScript, Nanjing, China) using the restriction sites of
HindIII and BamHI, and the resulting plasmid was
termed pRNA-H1.1-TCTP. U251 cells in the logarithmic growth phase
were seeded into 6-well plates. pRNA-H1.1-TCTP was transfected into
the U251 cells using Lipofectamine 2000, according to the
manufacturer's instructions (Invitrogen Life Technologies,
Carlsbad, CA, USA). After 24 h, complete DMEM, containing 400
µg/ml G418 (Invitrogen Life Technologies) was added into
each well for screening for 7–14 days, and the clones exhibiting
positive expression of TCTP were selected and identified by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting. In the process of the experiment, an
untransfected group (parental) and an empty vector-transfected
group (pRNA-H1.1-control) were set up as the controls.
 | Table IshRNA sequences. |
Table I
shRNA sequences.
| Primer | Sequence
(5′–3′) |
|---|
| TCTP shRNA |
| Forward |
GATCCCCGATGGTCAGTAGGACAGAATTCAAGAGATTCTGTCCTACTGACCATCTTTTT |
| Reverse |
AGCTAAAAAGATGGTCAGTAGGACAGAATCTCTTGAATTCTGTCCTACTGACCATCGGG |
| Control shRNA |
| Forward |
GATCCCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTT |
| Reverse |
AGCTAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAGGG |
Western blot analysis
Following lysis of the cells using NP-40 lysis
buffer (Beyotime Institute of Biotechnology, Shanghai, China), the
total cellular proteins were extracted, and the protein
concentrations were determined using a Bicinchoninic Acid Protein
Assay kit (Beyotime Institute of Biotechnology). Equal quantities
of proteins (40 µg) were subjected to 10 or 12% SDS-PAGE,
and the proteins were then transferred onto a polyvinylidene
difluoride membrane (EMD Millipore, Bedford, MA, USA), followed by
incubation overnight at 37°C with rabbit anti-human poly clonal
antibodies against TCTP antibody (1:200; cat. no. sc-133131; Santa
Cruz biotechnology Inc., Santa Cruz, CA, USA), cyclin D1 antibody
(1:1,000; cat. no. WL0205), cyclin E antibody (1:1,000; cat. no.
WL0055), cyclin B antibody (1:1,000; cat. no. WL0023), Bax
polyclonal antibody (1:1,000; cat. no. WL0101), Bcl-2 antibody
(1:1,000; cat. no.WL0104), cleaved-caspase-3 antibody (1:1,000;
cat. no. WL0146), MMP-2 antibody (1:1,000; cat. no. WL0657), MMP-9
antibody (1:1,000; cat. no. WL0884) or β-actin antibody (1:1,000;
all from Wanleibio, Shenyang, China) at 4°C. Subsequently, the
membrane was incubated with the corresponding horseradish
peroxidase-labeled goat anti-rabbit IgG secondary antibody at a
1:5,000 dilution (Beyotime Institute of Biotechnology) for 45 min
at 37°C. Signals were detected using enhanced chemiluminescence
(ECL) solution (Qihai Biotec, Shanghai, China) and the band
intensities were normalized against those of β-actin using
Gel-Pro-Analyzer version 4.0 software (Media. Cybernetics, Inc.,
Bethesda, MD, USA).
RT-qPCR
Total cellular RNA was extracted from the cells in
each group, strictly according to the instructions of the total RNA
extraction kit (Tiangen Biotech, Co., Ltd., Beijing, China). The
cDNA (20 µl) was obtained by RT using M-MLV Reverse
Transcriptase (BioTeke, Beijing, China), and quantitative
fluorescence analysis was performed using SYBR Green MasterMix (10
µl; Solarbio, Beijing, China) in an Exicycler™ 96
quantitative fluorescence analyzer (Bioneer, Daejeon, Korea). The
concentration of each primer was 10 µM. The reaction
conditions were as follows: 95°C for 10 min; 95°C for 10 sec, 60°C
for 20 sec and 72°C for 30 sec, for a total of 40 cycles. With
β-actin as the internal control, the 2−ΔΔCt method
(22) was used to analyze the mRNA
expression levels of TCTP. The sequences of the primers used are
listed in Table II.
 | Table IISequences of the primers used for
quantitative polymerase chain reaction. |
Table II
Sequences of the primers used for
quantitative polymerase chain reaction.
| Primer | Sequence
(5′–3′) |
|---|
| TCTP |
| Forward |
GCCGTCGTCGTCTCCCTTCA |
| Reverse |
ACCCGTCCGCGATCTCCCG |
| β-actin |
| Forward |
CTTAGTTGCGTTACACCCTTTCTTG |
| Reverse |
CTGTCACCTTCACCGTTCCAGTTT |
MTT assay for cell proliferation
Cells in the logarithmic growth phase were harvested
and seeded into 96-well plates at a density of 3×103
cells/well. Following culture of the cells for 12, 24, 48, 72 and
96 h, MTT solution (final concentration, 0.2 mg/ml; Sigma-Aldrich,
St. Louis, MO, USA) was added into the each well. The cells were
continuously cultured at 37°C for 4 h, and the supernatant was then
discarded. The purple crystals were dissolved using 200 µl
dimethyl sulfoxide (Sigma-Aldrich), and the optical density
(OD490) values were measured using a microplate reader
(ELX-800; Bio-Tek Instruments Inc, Winooski, VT, USA). A cell
growth curve was plotted and statistical analysis was
performed.
Colony formation assay
The cells in each group were seeded (200 cells/dish)
into a 35 mm petri-dish for culture. Subsequent to the formation of
colonies, 4% paraformaldehyde (Guoyao, Shenyang, China) was used to
fix the cells for 20 min, followed by Wright-Giemsa staining
(Jiancheng Bioengineering Institute, Nanjing, China) for 5–8 min.
The number of colonies, defined as a cell group with ≥50 cells,
were counted and recorded under an inverted microscope (AE31; Motic
Electric, Xiamen, China). The colony formation rate was then
calculated according to the following formula: Colony formation
rate = (number of colonies / number of seeded cells) × 100%.
Detection of the cell cycle using flow
cytometry
The cells were cultured in DMEM medium and were
harvested on reaching ~90% confluence. The cell cycle was detected
using a flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA,
USA) using a Cell Cycle Analysis kit (Beyotime Institute of
Biotechnology). In brief, the cells were harvested, washed with
phosphate-buffered saline (PBS), and fixed with pre-cooled 70%
ethanol at 4°C for 2 h. The fixed cells were then resus-pended in
500 µl binding buffer, containing 25 µl propidium
iodide (PI) and 10 µl RNase A, at 37°C in the dark for 30
min, and detected using flow cytometry.
Hoechst staining
A Hoechst staining kit (Beyotime Institute of
Biotechnology) was used to observe the apoptotic nuclear morphology
of the cells. Briefly, cells in the logarithmic growth phase were
seeded onto cell slides in 12-well plates, at a density of
1×105 cells per well, and were cultured for 24 h. Then,
the supernatant was discarded. The cells were fixed with 0.5 ml
fixing solution at room temperature for 20 min and were then
treated with Hoechst staining solution for 5 min. Anti-fluorescence
quenching mounting solution (Beyotime Institute of Biotechnology)
was added dropwise onto the slide, which was then covered with the
coverslip containing the cells. The cells were visualized and
images were captured under a fluorescence microscope (Olympus BX61;
Olympus Corporation, Tokyo, Japan).
Detection of apoptosis using an Annexin
V-FITC/PI assay
The cells were seeded into T25 cell culture flasks.
When they had reached 90% confluence, the cells were harvested. The
cells were resuspended in 500 µl Binding Buffer, according
to the instructions of the apoptosis detection kit (KeyGEN Biotech,
Co., Ltd., Nanjing, China). 5 µl Annexin V-FITC and 5
µl PI were immediately added with thorough mixing. Following
15 min incubation at 37°C in the dark, the cells were detected
using flow cytometry (FACSCalibur; BD Biosciences).
Wound healing assay
The cells in each group were seeded into 6-well
plates for culture. At a confluence of 80–90%, the culture medium
was discarded. Subsequently, a scratch was created on the cell
layer using a sterile 200 µl pipette tip. Images of the
cells in each group were captured (AE31; Motic Electric) following
being washed twice with serum-free medium. After 12 and 24 h of
continuous culture, the migration distances of the cells were
measured, and the cell migration rates were calculated using the
following formula: Cell migration rate = (1−distance following
healing / distance prior to healing) × 100%. Each experiment was
repeated three times.
Transwell assay
Following harvesting, 200 µl cell suspension
containing 2×104 cells (resuspended in serum-free
culture medium) were seeded in upper Transwell chambers (Corning
Incorporated Life Sciences, Tewksbury, MA, USA), which were coated
with Matrigel (BD Biosciences), while 800 µl DMEM medium
containing 30% FBS was added to the lower chamber. Following
culture for 24 h, the uninvaded cells on the upper side of the
microporous membrane were removed using a cotton swab. The
remaining cells were fixed with 4% paraformaldehyde and stained
with 0.5% crystal violet staining solution (Amresco, Solon, OH,
USA). The numbers of invaded cells were recorded under an inverted
microscope (AE31; Motic Electric) in five randomly-selected fields
(magnification, ×200), to obtain the average.
Statistical analysis
The data are presented as the mean ± standard
deviation. One-way analysis of variance was used for comparisons
between groups, and Bonferroni's post hoc-test was used for the
comparison of multiple variables. The image and data were processed
using Graphpad Prism 5.0 software (GraphPad Software, Inc., San
Diego, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Establishing and identifying the glioma
cell line stably transfected with TCTP shRNA
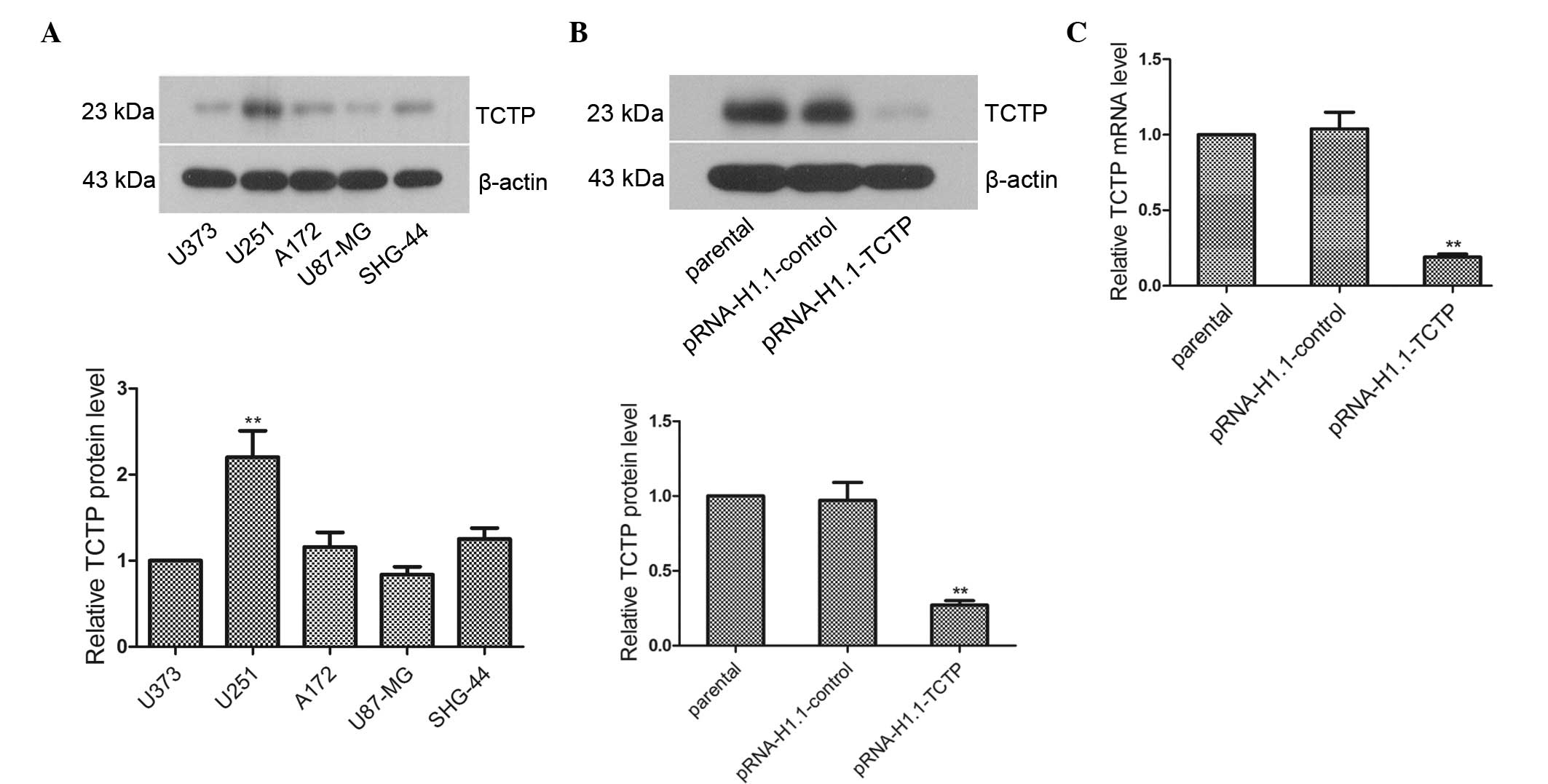
In the present study, total proteins were extracted
from the U373, U251, A172, U87-MG and SHG-44 cells to analyze the
expression of TCTP in these cells. The results of the western
blotting revealed that the expression levels of TCTP in the A172,
U87-MG and SHG-44 cells were essentially the same as that in the
U373 cells (Fig. 1A; P>0.05),
while the level of expression in the U251 cells was significantly
higher than that in the U373 cells (P<0.01). Thus, U251 cells
with high expression levels of TCTP were selected to investigate
TCTP gene function. The TCTP shRNA eukaryotic expression plasmid,
pRNA-H1.1-TCTP, was subsequently transfected into the U251 cells.
Western blot and RT-qPCR analyses revealed that the protein and
mRNA expression levels of TCTP in the pRNA-H1.1-TCTP group were
significantly decreased, and were 0.28-fold (Fig. 1B; P<0.01) and 0.18-fold
(Fig. 1C; P<0.01) lower than
that in the pRNA-H1.1-control group, respectively. Thus, a U251
glioma cell line stably transfected with TCTP shRNA was
successfully established.
Downregulation of the expression of TCTP
suppresses glioma cell proliferation
The impact of the downregulated expression of TCTP
on glioma cell proliferation was investigated. The MTT results
demonstrated that at 48, 72 and 96 h, cell proliferation in the
pRNA-H1.1-TCTP group was significantly lower, compared with that in
the pRNA-H1.1-control group (Fig.
2A; P<0.05). The effect of downregulated expression of TCTP
on the clonogenic capacity of glioma cells, detected using a colony
formation assay, is shown in Fig.
2B. The colony formation rate of the cells in the
pRNA-H1.1-TCTP group was significantly lower than that in the
pRNA-H1.1-control group (32.0±4.9, vs. 60.3±6.9%, respectively;
P<0.01). The present study further detected the cell cycle
distribution using flow cytometry. The percentage of G2/M phase
cells in the pRNA-H1.1-TCTP group was significantly reduced,
compared with that in the pRNA-H1.1-control group (0.56±0.08, vs.
8.97±0.94)%, respectively; Fig.
2C; P<0.01). The percentage of S phase cells was essentially
unchanged (26.17±2.48, vs. 27.00±2.23%), while the percentage of
G0/G1 phase cells was significantly increased (73.27±2.47, vs.
63.03±2.11%; P<0.01). Western blotting revealed that the
expression levels of Cyclin D1, Cyclin E and Cyclin B in the
pRNA-H1.1-TCTP cells were significantly reduced (Fig. 2D; P<0.01). These results
suggested that downregulation of the expression of TCTP caused cell
cycle arrest in the G0/G1 phase and ultimately inhibited glioma
cell proliferation.
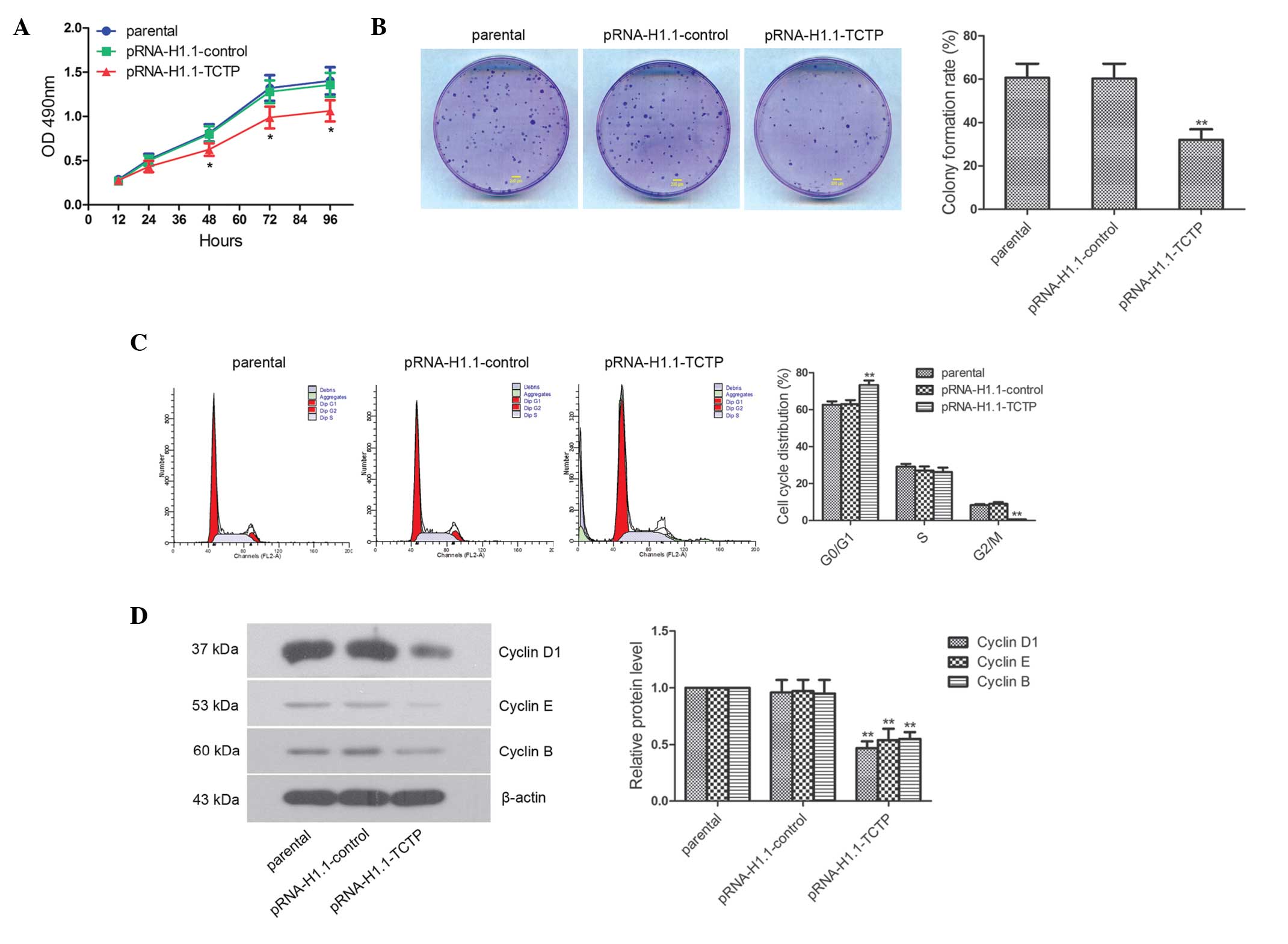 | Figure 2Downregulated expression of TCTP
effectively inhibits glioma cell proliferation. (A) Cells of the
three groups were seeded in 96-well plates, with five duplicate
wells for each setting, and the OD490 values 12, 24, 48,
72 and 96 hafter seeding were detected using an MTT assay. (B)
Clonogenic capacity of each group was measured, and a
representative image of three repeated experiments are shown (scale
bar, 200 µm). (C) Cell cycle was detected using flow
cytometry, and the representative result of three repeated
experiments are shown. (D) Expression levels of Cyclin D1, Cyclin E
and Cyclin B were detected by western blot analysis. The data are
presented as the mean ± standard deviations. *P<0.05
and **P<0.01, compared with the pRNA-H1.1-control
group. TCTP, translationally controlled tumor protein; shRNA, short
hairpin ribonucleic acid; OD, optical density. |
Downregulation of the expression of TCTP
effectively induces the apoptosis of brain glioma cells
The apoptosis and expression levels of
apoptosis-associated factors were analyzed using flow cytometry and
Hoechst staining. The Hoechst staining revealed that the nuclei of
the cells in the parental group and pRNA-H1.1-control group were
homogeneous blue. Typical apoptotic morphological changes,
including condensed chromatin and shrunken, crumpled and condensed
nuclei, were observed in the pRNA-H1.1-TCTP group. The apoptotic
rate of the cells in the pRNA-H1.1-TCTP group was significantly
higher than that in the pRNA-H1.1-control group (29.6±3.03, vs.
6.36±0.79%, respectively; Fig. 3A;
P<0.01). Flow cytometry consistently indicated that the
apoptotic rate of the cells in the pRNA-H1.1-TCTP group was
significantly higher than that in the pRNA-H1.1-control group
(24.60±2.68, vs. 6.88±0.86%, respectively; Fig. 3B; P<0.01). Western blot analysis
demonstrated that the expression levels of Bax and
cleaved-caspase-3 in the cells in the pRNA-H1.1-TCTP group were
significantly increased (Fig. 3C;
P<0.01), whereas the expression of Bcl-2 was significantly
decreased (P<0.01). These results suggested that downregulation
of the expression of TCTP effectively induced apoptosis in the
glioma cells.
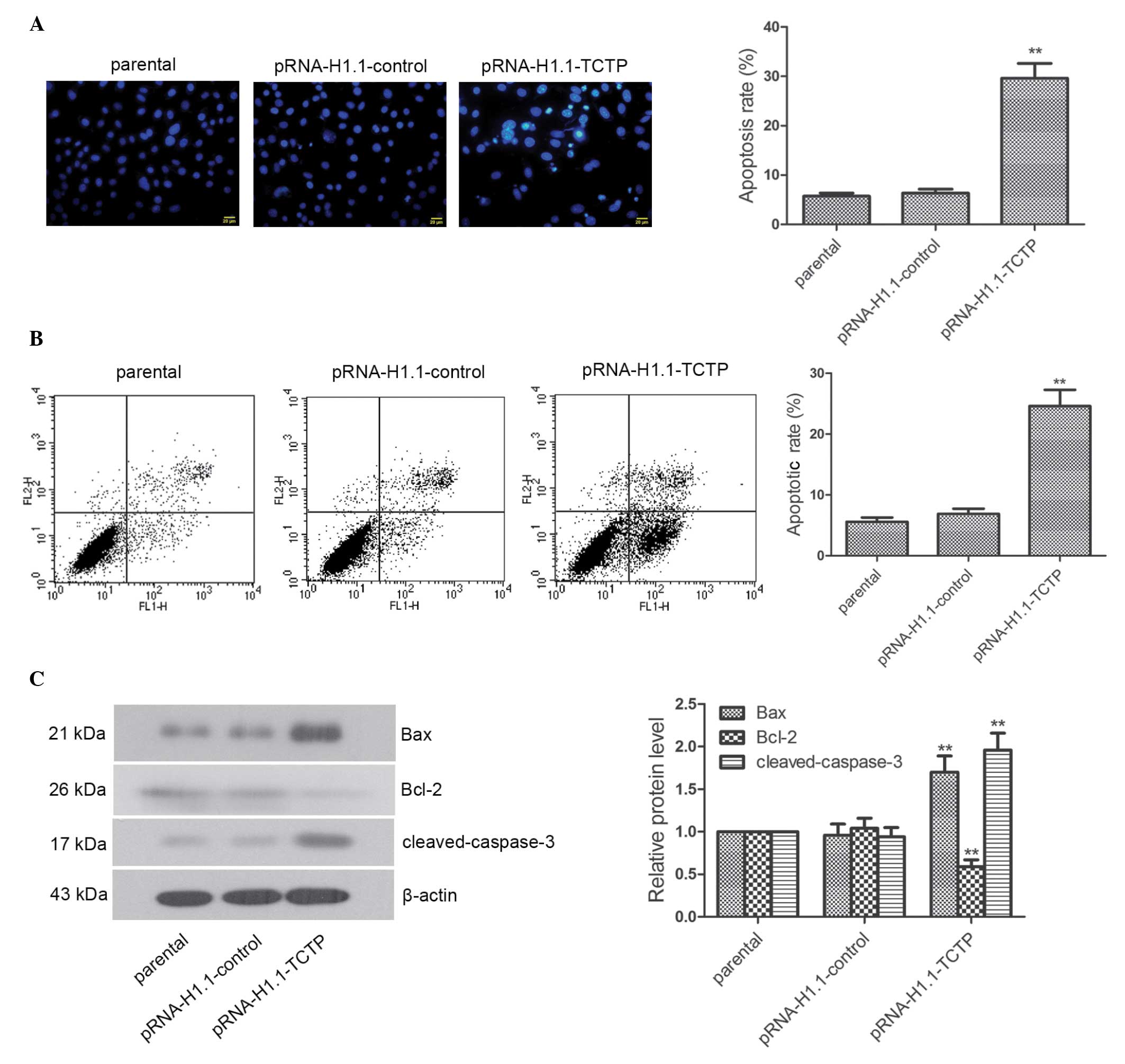 | Figure 3Downregulated expression of TCTP
significantly induces apoptosis in glioma cells. (A) Following the
downregulation of TCTP, Hoechst staining was performed and
apoptosis was observed under a fluorescence microscope. The nuclei
of the living cells were homogeneous blue. However, typical
apoptotic morphological changes were observed, including condensed
chromatin and shrunken, crumpled and condensed nuclei (scale bar,
20 µm). (B) Apoptosis was determined using flow cytometry,
and a representative result of the repeated experiments is shown.
(C) Expression levels of Bax, Bcl-2, and cleaved-caspase-3 were
detected using western blot analysis, with β-actin as the internal
control for grayscale analysis. The data are presented as the mean
± standard deviations. **P<0.01, compared with the
pRNA-H1.1-control group. TCTP, translationally controlled tumor
protein; Bax, B-cell-associated X protein; Bcl-2, B-cell
lymphoma-2. |
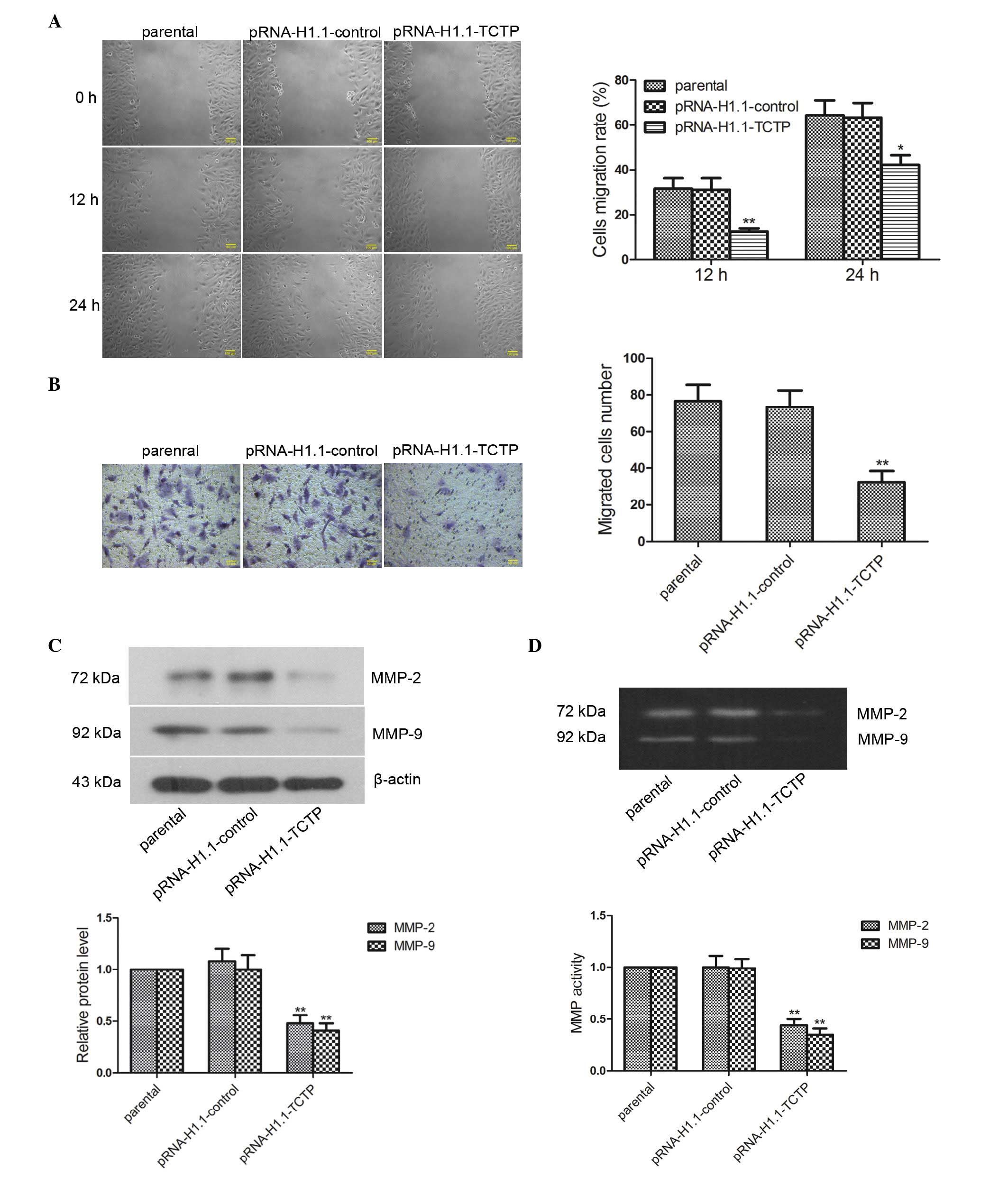
Downregulation of the expression of TCTP
inhibits the migration and invasion of glioma cells
The present study investigated the impact of
downregulated expression of TCTP on glioma cell migration. The cell
migration rate of the cells was examined using a wound healing
assay, the results of which revealed that the migration rate of the
cells in the pRNA-H1.1-TCTP group 12 h (Fig. 4A; P<0.01) and 24 h (P<0.05)
post-wounding were significantly lower than those in the
pRNA-H1.1-control group. The effect of TCTP shRNA on the
invasiveness of the glioma cells was further detected using a
Transwell assay, the results of which revealed that the number of
invaded cells in the pRNA-H1.1-TCTP group was significantly lower
than that in the pRNA-H1.1-control group (32.40±6.07, vs.
73.40±9.04), respectively; Fig.
4B; P<0.01). Western blot analysis revealed that the protein
expression levels of MMP-2 and MMP-9 in the cells in the
pRNA-H1.1-TCTP group were significantly lower than those in the
pRNA-H1.1-control group (Fig. 4C;
P<0.01). Gelatin zymography revealed bands at 72 and 92 kDa
(corresponding to MMP-2 and MMP-9, respectively) in the cells of
all groups. Additionally, the activities of MMP-2 and MMP-9 in the
cells of the pRNA-H1.1-TCTP group were significantly decreased
(Fig. 4D; P<0.01). These
results demonstrated that downregulation of the expression of TCTP
inhibited glioma cell migration and invasion.
Discussion
TCTP is widely expressed in several types of tumor
cell and is important in cell cycle regulation, malignant
metastasis and anti-apoptosis (23). However, its role in the malignant
metastasis of gliomas remains to be fully elucidated. In the
present study, the expression levels of TCTP in various glioma cell
lines were detected using western blotting. To investigate the role
of TCTP on glioma cell proliferation, cell cycle regulation,
apoptosis and invasion, and the associated mechanism, high
expression levels of TCTP in the U251 cells was down-regulated
using shRNA.
An important difference between cancer cells and
normal cells is that the growth and division of cancer cells is out
of control, and the mechanism of programmed cell death is lacking.
The overexpression and downregulation of TCTP can affect tumor cell
proliferation (24,25). Studies have reported that hepatoma
cell proliferation is significantly inhibited and cell cycle is
arrested in the G0/G1 phase following silencing of TCTP using an
antisense oligonucleotide (26),
and the arrest of the cell cycle is considered to be a predominant
reason for the inhibition of tumor cell growth (27–29).
The present study investigated the impact of downregulated
expression of TCTP on glioma cell proliferation and the cell cycle.
The results demonstrated significantly decreased cell proliferation
following downregulation of TCTP. Flow cytometric analysis
determined that the cell cycle was arrested in the G0/G1 phase and
that the expression levels of Cyclin D1, Cyclin E and Cyclin B in
the cells were significantly lower, compared with those in the
control group. This indicated that TCTP regulated the cell cycle by
regulating the expression of cell cycle proteins, thereby promoting
glioma cell proliferation, which is consistent with the results of
previous studies (24–26).
It has been demonstrated that adenovirus
vector-driven overexpression of TCTP can effectively resist
apoptosis in HeLa cells through the mitochondrial pathway, induced
by the chemotherapy drug etoposide, while the activities of
cytochrome c, caspase-3 and caspase-9 are simultaneously
inhibited (30). TCTP can anchor
to the mitochondrial membrane to inhibit Bax dimerization, thereby
exerting an anti-apoptotic effect through the inhibition of
mitochondrial injury (31).
Following silencing of the expression of TCTP using a small
interfering RNA in breast cancer, squamous cell carcinoma, prostate
cancer and lung cancer cells, tumor cell proliferative capability
has been observed to decrease, and apoptosis significantly increase
(20,32–34).
The results of the present study also demonstrated that, following
downregulation of the expression of TCTP in glioma cells, the
expression levels of Bax and cleaved-caspase-3 increased, while the
expression of Bcl-2 decreased, suggesting that high expression
levels of TCTP in glioma cells may exert an anti-apoptotic role by
inhibiting Bax and cleaved-caspase-3, and activating Bcl-2, thereby
promoting glioma onset and progression.
The metastasis and invasion of tumors is closely
associated with their degree of malignancy. MMP-2 and MMP-9 can
degrade a variety of extracellular matrices to function in tumor
metastasis and invasion (35),
which are important in tumor metastasis (36). Previous studies have demonstrated
that, following downregulation of the expression of TCTP in colon
cancer cells, the abilities of cell proliferation, migration and
invasiveness also decrease (37,38).
The present study also observed that TCTP is closely associated
with glioma cell migration and invasion, as downregulation of TCTP
significantly inhibited glioma cell migration and invasion. In
addition, the expression and activity levels of MMP-2 and MMP-9
were significantly decreased. These results indicated that TCTP may
facilitate glioma cell migration and invasion through MMP-2 and
MMP-9.
In conclusion, shRNA-mediated downregulation of the
expression of TCTP in brain glioma cells effectively inhibited
glioma cell proliferation, promoted apoptosis and inhibited cell
migration and invasion. These findings demonstrate the importance
of TCTP in the regulation of proliferation, cell cycle, apoptosis
and invasion of glioma cells, and offer preliminary evidence for
the mechanism underlying its interaction with cell cycle proteins,
MMPs and downstream apoptosis-associated factors, and provide a
theoretical basis and experimental evidence for using TCTP as a
target for gene therapy in glioma.
References
|
1
|
Fine HA, Dear KB, Loeffler JS, Black PM
and Canellos GP: Meta-analysis of radiation therapy with and
without adjuvant chemotherapy for malignant gliomas in adults.
Cancer. 71:2585–2597. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu X, Li Y, Wan X, Kayira TM, Cao R, Ju X,
Zhu X and Zhao G: Down-regulation of neogenin accelerated glioma
progression through promoter Methylation and its overexpression in
SHG-44 Induced Apoptosis. PLoS One. 7:e380742012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turbyville TJ, Gürsel DB, Tuskan RG,
Walrath JC, Lipschultz CA, Lockett SJ, Wiemer DF, Beutler JA and
Reilly KM: Schweinfurthin A selectively inhibits proliferation and
Rho signaling in glioma and neurofibromatosis type 1 tumor cells in
a NF1-GRD-dependent manner. Mol Cancer Ther. 9:1234–1243. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohgaki H and Kleihues P: Epidemiology and
etiology of gliomas. Acta Neuropathol. 109:93–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwartzbaum JA, Fisher JL, Aldape KD and
Wrensch M: Epidemiology and molecular pathology of glioma. Nat Clin
Pract Neurol. 2:494–503. 2006.quiz 491 p following 516. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gross B, Gaestel M, Böhm H and Bielka H:
cDNA sequence coding for a translationally controlled human tumor
protein. Nucleic Acids Res. 17:83671989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chung S, Kim M, Choi W, Chung J and Lee K:
Expression of translationally controlled tumor protein mRNA in
human colon cancer. Cancer Lett. 156:185–190. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bazile F, Pascal A, Arnal I, Le Clainche
C, Chesnel F and Kubiak JZ: Complex relationship between TCTP,
microtubules and actin microfilaments regulates cell shape in
normal and cancer cells. Carcinogenesis. 30:555–565. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang F, Liu B, Wang Z, Yu XJ, Ni QX, Yang
WT, Mukaida N and Li YY: A novel regulatory mechanism of Pim-3
kinase stability and its involvement in pancreatic cancer
progression. Mol Cancer Res. 11:1508–1520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu LK, Wu HF, Guo ZR, Chen XJ, Yang D,
Shu YQ and Zhang JN: Targeted efficacy of dihydroartemisinin for
transla-tionally controlled protein expression in a lung cancer
model. Asian Pac J Cancer Prev. 15:2511–2515. 2014. View Article : Google Scholar
|
|
12
|
Cans C, Passer BJ, Shalak V,
Nancy-Portebois V, Crible V, Amzallag N, Allanic D, Tufino R,
Argentini M, Moras D, et al: Translationally controlled tumor
protein acts as a guanine nucleotide dissociation inhibitor on the
translation elongation factor eEF1A. Proc Natl Acad Sci USA.
100:13892–13897. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gachet Y, Tournier S, Lee M,
Lazaris-Karatzas A, Poulton T and Bommer UA: The growth-related,
translationally controlled protein P23 has properties of a tubulin
binding protein and associates transiently with microtubules during
the cell cycle. J Cell Sci. 112:1257–1271. 1999.PubMed/NCBI
|
|
14
|
Liu H, Peng HW, Cheng YS, Yuan HS and
Yang-Yen HF: Stabilization and enhancement of the antiapoptotic
activity of mcl-1 by TCTP. Mol Cell Biol. 25:3117–3126. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagano-Ito M and Ichikawa S: Biological
effects of Mammalian translationally controlled tumor protein
(TCTP) on cell death, proliferation and tumorigenesis. Biochem Res
Int. 2012:2049602012. View Article : Google Scholar
|
|
16
|
Li F, Zhang D and Fujise K:
Characterization of fortilin, a novel antiapoptotic protein. J Biol
Chem. 276:47542–47549. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miao X, Chen YB, Xu SL, Zhao T, Liu JY, Li
YR, Wang J, Zhang J and Guo GZ: TCTP overexpression is associated
with the development and progression of glioma. Tumour Biol.
34:3357–3361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu X, Yao L, Ma G, Cui L, Li Y, Liang W,
Zhao B and Li K: TCTP promotes glioma cell proliferation in vitro
and in vivo via enhanced β-catenin/TCF-4 transcription. Neuro
Oncol. 16:217–227. 2014. View Article : Google Scholar :
|
|
19
|
Acunzo J, Baylot V, So A and Rocchi P:
TCTP as therapeutic target in cancers. Cancer Treat Rev.
40:760–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lucibello M, Gambacurta A, Zonfrillo M,
Pierimarchi P, Serafino A, Rasi G, Rubartelli A and Garaci E: TCTP
is a critical survival factor that protects cancer cells from
oxidative stress-induced cell-death. Exp Cell Res. 317:2479–2489.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Graidist P, Phongdara A and Fujise K:
Antiapoptotic protein partners fortilin and MCL1 independently
protect cells from 5-fluorouracil-induced cytotoxicity. J Biol
Chem. 279:40868–40875. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Chan TH, Chen L and Guan XY: Role of
translationally controlled tumor protein in cancer progression.
Biochem Res Int. 2012:3693842012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tuynder M, Fiucci G, Prieur S, Lespagnol
A, Géant A, Beaucourt S, Duflaut D, Besse S, Susini L, Cavarelli J,
et al: Translationally controlled tumor protein is a target of
tumor reversion. Proc Natl Acad Sci USA. 101:15364–15369. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen SH, Wu PS, Chou CH, Yan YT, Liu H,
Weng SY and Yang-Yen HF: A knockout mouse approach reveals that
TCTP functions as an essential factor for cell proliferation and
survival in a tissue-or cell type-specific manner. Mol Biol Cell.
18:2525–2532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu WL, Cheng HX, Han N, Liu DL, Zhu WX,
Fan BL and Duan FL: Messenger RNA expression of translationally
controlled tumor protein (TCTP) in liver regeneration and cancer.
Anticancer Res. 28:1575–1580. 2008.PubMed/NCBI
|
|
27
|
Hsiao YC, Hsieh YS, Kuo WH, Chiou HL, Yang
SF, Chiang WL and Chu SC: The tumor-growth inhibitory activity of
flavanone and 2′-OH flavanone in vitro and in vivo through
induction of cell cycle arrest and suppression of cyclins and CDKs.
J Biomed Sci. 14:107–119. 2007. View Article : Google Scholar
|
|
28
|
Ayyagari VN and Brard L: Bithionol
inhibits ovarian cancer cell growth in vitro-studies on
mechanism(s) of action. BMC Cancer. 14:612014. View Article : Google Scholar
|
|
29
|
He L, Lu N, Dai Q, Zhao Y, Zhao L, Wang H,
Li Z, You Q and Guo Q: Wogonin induced G1 cell cycle arrest by
regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in
human colorectal cancer carcinoma cells. Toxicology. 312:36–47.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jung J, Kim HY, Maeng J, Kim M, Shin DH
and Lee K: Interaction of translationally controlled tumor protein
with Apaf-1 is involved in the development of chemoresistance in
HeLa cells. BMC Cancer. 14:1652014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Susini L, Besse S, Duflaut D, Lespagnol A,
Beekman C, Fiucci G, Atkinson AR, Busso D, Poussin P, Marine JC, et
al: TCTP protects from apoptotic cell death by antagonizing bax
function. Cell Death Differ. 15:1211–1220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu D, Guo Z, Min W, Zhou B, Li M, Li W and
Luo D: Upregulation of TCTP expression in human skin squamous cell
carcinoma increases tumor cell viability through anti-apoptotic
action of the protein. Exp Ther Med. 3:437–442. 2012.PubMed/NCBI
|
|
33
|
Rho SB, Lee JH, Park MS, Byun HJ, Kang S,
Seo SS, Kim JY and Park SY: Anti-apoptotic protein TCTP controls
the stability of the tumor suppressor p53. FEBS Lett. 585:29–35.
2011. View Article : Google Scholar
|
|
34
|
Kaarbo M, Storm ML, Qu S, Wæhre H, Risberg
B, Danielsen HE and Saatcioglu F: TCTP is an androgen-regulated
gene implicated in prostate cancer. PLoS One. 8:e693982013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakopoulou L, Tsirmpa I, Alexandrou P,
Louvrou A, Ampela C, Markaki S and Davaris PS: MMP-2 protein in
invasive breast cancer and the impact of MMP-2/TIMP-2 phenotype on
overall survival. Breast Cancer Res Treat. 77:145–155. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo Y, Liang F and Zhang ZY: PRL1 promotes
cell migration and invasion by increasing MMP2 and MMP9 expression
through Src and ERK1/2 pathways. Biochemistry. 48:1838–1846. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chu ZH, Liu L, Zheng CX, Lai W, Li SF, Wu
H, Zeng YJ, Zhao HY and Guan YF: Proteomic analysis identifies
translationally controlled tumor protein as a mediator of
phosphatase of regenerating liver-3-promoted proliferation,
migration and invasion in human colon cancer cells. Chin Med J
(Engl). 124:3778–3785. 2011.
|
|
38
|
Ma Q, Geng Y, Xu W, Wu Y, He F, Shu W,
Huang M, Du H and Li M: The role of translationally controlled
tumor protein in tumor growth and metastasis of colon
adenocarcinoma cells. J Proteome Res. 9:40–49. 2010. View Article : Google Scholar
|