Introduction
Cataracts are a type of visual impairment due to
reduction of lens transparency and changes of crystalline lens
color (1). They are the premier
cause of blindness worldwide (2),
accounting for 47.8% of all causes of blindness (3). Although high-quality surgical
treatments of cataracts have had certain positive effects, the
number of patients with cataract-induced blindness is increasing
(4). Ultraviolet radiation (UV),
oxidative stress and other factors lead to the formation of
cataracts (5,6). UV can induce DNA damage and result in
cell apoptosis, thereby disrupting the physiological functions of
the crystalline lenses and causing a series of physiological
changes. Loss of the crystalline lens microstructure interferes
with the transmission of light to the retina, leading to visual
impairment. UV exposure is inevitable in daily life and is closely
linked to eye diseases. Therefore, studying UV-induced apoptosis in
crystalline lenses may provide clues for the exploration of the
causes of cataract formation and lead to the development of novel
treatments.
ELL-associated factor 2 (Eaf2) is a potential tumor
suppressor. It was discovered as a protein binding to ELL to form a
complex. Eaf2, as a potential regulator of transcription, interacts
with ELL and increases the extension activity of RNA polymerase II
(7). Eaf2 is involved in multiple
physiological processes, including regulation of transcription
activation, cell apoptosis and embryonic development (8). Compared to normal cells, prostate
cancer cell lines have significantly lower Eaf2 expression levels.
The loss of the Eaf2 gene promotes tumorigenesis of prostate cancer
(9), whereas over-expression of
Eaf2 in prostate cancer cells can induce apoptosis (10). In vivo studies showed that
Eaf2 can inhibit the growth and induce apoptosis of xenografted
prostate tumors (10). Eaf2
knockout causes the formation of tumors, including lung
adenocarcinoma, B-cell lymphoma and hepatocellular carcinoma
(11). In addition, Eaf2 has
important roles in embryonic development, in particular during the
development of the eyes (12,13).
Eaf2 expression is undetectable in proliferating epithelial cells
of the anterior lenses, but can be detected in terminally
differentiated and non-proliferating lens fibroblasts. These
studies indicated that Eaf2 has important roles in the regulation
of crystalline lens development and maturation (13).
Eaf2 has an important role in the regulation of
crystalline lens development. However, its roles in UV-induced
cataract formation have yet to be fully elucidated. In the present
study, the roles of Eaf2 in UV-induced apoptosis were investigated
in crystalline lenses of mice; furthermore, the activity of
caspase-3 and caspase-9 and the expression levels of B-cell
lymphoma 2 (bcl-2), bcl-2-associated X protein (bax) and
phosphorylated extracellular signal-regulated kinase (p-ERK) were
assessed in wild-type (WT) and Eaf2 knockout (Eaf2 KO) mice. The
present study laid a theoretical foundation for the development of
drugs for cataract treatment.
Materials and methods
Animals
A total of 40 14-week-old WT or Eaf2 KO mice
(14) were divided into four
groups (n=10 in each): i) WT-nonUV, ii) WT-UV, iii) Eaf2 KO-nonUV
and iv) Eaf2 KO-UV. The right eyes of the WT-UV mice and Eaf2 KO-UV
mice were exposed to UV radiation, while the left eyes received no
radiation. WT-nonUV mice and Eaf2 KO-nonUV mice were not exposed to
UV. The Eaf2 KO mice were obtained from Professor Yi Sin Liu
(University of Southern California, Los Angeles, CA, USA). The mice
were maintained in an environment with a constant temperature of 23
± 2°C, a relative humidity of 50±5 % and a 12 h light-dark cycle.
Chow and water were provided ad libitum. The mice were
sacrificed by decapitation. All animal experiments were performed
according to the Guide for the Care and Use of Laboratory Animals.
The present met the standards of, and was approved by, the ethics
committee of China Medical University (Shenyang, China).
Construction of the Eaf2 overexpression
plasmid (Eaf2 OE)
The Eaf2 coding sequence (CDS) was amplified by
polymerase chain reaction (PCR) using murine cDNA, which was
reverse transcribed from total RNA extracted from the rat cells.
This was maintained in our laboratory as a template. The primers
used were as follows: Eaf2-CDS forward,
5′-GTATGAAAGCTTAAGGCCAAAAGCGG-3′ and reverse,
5′-GCCCGAATTCATCTCACAAATGTTTTCTCTGT-3′. The primers were
synthesized by Sangon Biotech (Shanghai, China). The PCR was
performed using a Life Express PCR Instrument (Bioer, Hangzhou,
China) and the following cycling conditions were used: 95 °C for 5
min; 95°C for 30 sec, 55°C for 30 sec, 72°C for 60 sec, 30 cycles;
72°C for 5 min and 4°C for 2 min. The products were purified using
a multifunctional DNA purification kit (BioTeke, Beijing, China).
The sequencing was performed by Sangon Biotech). The coding
sequence was inserted into the p enhanced green fluorescence
protein-N1 vector (Clontech, Mountain View, CA, USA) by the
restriction enzymes FastDigest HindIII and FastDigest
EcoRI (Fermentas, Burlington, CA, USA). The vector was
sequenced for verification and was named as Eaf2 OE.
Cell culture and transfection
The rat crystalline lens epithelial cell line α-TN4
was cultured in Dulbecco's modified Eagle's medium (Gibco
Invitrogen Life Technologies, Carlsbad, CA, USA) containing 10%
(v/v) fetal bovine serum (Hyclone, Logan, UT, USA). The cells were
incubated in an incubator at 37°C in an atmosphere containing 5%
(v/v) CO2. Cells were seeded into six-well plates and
transfected at 70–80% confluence with the empty vector (Vector) or
the Eaf2 overexpression plasmid (Eaf2 OE) using Lipofectamine 2000
reagent (Invitrogen Life Technologies) according to the
manufacturer's instructions. 48 h after transfection, the
transfection efficiency was analyzed by quantitative real-time PCR
and western blotting.
Mouse model of UV-induced cataracts
UV at a wavelength of 302 nm and intensity of 200
mW/cm2 was generated by a UV transilluminator
(Spectroline XX-15N/F; Spectronics, Westbury, NY, USA). The UV
transilluminator was covered with aluminum foil, leaving only a
small hole with a diameter of 5 mm for irradiating the eyes of the
mice. The mice were not anesthetized, and only the right eyes were
irradiated with UV. The left eyes were not irradiated and used as a
control. 5 min prior to UV irradiation, the eyes of the mice were
injected with 1% (w/v) tropicamide (cat. no. T9778; Sigma-Aldrich,
St. Louis, MO, USA) and 0.1% (w/v) atropine sulfate to induce
mydriasis. Prior to UV irradiation, the mice were checked with a
slit lamp to exclude pre-existing cataracts. One eye of each mouse
was exposed to UV for 100 sec twice per week for three weeks. 48 h
after the last UV irradiation, the mouse lens opacity was observed
by slit lamp examination.
Preparation of lens tissue
48 h after UV irradiation, the eyeballs of all the
mice were surgically removed, together with a ~2-mm optic nerve.
The eyeballs were incubated in fixing buffer, containing acetic
acid, formaldehyde solution (Kemiou Chemical Reagent Co., Lt.,
Tianjing China), physiological saline (Cisen Pharmaceutical Co.,
Ltd., Shandong, China) and 75% ethanol at a ratio of 1:2:7:10.
After fixing, the crystalline lenses were removed under a
microscope OLYMPUS DP73 (Olympus Corporation, Tokyo, Japan) for
subsequent experiments.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) apoptosis analysis
Apoptosis was analyzed using the In Situ Cell Death
Detection kit (cat. no. 11684817910; Roche, Basel, Switzerland).
Fixed eyes were paraffin-embedded and cut into tissue sections of 5
µm. Cultured cells were seeded and fixed with 4% (w/v)
paraformaldehyde. Prior to the TUNEL assay, the samples were
permeabilized with 0.1% (v/v) TritonX-100 (Beyotime Institute of
Biotechonlogy, Shanghai, China) and blocked with 3% (v/v)
H2O2. The TUNEL reagent was mixed with Enzyme
Solution and Label Solution and then added to the sample surfaces
dropwise. The samples were incubated at 37°C in dark in a humid
environment for 60 min. The Converter-POD was added to the sample
surfaces dropwise and incubated at 37°C for 30 min followed by
diaminobenzidine substrate (Solarbio, Beijing, China). The samples
were counterstained with hematoxylin (Solarbio), mounted with
neutral balsam and subjected to microscopic imaging.
Western blot analysis
Proteins in tissues were extracted using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) containing 1% phenylmethanesul-fonylfluoride
(Beyotime Institute of Biotechnology). Proteins in cultured cells
were extracted using NP-40 lysis buffer (Beyotime Institute of
Biotechnology). Proteins extracted were quantified using a
Bicinchoninic Acid Protein Assay kit (cat. no. P0012; Beyotime
Institute of Biotechnology). Equal amounts of each protein sample
were subjected to SDS-PAGE. The separated proteins were then
transferred to polyvinylidene fluoride (PVDF) membranes (Millipore,
Bedford, MA, USA). PVDF membranes were blocked with 5% (w/v)
skimmed milk or 5% (w/v) bovine serum albumin (Biosharp, Hefei,
China) and incubated at 4°C overnight with the corresponding
primary rabbit antibodies against bax (rabbit anti-mouse polyclonal
antibody; 1:500; cat. no. WL0101; Wanleibio, Shenyang, China),
bcl-2 (rabbit anti-mouse polyclonal antibody; 1:500; cat. no.
WL0104; Wanleibio), ERK (rabbit anti-mouse polyclonal antibody;
1:1,000; cat. no. WL0323; Wanleibio), p-ERK (rabbit anti-mouse
polyclonal antibody; 1:1,000; cat. no. WLP002; Wanleibio), Eaf2
(rabbit anti-mouse polyclonal antibody; 1:1,000; cat. no. WL0333;
Wanleibio), cleaved-caspase-3 (rabbit anti-mouse polyclonal
antibody; 1:1,000; cat. no. ab2575; Abcam, Cambridge, MA, USA) and
cleaved-caspase-9 (rabbit anti-mouse polyclonal antibody; 1:1,000;
cat. no. WL0001; Wanleibio). The PVDF membranes were then incubated
with horseradish peroxidase-labeled goat anti-rabbit secondary
antibody (1:5,000; cat. no. A0208; Beyotime Institute of
Biotechnology) at 37°C for 45 min. Using β-actin as an internal
reference, the objective proteins were detected by an enhanced
chemiluminescence detection system (cat. no. E002-5; 7Sea Biotech,
Shanghai, China). The signal intensities of the protein bands were
analyzed using Gel-Pro-Analyzer 4.5 software.
Caspase-3 and caspase-9 activity
assay
The crystalline lens tissues were mixed with nine
volumes of phosphate-buffered saline, homogenized using a High
speed Homogenizer (Scientz, Ningbo, China) and then frozen and
thawed three times in liquid nitrogen. The samples were centrifuged
at 10010 × g at 4°C for 10 min, and the supernatants were retained
for the experiments. The protein concentrations were measured using
the Bradford Protein Assay kit (Beyotime Institute of
Biotechnology) and diluted to equal concentrations. Caspase-3 and
caspase-9 activities were analyzed using Caspase-3 Activity Assay
kit (cat. no. C1116) and Caspase-9 Activity Assay kit (cat. no.
C1158; Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. The absorbance at 409 nm was measured
using a microplate reader (ELX-800; BioTek, Winooski, VT, USA).
Reverse transcription quantitative PCR
(RT-qPCR)
Total RNA was extracted using the RNA simple Total
RNA kit (cat. no. DP419; Tiangen, Beijing, China), according to the
manufacturer's instructions. The total RNA was reverse transcribed
into cDNA by Moloney mouse leukemia virus reverse transcriptase
(BioTeke) and oligo (dT)15 (Sangon Biotech). Eaf2 mRNA
levels were determined by RT-qPCR using the SYBR GREEN method with
cDNA as template. SYBR GREEN reagent was purchased from Solarbio.
The primers used are as follows: Eaf2 forward,
5′-CTTGCATACCTGGACCGT-3′ and reverse, 5′-GTTCACCTTTGCCAACCTCA-3′;
β-actin forward, 5′-CTGTGCCCATCTACGAGGGCTAT-3′ and reverse,
5′-TTTGATGTCACGCACGATTTCC-3′. The primers were synthesized by
Sangon Biotech. The RT-qPCR reaction volume (20 µl),
contained cDNA (1 µl), forward primer (10 µM; 0.5
µl), reverse primer (10 µM; 0.5 µl), SYBR
GREEN mastermix (10 µl) and water. An Exicycler™ 96, BIONEER
Real-Time PCR system was used (Bioneer Corporation, Daejeon, Korea)
and the reaction conditions were as follows: 95°C for 10 min
followed by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C
for 30 sec, and final incubation at 4°C 5 min. The relative mRNA
expression levels for each sample were calculated using the
2−ΔΔCt method with β-actin as an internal reference
(15).
Statistical analysis
Values are expressed as the mean ± standard
deviation. All experiments were repeated three times. Differences
between groups were analyzed using one-way analysis of variance and
Bonferroni's Multiple Comparison. Statistical analysis was
performed using GraphPad Prism 5 software (GraphPad Software, Inc.,
San Diego, CA, USA). P<0.05 was considered to indicate a
statistically significant difference between values.
Results
UV induces apoptosis in mouse cataract
lenses
In order to study the role of Eaf2 in UV-induced
formation of cataracts in mice, UV was used to irradiate the
crystalline lenses of WT mice and Eaf2 KO mice, and a TUNEL assay
was used to quantify the apoptosis in the crystalline lenses. For
Eaf2 KO and WT mice, the apoptosis rates of the crystalline lenses
after receiving UV irradiation were higher than those in animals
that were not exposed to UV (Fig.
1). These results indicated that UV induced apoptosis in the
crystalline lenses. In addition, the apoptotic rates in crystalline
lenses in the Eaf2 KO-UV group were lower than those in the WT-UV
group (Fig. 1). These results
implied that Eaf2 knockout reduces UV-induced apoptosis in the
crystalline lenses, thereby mitigating the formation of UV-induced
cataracts.
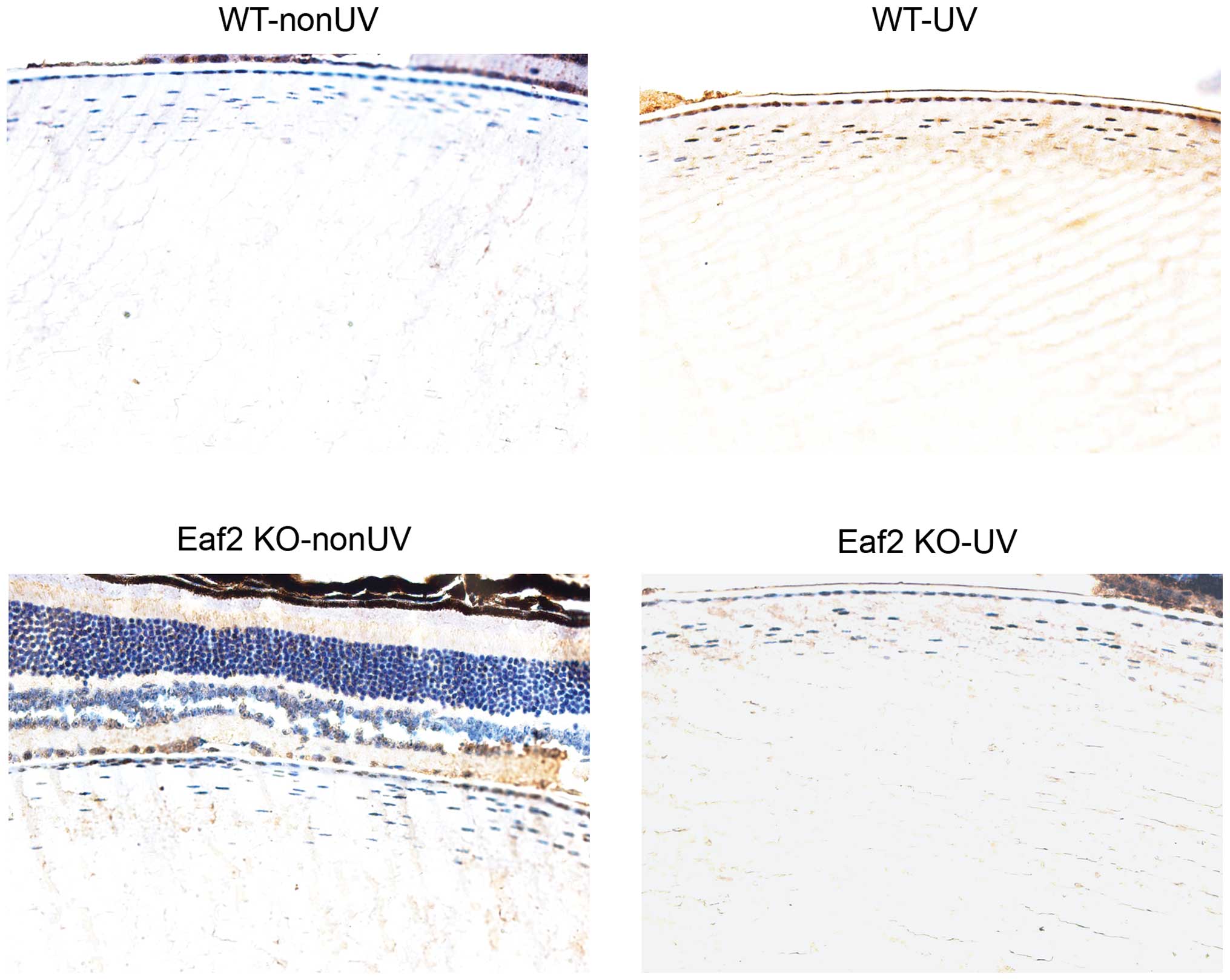 | Figure 1UV radiation induces apoptosis in
crystalline lenses. After UV irradiation, apoptosis in the
crystalline lenses of mice in each group was detected by terminal
deoxynucleotidyl transferase dUTP nick end labeling assay
(magnification, x400). WT-nonUV, wild-type mice without UV
radiation; WT-UV, wild-type mice with UV radiation; Eaf2 KO-nonUV,
Eaf2-KO mice without UV irradiation; Eaf2 KO-UV, Eaf2-KO mice with
UV radiation. UV, ultraviolet; Eaf2, ELL associated factor 2; WT,
wild-type; KO, knockout. |
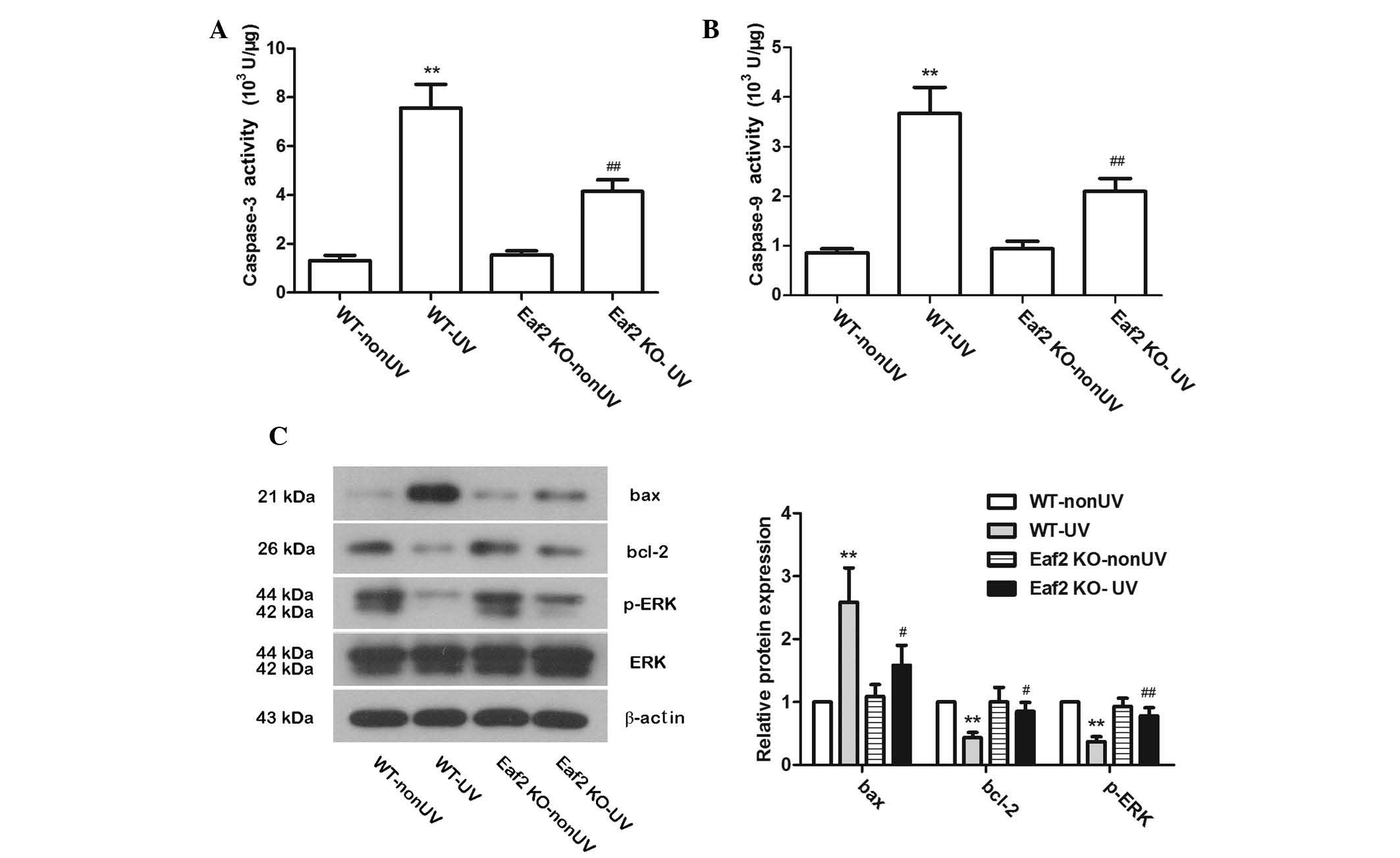
Eaf2 knockout reduces UV-induced
apoptosis
To further study the role of Eaf2 in UV-induced
cataract formation in mice, the activities of caspase-3 and
caspase-9 were examined. In WT-UV and Eaf2 KO-UV mice, after UV
irradiation, the activities of caspase-3 and caspase-9 were
significantly increased. Compared with WT-UV mice, Eaf2 KO-UV mice
had signifi-cantly lower caspase-3 and caspase-9 activities
(Fig. 2A and B; P<0.01). These
results suggested that Eaf2 knockout can reduce UV-induced
apoptosis, which was consistent with the results of the TUNEL
assays. Protein levels of bax, bcl-2, ERK and p-ERK were also
detected by western blotting. In WT-UV mice, bax protein levels
were increased, while bcl-2 and p-ERK protein expression levels
were decreased as compared with those in the WT-nonUVmice. Of note,
compared with WT-UV mice, Eaf2 KO-UV mice had decreased bax levels
and elevated bcl-2 and p-ERK levels (Fig. 2C). These results, on the molecular
level, suggested that Eaf2 knockout can reduce UV-induced apoptosis
in crystalline lenses.
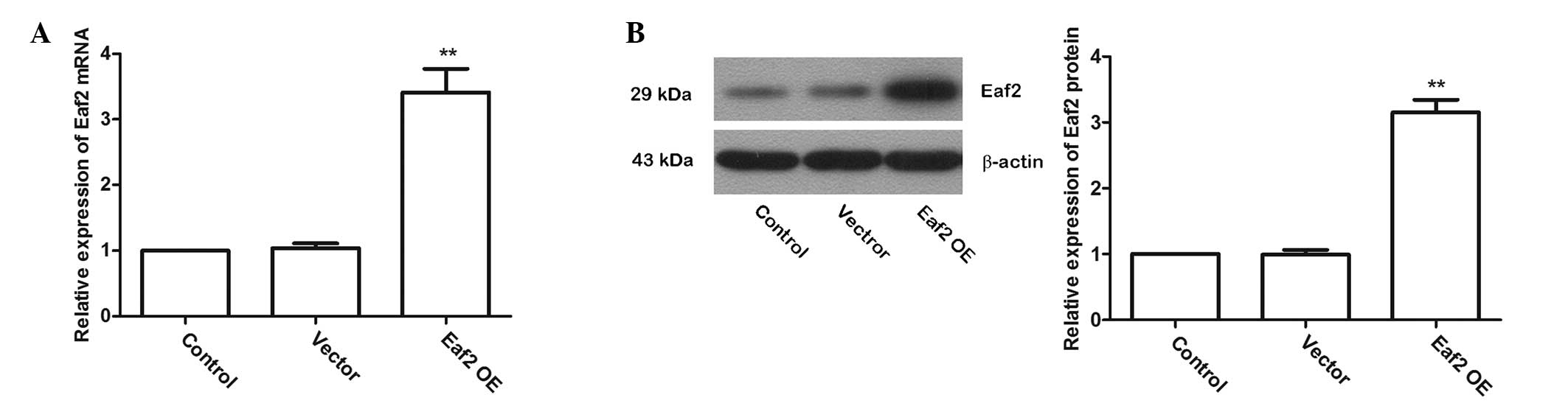
Eaf2 overexpression
To investigate the role of Eaf2 in UV-induced
apoptosis in crystalline lenses, a plasmid named Eaf2 OE to
over-express Eaf2 in murine crystalline lens cells was constructed
and the over-expression efficiency was detected by RT-qPCR and
western blotting. The RT-qPCR results showed that after
transfection with Eaf2 OE, the Eaf2 mRNA levels were significantly
increased (Fig. 3A; P<0.01).
The western blotting results also showed that after transfection
with Eaf2 OE, Eaf2 protein levels were significantly increased
(Fig. 3B; P<0.01). These
results indicated that transfection of Eaf2 OE effectively
increased the levels of Eaf2 in murine crystalline lens cells.
Eaf2 overexpression induces apoptosis in
murine crystalline lens cells
The TUNEL assay was used to detect cell apoptosis
after transfection with Eaf2 OE. Compared with non-transfected
(Control) cells and cells transfected with empty vector (Vector),
cells transfected with Eaf2 OE exhibited a significantly increased
level of apoptosis (Fig. 4A).
Western blotting was then used to examine protein levels of cleaved
caspase-3 and cleaved caspase-9. After transfection with Eaf2 OE,
the levels of cleaved caspase-3 and cleaved caspase-9 were
significantly increased (Fig. 4B;
P<0.01), indicating an increase in the activation of caspase-3
and caspase-9. Western blotting was also used to detect the protein
levels of the pro-apoptotic protein bax and the anti-apoptotic
protein bcl-2. In cells over-expressing Eaf2, bax expression was
significantly elevated, whereas bcl-2 expression levels were
significantly reduced (Fig. 4C;
P<0.01). These results illustrated that Eaf2 promoted the
activation of caspase-3 and caspase-9 and affected the expression
of apoptosis-associated proteins, thereby inducing apoptosis.
Discussion
UV (5), smoking
(16,17), vitamin deficiency (18), oxidative stress (19) and other factors may cause
cataracts. Among these factors, UV is an inevitable risk factor
encountered in daily life. In the present study, the role of Eaf2
in UV-induced cataract formation in mice was investigated. The
In vivo experiment suggested that Eaf2 knockout was able to
mitigate UV-induced cataract formation in mice. Furthermore, the
results of the in vitro study suggested that Eaf2 activates
caspases, regulates the expression levels of apoptosis-associated
proteins and promotes apoptosis of crystalline lens cells. These
results provided a theoretical basis for the development of novel
cataract treatments.
UV-induced apoptosis in the crystalline lenses is an
important cause of UV-induced cataracts. The present study found
that UV irradiation can induce apoptosis of crystalline lens cells
in WT and Eaf2-KO mice. It has been reported that UV-induced
cataracts are associated with the activation of P53 and caspase-3
(20). UV activates P53 and
caspase-3 and ultimately induces apoptosis in crystalline lens
cells, resulting in cataracts. In addition, UV alters the
translocation of nuclear factor (NF)-κB. Blocking the NF-κB pathway
with an NF-κB inhibitor decreased the degree of UV-induced cell
death (21). UV can also cause DNA
damage (22), interfering with DNA
replication and transcription. When a large number of cells undergo
apoptosis, the physiological functions of the crystalline lens are
disturbed, causing cataract lesions.
Eaf2 has an extensive role in the development of
crystalline lenses. During mouse embryo development, Eaf2 has a
spatial regulation mode in the developing crystalline lens
(13). A study showed that in
Xenopus laevis, Eaf2 deficiency leads to the loss of the
eyes (12). These studies provided
important evidence for the importance of Eaf2 in the developing
eye. The present study found that the extent of apoptosis in Eaf2
KO-UV mice was significantly lower than that in WT-UV mice. These
results implied that Eaf2 knockout, to a certain degree, has a
mitigating role in UV-induced cataracts. Furthermore, the present
study examined the UV-induced apoptosis in Eaf2-KO murine
crystalline lenses. UV irradiation activated caspase-3 and
caspase-9, promoted the expression of bax, and inhibited the
expression of bcl-2 as well as the activation of ERK. These results
are consistent with the results of a previous study (20). Eaf2-KO reduced the UV-induced
activation of caspase-3 and caspase-9, changes in bax and bcl-2
expression levels, and ERK pathway activation. These results
suggested that Eaf2 may modulate UV-induced apoptosis through
regulating caspase activation, the expression of
apoptosis-associated proteins and the activation of the ERK
pathway. Further study of gene functions showed that Eaf2 promoted
caspase-3 and caspase-9 activation, altered bax and bcl-2
expression and thus affected apoptosis of crystalline lens cells.
Similar to the present study, Xiao et al (14) showed that Eaf2 can affect the
activation of caspase-3, as well as the expression levels of bax,
BH3 interacting-domain death agonist and P53, thus affecting
UV-induced apoptosis in crystalline lenses. In the process of
UV-induced cataract formation, UV can lead to DNA damage, which
affects the distribution of Eaf2. It has been indicated that UV
irradiation may promote Eaf2 translocation to the nucleolus
(8). Studies have shown that Eaf2
can also act as a tumor suppressor gene either by regulating ERK
phosphorylation (23) or by
binding to the retinoblastoma protein to inhibit the Ras pathway
(24). However, how Eaf2 induces
apoptosis during UV-induced cataract formation is worth pursuing in
in-depth studies.
In the present study, the effects of Eaf2 in
UV-induced apoptosis of murine crystalline lens cells were
investigated. The results showed that Eaf2 promoted the activation
of caspase-3 and caspase-9, increased the expression of the
pro-apoptotic protein bax and inhibited the expression of the
anti-apoptotic protein bcl-2, thereby promoting apoptosis of
crystalline lens cells. Eaf2-KO inhibited UV-induced activation of
caspase-3 and caspase-9 as well as changes in the expression levels
of apoptosis-associated proteins, and Eaf2 KO was shown to mitigate
UV-induced cataracts. These results laid a theoretical foundation
for developing novel drugs for cataract treatment.
Acknowledgments
This study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81270988).
References
|
1
|
Wormstone IM, Collison DJ, Hansom SP and
Duncan G: A focus on the human lens in vitro. Environ Toxicol
Pharmacol. 21:215–221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moghaddam MS, Kumar PA, Reddy GB and Ghole
VS: Effect of Diabecon on sugar-induced lens opacity in organ
culture: Mechanism of action. J Ethnopharmacol. 97:397–403. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Resnikoff S, Pascolini D, Etya'ale D,
Kocur I, Pararajasegaram R, Pokharel GP and Mariotti SP: Global
data on visual impairment in the year 2002. Bull World Health
Organ. 82:844–851. 2004.
|
|
4
|
McCarty CA: Cataract in the 21st Century:
Lessons from previous epidemiological research. Clin Exp Optom.
85:91–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyer LM, Söderberg P, Dong X and Wegener
A: UVR-B induced cataract development in C57 mice. Exp Eye Res.
81:389–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varma SD, Chand D, Sharma YR, Kuck JF Jr
and Richards RD: Oxidative stress on lens and cataract formation:
Role of light and oxygen. Curr Eye Res. 3:35–57. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong SE, Banks CA, Shilatifard A, Conaway
JW and Conaway RC: ELL-associated factors 1 and 2 are positive
regulators of RNA polymerase II elongation factor ELL. Proc Natl
Acad Sci USA. 102:10094–10098. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhuang F, Yen P, Zhao J, Nguyen M, Jiang M
and Liu YH: Dynamic intracellular distribution of Eaf2 and its
potential involvement in UV-Induced DNA damage response. DNA Cell
Biol. 27:649–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ai J, Pascal LE, O'Malley KJ, Dar JA,
Isharwal S, Qiao Z, Ren B, Rigatti LH, Dhir R, Xiao W, et al:
Concomitant loss of EAF2/U19 and Pten synergistically promotes
prostate carcinogenesis in the mouse model. Oncogene. 33:2286–2294.
2014. View Article : Google Scholar
|
|
10
|
Xiao W, Zhang Q, Jiang F, Pins M,
Kozlowski JM and Wang Z: Suppression of prostate tumor growth by
U19, a novel testosterone-regulated apoptosis inducer. Cancer Res.
63:4698–4704. 2003.PubMed/NCBI
|
|
11
|
Xiao W, Zhang Q, Habermacher G, Yang X,
Zhang AY, Cai X, Hahn J, Liu J, Pins M, Doglio L, et al: U19/Eaf2
knockout causes lung adenocarcinoma, B-cell lymphoma,
hepatocellular carcinoma and prostatic intraepithelial neoplasia.
Oncogene. 27:1536–1544. 2008. View Article : Google Scholar
|
|
12
|
Maurus D, Héligon C, Bürger-Schwärzler A,
Brändli AW and Kühl M: Noncanonical Wnt-4 signaling and EAF2 are
required for eye development in Xenopus laevis. EMBO J.
24:1181–1191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li M, Wu X, Zhuang F, Jiang S, Jiang M and
Liu YH: Expression of murine ELL-associated factor 2 (Eaf2) is
developmentally regulated. Dev Dyn. 228:273–280. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao F, Zhang JS, Zhao JY and Wu D:
Regulation of Eaf2 in mouse lens cells apoptosis induced by
ultraviolet radiation. Int J Ophthalmol. 5:570–575. 2012.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Kelly SP, Thornton J, Edwards R, Sahu A
and Harrison R: Smoking and cataract: Review of causal association.
J Cataract Refract Surg. 31:2395–2404. 2005. View Article : Google Scholar
|
|
17
|
Krishnaiah S, Vilas K, Shamanna BR, Rao
GN, Thomas R and Balasubramanian D: Smoking and its association
with cataract: Results of the Andhra Pradesh eye disease study from
India. Invest Ophthalmol Vis Sci. 46:58–65. 2005. View Article : Google Scholar
|
|
18
|
Ishikawa Y, Hashizume K, Kishimoto S,
Tezuka Y, Nishigori H, Yamamoto N, Kondo Y, Maruyama N, Ishigami A,
Kurosaka D, et al: Effect of vitamin C depletion on UVR-B induced
cataract in SMP30/GNL knockout mice. Exp Eye Res. 94:85–89. 2012.
View Article : Google Scholar
|
|
19
|
Truscott RJ: Age-related nuclear
cataract-oxidation is the key. Exp Eye Res. 80:709–725. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ayala M, Strid H, Jacobsson U and
Söderberg PG: p53 expression and apoptosis in the lens after
ultraviolet radiation exposure. Invest Ophthalmol Vis Sci.
48:4187–4191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee DH, Cho KS, Park SG, Kim EK and Joo
CK: Cellular death mediated by nuclear factor kappa B (NF-kappaB)
translocation in cultured human lens epithelial cells after
ultraviolet-B irradiation. J Cataract Refract Surg. 31:614–619.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mesa R and Bassnett S: UV-B-induced DNA
damage and repair in the mouse lens. Invest Ophthalmol Vis Sci.
54:6789–6797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su F, Correa BR, Luo J, Vencio RZ, Pascal
LE and Wang Z: Gene Expression Profiling Reveals Regulation of ERK
Phosphorylation by androgen-induced tumor suppressor U19/EAF2 in
the mouse prostate. Cancer Microenviron. 6:247–261. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai L, Wang D, Fisher AL and Wang Z:
Identification of a genetic interaction between the tumor
suppressor EAF2 and the retinoblastoma protein (Rb) signaling
pathway in C. elegans and prostate cancer cells. Biochem Biophys
Res Commun. 447:292–298. 2014. View Article : Google Scholar : PubMed/NCBI
|